Summary
The bromodomain and extraterminal (BET) protein BRD4 is a therapeutic target in acute myeloid leukemia (AML). Here, we demonstrate that the AML maintenance function of BRD4 requires its interaction with NSD3, which belongs to a subfamily of H3K36 methyltransferases. Unexpectedly, AML cells were found to only require a short isoform of NSD3 that lacks the methyltransferase domain. We show that NSD3-short is an adaptor protein that sustains leukemia by linking BRD4 to the CHD8 chromatin remodeler, by utilizing a PWWP domain-mediated interaction with methylated H3K36, and by employing an acidic transactivation domain. Genetic targeting of NSD3 or CHD8 mimics the phenotypic and transcriptional effects of BRD4 inhibition. Furthermore, BRD4, NSD3, and CHD8 colocalize across the AML genome and are each released from super-enhancer regions upon chemical inhibition of BET bromodomains. These findings suggest that BET inhibitors exert therapeutic effects in leukemia by evicting BRD4-NSD3-CHD8 complexes from chromatin to suppress transcription.
Introduction
BRD4 belongs to the bromodomain and extraterminal (BET) family of transcriptional coactivators, which use tandem bromodomain modules to recognize acetyl-lysine side chains on various nuclear proteins (Shi and Vakoc, 2014; Wu and Chiang, 2007). Original studies demonstrated a critical role for histone tail acetylation in tethering BRD4 to chromatin (Dey et al., 2003), however emerging evidence suggests that acetylation of transcription factors (TFs) is also a major mechanism that directs BRD4 to enhancer and promoter regions across the genome (Brown et al., 2014; Huang et al., 2009; Roe et al., 2015; Shi et al., 2014). When bound to regulatory elements, BRD4 activates transcription of nearby genes, in part via the direct interaction of its C-terminal domain (CTD) with the kinase P-TEFb (Bisgrove et al., 2007; Jang et al., 2005; Yang et al., 2005). Proteomic screens have revealed additional factors that associate with the BRD4 extraterminal (ET) domain, including NSD3, JMJD6, and GLTSCR1, although the functional relevance of these interactions is uncertain (Liu et al., 2013; Rahman et al., 2011).
Despite the apparent role of BRD4 as a general transcriptional coactivator, blocking the function of BET bromodomains with small molecules (e.g. JQ1 or I-BET) disproportionately suppresses the expression of specific genes and leads to distinct cellular phenotypes (Filippakopoulos et al., 2010; Nicodeme et al., 2010). One striking effect of BET inhibitors is their preferential toxicity to cancer cells, which was first demonstrated in midline carcinoma cells harboring the BRD4-NUT fusion oncogene and subsequently in various hematopoietic cancers that lack BRD4 rearrangements, such as acute myeloid leukemia (AML) (Dawson et al., 2011; Delmore et al., 2011; Filippakopoulos et al., 2010; Mertz et al., 2011; Zuber et al., 2011b). Indeed, AML cells are hypersensitive to BRD4 knockdown and to pharmacological BET inhibition (Dawson et al., 2011; Mertz et al., 2011; Zuber et al., 2011b), an observation that has motivated several ongoing clinical trials of BET inhibitors in human AML patients (Clinicaltrials.gov Identifiers: NCT02158858, NCT02308761, and NCT01943851). The therapeutic potential of targeting BRD4 in AML stems from its role in maintaining the expression of several key oncogenes, including MYC, BCL2, and CDK6 (Dawson et al., 2011; Mertz et al., 2011; Zuber et al., 2011b). In leukemia cells, each of these loci possesses large clusters of BRD4-occupied enhancers, termed super-enhancers, which are assembled through the coordinated action of hematopoietic transcription factors and the lysine acetyltransferase activity of p300 (Dawson et al., 2014; Loven et al., 2013; Roe et al., 2015; Shi et al., 2013b). While molecular mechanisms that target BRD4 to specific genomic sites in AML have been identified (Roe et al., 2015), the effector proteins required for BRD4-dependent transcriptional activation in this disease are unknown.
NSD3 (encoded by WHSC1L1) is a member of the NSD family of histone H3 lysine 36 (H3K36) methyltransferases, which function as oncoproteins in a variety of cancer contexts (Li et al., 2009; Lucio-Eterovic and Carpenter, 2011). NSD3 exists as three different isoforms (long, short, and whistle), with the long isoform possessing a H3K36 methyltransferase SET domain and seven chromatin reader modules (five PHD fingers and two PWWP domains) (Angrand et al., 2001; Kim et al., 2006). NSD3-whistle is a testes-specific isoform expressed from a downstream promoter, which produces a protein that retains the catalytic SET domain and the adjacent PWWP and PHD domains (Kim et al., 2006). NSD3-short, which is produced via alternative splicing, is less than half the size of NSD3-long and lacks the catalytic SET domain and six of the chromatin reader modules, but retains a single N-terminal PWWP domain that binds to histone H3 when it is methylated at lysine 36 (Vermeulen et al., 2010; Wu et al., 2011). While functions of the different NSD3 isoforms have been largely unexplored, one study suggests that NSD3-long can promote neural crest specification and migration through its H3K36 methyltransferase activity (Jacques-Fricke and Gammill, 2014). A rare subset of AML patients have been found to harbor a translocation involving NUP98 and WHSC1L1, which produce fusions of NUP98 with NSD3-long and NSD3-short (Rosati et al., 2002). In midline carcinoma, rare chromosomal translocations lead to the formation of NSD3-NUT fusions, which retain an N-terminal region common to NSD3-short and NSD3-long (French et al., 2014). WHSC1L1 also resides in a region on chromosome 8p11–12 that is commonly amplified in human breast and lung cancers, which has implicated NSD3 as a putative oncoprotein in these diseases (Tonon et al., 2005; Yang et al., 2010). Despite substantial evidence linking NSD3 to the pathogenesis of cancer, the molecular mechanisms underlying its function in these contexts is largely unknown. Prior studies have shown that NSD3 can associate with BRD4 in nuclear lysates, although the nature of this interaction and its functional relevance are unclear (French et al., 2014; Rahman et al., 2011).
Here we demonstrate that BRD4 utilizes an interaction with NSD3 to carry out its AML maintenance function. Remarkably, we found that AML cells only require the short isoform of NSD3, which we show contains a region that binds to the BRD4 ET domain. Despite lacking the catalytic SET domain and six of the chromatin reader modules present on the longer isoform, NSD3-short performs a critical gene regulatory function in this disease context using four interaction surfaces. This includes independent binding sites for BRD4 and for the CHD8 chromatin remodeling enzyme, a PWWP domain-mediated interaction with methylated H3K36, and a newly identified acidic transactivation domain. ChIP-seq analysis confirmed a global correlation among BRD4, NSD3, and CHD8 across the AML genome, with JQ1 causing the release of all three factors from super-enhancer regions located near oncogene loci. Based on these observations, we propose that NSD3-short is a multifunctional adaptor protein that performs an essential role in AML, and potentially other cancers, by linking BRD4 with CHD8 to sustain oncogenic transcriptional programs.
Results
A short isoform of NSD3 lacking lysine methyltransferase activity interacts with the BRD4 extraterminal domain and is essential for proliferation of AML cells
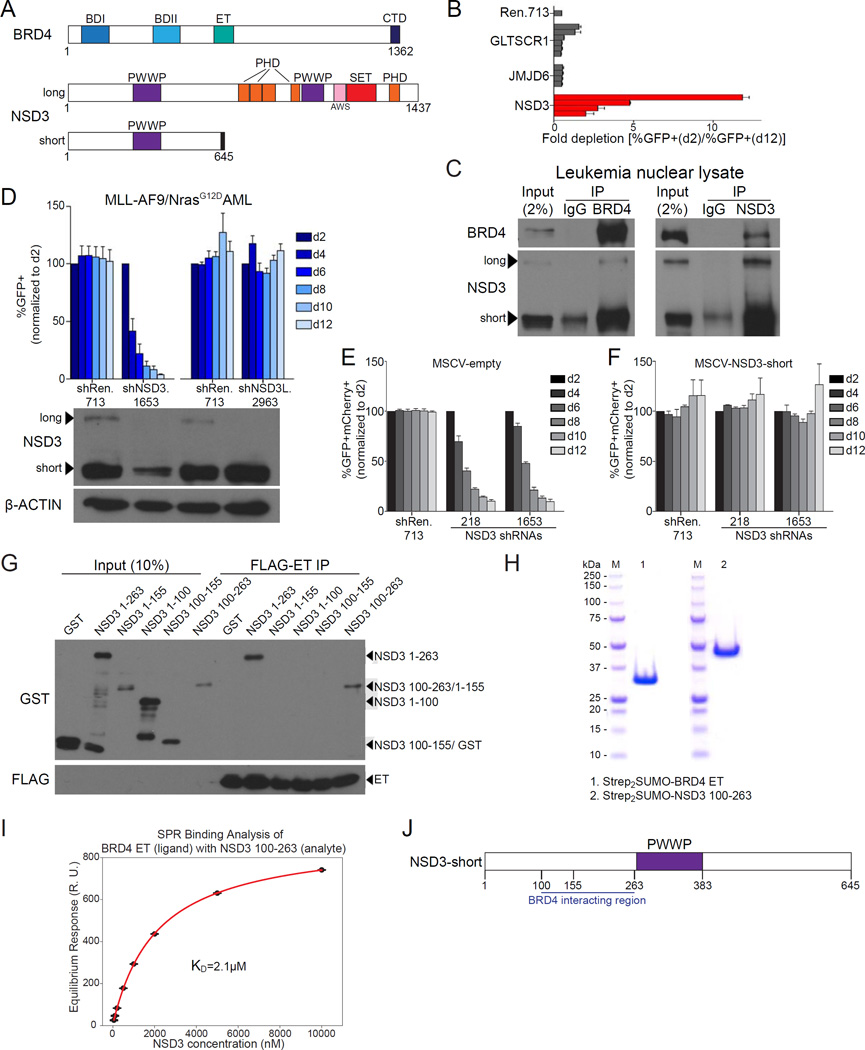
We previously used CRISPR-Cas9 scanning of Brd4 exons to implicate the BRD4 CTD and ET domains as requirements for the proliferation of RN2 cells, which is a cell line derived from a mouse model of MLL-AF9/NrasG12D AML (Figure 1A) (Shi et al., 2015; Zuber et al., 2011a). To examine the molecular function of the BRD4 ET domain in AML, we measured the sensitivity of RN2 cells to shRNA-based targeting of known ET-associated proteins GLTSCR1, JMJD6, and NSD3 (Liu et al., 2013; Rahman et al., 2011). Among these candidates, only NSD3 shRNAs (which target exons common to both long and short isoforms) reduced the proliferation of RN2 cells in vitro, an effect we also confirmed in vivo (Figure 1B, Figure S1A–S1B). Furthermore, NSD3 knockdown suppressed the growth of human AML cell lines HL-60, MOLM-13, and NOMO-1 (Figure S1C–S1F). These results prompted our investigation of NSD3 as a candidate effector of BRD4 that supports AML maintenance.
Figure 1. A short isoform of NSD3 lacking catalytic function is essential in AML and binds directly to the BRD4 extraterminal domain.
(A) Domain architectures of human BRD4 and NSD3. BDI: bromodomain I, BDII: bromodomain II, ET: extraterminal domain, CTD: C-terminal domain. PWWP: Pro-Trp-Trp-Pro chromatin reader module. PHD: plant homeodomain finger. AWS: Associated with SET domain region, SET: Su(var)3–9, enhancer-of-zeste and trithorax domain which catalyzes H3K36 methylation. A region unique to NSD3-short is represented by a black rectangle. (B) Competition-based assay in RN2 cells evaluating effects of the indicated LMN shRNAs (which express GFP) on cell proliferation. Each horizontal bar represents the average fold-decrease in GFP percentage for an independent shRNA over 10 days in culture. n=3. (C) Immunoprecipitation followed by Western blotting performed with the indicated antibodies. The nuclear lysate was prepared from the human AML cell line NOMO-1. IP: immunoprecipitation, IgG: isotype control immunoglobulin. Note: a background band appears in the control IP at ∼70 kDa, which is near the NSD3-short band. Shown is a representative experiment of three independent biological replicates. (D) (top) Competition-based assay to evaluate the effect of the indicated LMN shRNAs on RN2 cell proliferation. GFP percentages were normalized to day 2 (d2) measurements. shNSD3.1653 targets both long and short NSD3 isoforms and shNSD3L.2963 targets exclusively the long isoform. (bottom) Western blotting analysis of whole cell lysates prepared from RN2 cells following doxycycline (dox)-induced knockdown of NSD3. RN2 cells were transduced with TRMPV-Neo shRNAs followed by dox treatment for 48 hours. A representative experiment of three biological replicates is shown. (E–F) Competition-based assay evaluating the effect of the human NSD3-short cDNA (which is not recognized by the murine shRNAs and is expressed with the PIG vector linked to GFP) on the proliferation arrest induced by NSD3 shRNAs (expressed using the LMN-mCherry vector). Results were normalized to the d2 percentage of GFP+mCherry+ cells. Results shown are the average of three biological replicates. (G) FLAG-BRD4 ET domain pulldown assays evaluating interactions with the indicated GST-NSD3 fragments. FLAG-BRD4 ET domain was expressed in HEK293T followed by immobilization on anti-FLAG agarose beads and extensive washing. ET immobilized beads were then incubated with purified GST-NSD3 fragments expressed in E. coli. A representative experiment of three biological replicates is shown. (H) Recombinant protein purity as assessed by SDS-PAGE and Coomassie blue staining. Strep2SUMO-BRD4 ET and Strep2SUMO-NSD3 100–263 proteins were individually expressed in Sf9 cells and purified by affinity, ion exchange, and size exclusion chromatography. The final purity of these proteins was assessed by SDS-PAGE. Molecular weight markers are shown for reference. (I) Binding affinity of the BRD4 ET-NSD3 interaction as determined by surface plasmon resonance using purified proteins. Steady-state response values from the association phase of each injection were plotted as a function of analyte concentration and fit to determine the KD. Points indicate the average of three technical replicates with error bars representing the standard deviation. (J) Diagram of NSD3-short fragments evaluated in the BRD4 ET pulldown assay. All error bars shown except for (I) represent SEM. See also Figure S1.
Due to the inconsistency of results obtained when using commercial anti-NSD3 antibodies, we generated a polyclonal antibody that recognizes the long and short NSD3 isoforms and used this reagent throughout our study. Immunoprecipitation (IP) of endogenous BRD4 or NSD3 from leukemia nuclear lysates followed by Western blotting confirmed the association between these factors in this cell type (Figure 1C). We noticed that NSD3-short was consistently expressed at higher levels than NSD3-long, with both isoforms associating with BRD4 in IP experiments (Figure 1C-1D, Figure S1C). While shRNAs that target exons shared by NSD3-long and NSD3-short suppressed RN2 proliferation, shRNAs that selectively suppressed NSD3-long resulted in no significant phenotype (Figure 1D and Figure S1G). Moreover, we observed that the proliferation arrest of RN2 and HL-60 cells caused by knockdown of endogenous NSD3-long and -short was rescued by expressing an shRNA-resistant NSD3-short cDNA (Figure 1E-1F and Figure S1H–S1J). These unexpected results suggested that NSD3-short is the essential isoform that supports AML proliferation.
We next mapped the region of NSD3-short that associates with BRD4. FLAG-tagged BRD4 ET domains were immobilized on agarose beads, followed by incubation with purified GST-tagged fragments of NSD3-short. Pulldown experiments revealed that the BRD4-binding region resides between amino acids 100-263 of NSD3 (Figure 1G and S1K). Furthermore, we found that the BRD4 ET and NSD3 100-263 could be copurified in an apparent 1:1 ratio when coexpressed in Sf9 insect cells (Figure S1L). Surface plasmon resonance (SPR) analysis of purified BRD4 ET and NSD3 100-263 further validated the interaction between these proteins with an estimated dissociation constant of 2.1 µM (Figure 1H-1I, Figure S1M). These experiments confirmed that NSD3-short binds to the BRD4 ET domain, presumably in a direct manner (Figure 1J).
NSD3-short is an adaptor protein that links BRD4 to the CHD8 chromatin remodeling enzyme
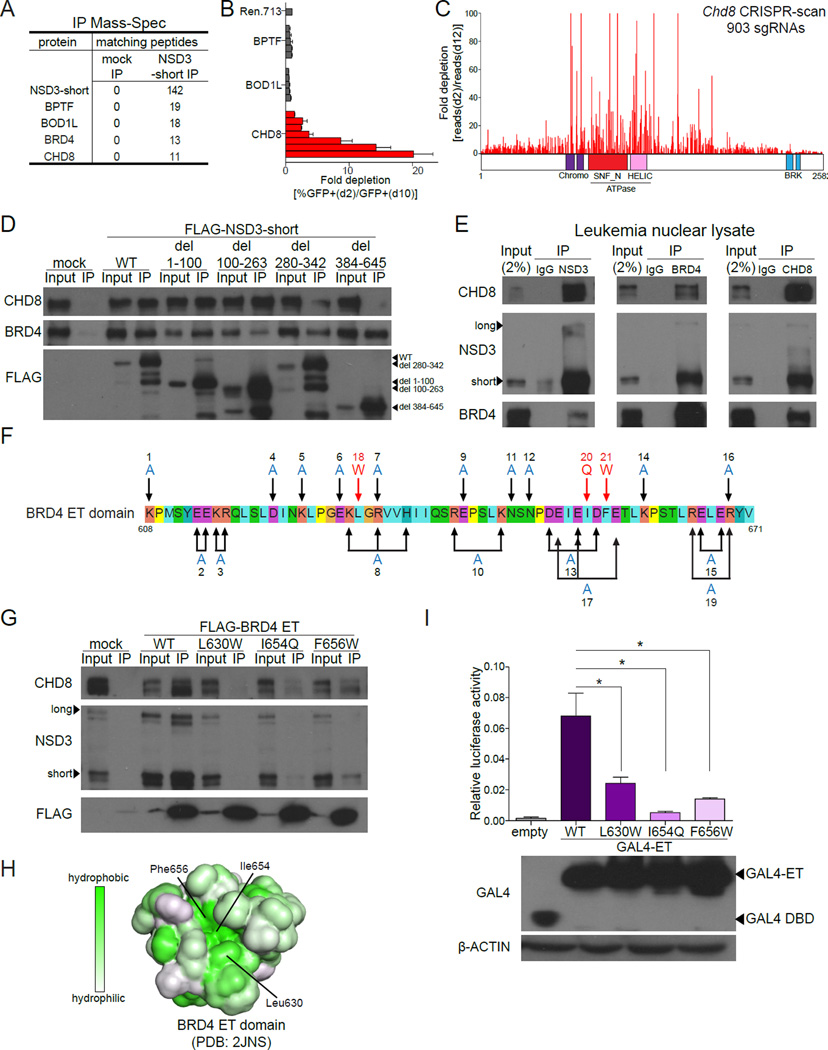
Since NSD3-short lacks the lysine methyltransferase activity present on the long isoform, we considered whether NSD3-short functions as an adaptor protein that links BRD4 to other regulators. To evaluate this, we transiently expressed FLAG-tagged NSD3-short in HEK293T cells, followed by IP-mass spectrometry analysis to identify associated proteins. As expected, NSD3-short and BRD4 were among the top five proteins recovered in this analysis (Figure 2A). The other highly enriched proteins found associated with NSD3-short included BPTF, BOD1L, and CHD8 (Figure 2A and Figure S2A). To examine the relevance of these factors in leukemia cells, we performed shRNA-based targeting of each protein and measured the effect on RN2 cell proliferation. Notably, knockdown of CHD8 resulted in a proliferation arrest whereas knockdown of BPTF or BOD1L resulted in no significant phenotype (Figure 2B, S2B–S2C).
Figure 2. NSD3-short is an adaptor protein that links BRD4 to the CHD8 chromatin remodeling enzyme.
(A) Mass spectrometry analysis of proteins identified using anti-FLAG IP performed with nuclear lysates prepared from HEK293T cells transfected with FLAG-NSD3-short or empty vector (for mock IP). The list was ranked by the total number of matched peptides recovered. Complete results are provided in Table S1. (B) Competition-based assay in RN2 cells evaluating the effect of LMN shRNAs targeting the indicated proteins. Each bar represents the average fold-decrease in the percentage of GFP+ cells over 8 days for individual shRNAs. n=3. (C) CRISPR-scan of Chd8 with all possible sgRNAs. Deep sequencing based measurement of the impact of 907 Chd8 sgRNAs on the proliferation of Cas9-expressing RN2 cells. The location of each sgRNA relative to the CHD8 protein is indicated along the x axis. Shown is a representative experiment of two biological replicates. (D) IP-Western blotting performed with anti-FLAG antibodies and nuclear lysates prepared from RN2 cells stably expressing the indicated FLAG-NSD3 constructs or empty vector. (E) Endogenous IP-Western blotting performed with the indicated antibodies and nuclear lysates prepared from NOMO-1 cells. (F) The amino acid sequence of the human BRD4 ET domain indicating the surface residues that were subjected to mutagenesis. Combinations of mutations were used in some cases in an attempt to disrupt specific clusters of charged residues. (G) IP of the indicated FLAG-BRD4 ET domains expressed transiently in HEK293T cells followed by Western blotting with the indicated antibodies. (H) The molecular surface of the BRD4 ET domain (PDB: 2JNS) with hydrophobicity indicated in green (Lin et al., 2008). (I) (top) Luciferase reporter assay evaluating the activation function of the indicated GAL4-ET domain fusions on a minimal plasmid-based reporter harboring GAL4 recognition sequences. HEK293T cells were co-transfected with p9xGAL4-UAS-luciferase (firefly) reporter and the indicated GAL4 fusion expression plasmids expressing Renilla luciferase from a constitutive promoter. Plots indicate firefly luciferase activity normalized to the Renilla luciferase control. n=3. *p<0.05, two-tailed Student’s t-test. (bottom) Western blotting analysis of HEK293T cells transfected with the indicated plasmids shown in the top panel. All error bars represent SEM and all IP-Western and Western blotting experiments shown are a representative experiment of at least three independent biological replicates. See also Figure S2.
CHD8 is a member of the SNF2 family of chromatin remodeling ATPases which, to our knowledge, has not previously been linked to BRD4, NSD3, or to leukemia maintenance. Due to the large size of Chd8, we were unable to perform cDNA rescue experiments to validate that the proliferation arrest observed using shRNAs was due to on-target CHD8 knockdown. Therefore, we performed negative selection CRISPR-Cas9 mutagenesis scanning of all Chd8 coding exons with a multiplexed library of 907 sgRNAs, which is a method for revealing functionally important domains of large proteins (Shi et al., 2015). Deep sequencing revealed that severe proliferation arrest of RN2 cells was correlated with CRISPR-based targeting of exons encoding the chromodomains and the ATPase domain of CHD8, but not with targeting of the BRK domains (Figure 2C, S2D). This analysis also revealed a region of functional importance located between the ATPase and BRK domains at residues 1440-1750 (Figure 2C). These results validate CHD8 as a leukemia dependency and led us to hypothesize that NSD3-short performs an essential role in this disease by linking CHD8 to BRD4.
IP-Western blotting experiments confirmed the association of FLAG-NSD3-short with BRD4 and CHD8 in HEK293T nuclear lysates (Figure S2E). A deletion analysis of NSD3-short identified a critical requirement for residues 384-645 (C-terminal to the PWWP domain) and a partial requirement for residues 280-342 in mediating the CHD8 interaction (Figure 2D). In these experiments, we noted that deletion of residues 100-263 of NSD3-short reduced, but did not abolish, the interaction with BRD4 (Figure 2D). However, NSD3-short del(100–263) can efficiently immunoprecipitate endogenous wild-type NSD3, which may allow an indirect BRD4 association under these conditions (Figure S2F). IP of endogenous proteins from leukemia nuclear extracts confirmed the association among these three factors (Figure 2E). By evaluating various truncated forms of FLAG-BRD4 in IP assays, we mapped its CHD8 interaction site to the ET domain, which suggests that NSD3-short could act as the bridge that links BRD4 to CHD8 (Figure S2G–S2H). To further evaluate this possibility, we performed a structure-guided mutagenesis of all surface residues of the ET domain and assayed the impact on NSD3 binding in vitro (Lin et al., 2008). While alanine substitutions at 26 different charged residues had no effect on the NSD3 interaction, replacement of three hydrophobic residues with bulkier side chains (L630W, I654Q, or F656W) was each sufficient to disrupt NSD3 binding (Figure 2F-2G, and Figure S2I). All three of these residues localize to a single hydrophobic groove on the surface of the ET domain, which is the likely binding surface for NSD3 (Figure 2H). When expressed in cells, we found that all three of the BRD4 ET mutations were unable to associate with CHD8 (Figure 2G). This result strongly suggests that NSD3-short acts as the intermediary between BRD4 and CHD8.
We next evaluated whether the three ET domain point mutations that disrupt NSD3 binding also resulted in a defect in BRD4-dependent transcriptional activation. When fused to the DNA binding domain of GAL4, we found that the BRD4 ET domain activated transcription of a plasmid-based luciferase reporter harboring GAL4 recognition motifs upstream of a minimal promoter (Figure 2I). In contrast to the wild-type ET domain, the L630W, I654Q, and F656W substitutions each led to reduced transcriptional activation (Figure 2I). Furthermore, we observed that GAL4-NSD3-short, and to a much lesser extent GAL4-NSD3-long, activate transcription in this assay (Figure S2J). While we cannot rule out that the three ET mutations compromise the interaction with multiple binding partners, these results support the functional importance of the NSD3-BRD4 interaction for transcriptional activation.
NSD3-short utilizes four distinct interaction surfaces to sustain AML cell proliferation
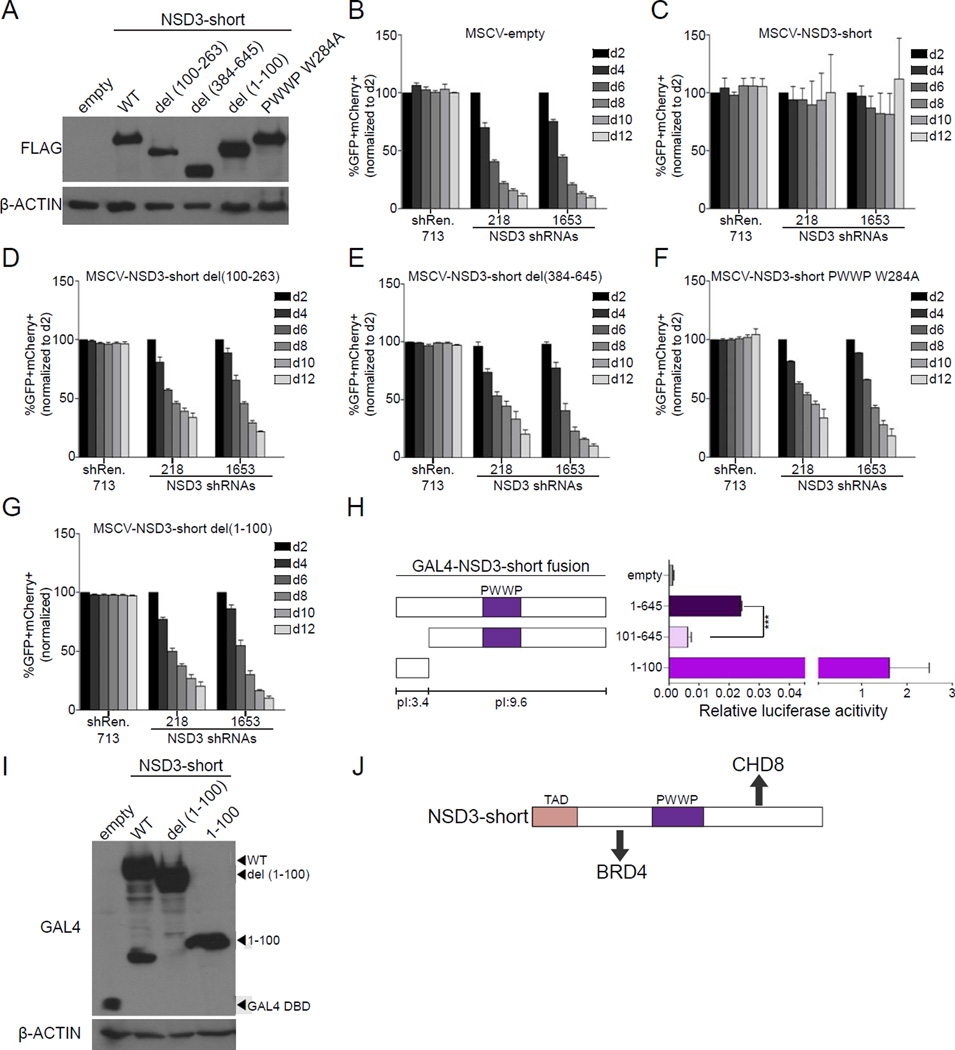
To further test the adaptor model of NSD3-short function, we employed the shRNA/cDNA rescue assay described above to evaluate how mutating different regions of NSD3-short influenced the proliferation of RN2 cells. While a wild-type NSD3-short cDNA was able to complement the knockdown of endogenous NSD3, a deletion of amino acids 100-263 (the BRD4 interacting region) or 384-645 (the CHD8 interacting region) resulted in a functionally defective NSD3 protein despite being expressed at normal levels (Figure 3A-3E, S3A). This suggests that NSD3-short requires interactions with both BRD4 and CHD8 to maintain leukemia cell proliferation.
Figure 3. NSD3-short utilizes four distinct interaction surfaces to sustain AML cell proliferation.
(A) Western blotting analysis of whole cell lysates prepared from RN2 cells transduced with the indicated PIG retroviral expression constructs. A representative experiment of three independent biological replicates is shown. (B–G) Competition-based assay tracking the abundance of GFP+mCherry+ cells during culturing of transduced RN2 cells. GFP is linked to the indicated cDNA and mCherry is linked to the indicated LMN shRNA. Plotted is the average of three independent biological replicates, normalized to d2. (H) Luciferase reporter assay with the indicated GAL4 fusions, performed as described in Figure 2I. n=3. ***p<0.001, two-tailed Student’s t-test. (I) Western blotting analysis of HEK293T cells transfected with the indicated plasmids. A representative experiment of three biological replicates is shown. (J) Diagram of the functionally important surfaces of NSD3-short. All error bars in this figure represent SEM. See also Figure S3.
We next considered whether the essential function of NSD3-short in AML requires its PWWP domain, which has been shown previously to interact with H3K36 methylated peptides (Vermeulen et al., 2010; Wu et al., 2011). Methyl-lysine recognition by PWWP domains requires an aromatic cage, which can be perturbed by substituting the second tryptophan of the PWWP motif with alanine (Figure S3B) (Qin and Min, 2014). When introduced into the PWWP motif of NSD3-short (W284A), this mutation resulted in a loss-of-function in the shRNA/cDNA rescue assay, without impairing the interaction of NSD3-short with BRD4 and CHD8 or the stability of NSD3-short protein (Figure 3A and 3F, Figure S3C, and Table S1). A CRISPR-based targeting of NSD3 in MOLM-13 cells further supports the role of this PWWP domain in leukemia maintenance (Figure S3D). These experiments suggest that NSD3-short requires H3K36-methyl recognition via its PWWP domain to carry out its essential function in AML.
The N-terminal 100 amino acids of NSD3-short is dispensable for its association with BRD4 and CHD8 (Figure 2D), however, we found that deleting this region compromised the function of NSD3-short in RN2 cells (Figure 3G). Interestingly, the 1–100 region of NSD3 is highly enriched for acidic amino acids (pI: 3.4), in contrast to the rest of NSD3-short (pI: 9.6). Since other transcription activation domains (TADs) are known to be enriched for acidic residues (e.g. VP16 and GCN4), we considered whether the 1–100 region of NSD3 functions as a TAD (Sigler, 1988). Using the GAL4-fusion based reporter assay described above, we observed that the activation function of GAL4-NSD3-short is significantly reduced upon deleting the first 100 amino acids of NSD3 (Figure 3H). In addition, a fusion of GAL4 with the 1–100 region of NSD3 alone led to potent transcriptional activation (∼1,300 fold), thus confirming this region of NSD3 as a TAD (Figure 3H). IP-mass spectrometry experiments failed to identify proteins associated with the NSD3 TAD (data not shown). Since many TADs are known to bind to multiple cofactors with low affinity, we suspect that the functionally relevant ligand(s) of the NSD3 TAD were not retained under these purification conditions. Nevertheless, this set of experiments suggests that NSD3-short utilizes four independent interaction surfaces to perform its essential function in leukemia cells: a BRD4 interacting region, a CHD8 interacting region, a PWWP domain-mediated interaction with H3K36 methylation, and an acidic TAD (Figure 3J).
Targeting of NSD3 or CHD8 leads to differentiation of AML cells and suppression of BRD4-dependent gene expression
To examine whether BRD4, NSD3, and CHD8 function in the same genetic pathway, we evaluated whether targeting of these three factors led to similar phenotypic and transcriptional effects in AML cells. Analogous to prior analyses of BRD4 inhibition in RN2 cells, we found that knockdown of NSD3-short or CHD8 resulted in a decrease in the expression of c-Myc at the mRNA and protein level (Figure 4A-4B, Figure S4A–S4C) (Zuber et al., 2011b). Using flow cytometry, we also found that NSD3 or CHD8 deficiency led to a decrease in the expression of c-kit and an increase in Mac-1 on the cell surface, which is a myeloid differentiation immunophenotype that has previously been associated with BRD4 inhibition (Figure 4C-4D, Figure S4D–S4E) (Zuber et al., 2011b). Moreover, targeting of NSD3 or CHD8 caused RN2 cells to undergo morphological changes associated with terminal myeloid differentiation (Figure 4E-4F, Figure S4F–S4G). This differentiation phenotype was prevented if c-Myc was expressed ectopically from a retroviral promoter (Figure 4E-4F, Figure S4F–G). Finally, RNA-seq analysis confirmed that targeting of NSD3 and CHD8 led to significant changes in gene expression signatures previously associated with BRD4 function in leukemia cells (Figure 4G-4H, Figure S4H–S4I) (Zuber et al., 2011b). Collectively, these results show that BRD4, NSD3, and CHD8 perform overlapping gene regulatory functions in AML, consistent with these factors acting in the same pathway.
Figure 4. Targeting of NSD3 or CHD8 leads to differentiation of AML cells and suppression of BRD4-dependent gene expression.
(A–B) (top) Competition-based assays to evaluate the effect of NSD3 or CHD8 LMN shRNAs on RN2 cell proliferation. GFP percentages are normalized to d2 measurements. All error bars represent the SEM of three independent biological replicates, (bottom) Western blotting analysis of whole cell lysates prepared from RN2 cells transduced with the indicated TRMPV-Neo constructs following 48 hours of dox treatment. A representative experiment of three biological replicates is shown. (C–D) Flow cytometry analysis of c-kit and Mac-1 stained RN2 cells following TRMPV-Neo shRNA induction with dox for 96 hours. Gating was performed on dsRed+/shRNA+ cells. A representative experiment of three biological replicates is shown. (E–F) Light microscopy of May-Grünwald/Giemsa-stained RN2 cells expressing the indicated NSD3 shRNAs or Chd8 sgRNAs, in the presence or absence of ectopic c-Myc expression. For sgRNA experiments, an RN2 line stably expressing Cas9 was used. shRNA expression was induced using the TRMPV-Neo vector treated with dox for 4 days. For F, cells were imaged 6 days following transduction with the indicated LRG sgRNAs. Imaging was performed with a 40x objective. A representative image of three independent biological replicates is shown. (G–H) Gene Set Enrichment Analysis (GSEA) of RNA-seq data obtained from RN2 cells expressing NSD3 TRMPV-Neo shRNAs (induced with dox for 48 hours) or RN2-Cas9 cells expressing Chd8 LRG sgRNAs (4 days following transduction). Two independent NSD3 shRNAs or Chd8 sgRNAs were compared to a Ren.713 shRNA or Rosa26 sgRNA, respectively in this analysis. NES: Normalized enrichment score. For each of the indicated gene sets shown, the false discovery rate and nominal p-value were <0.01. See also Figure S4 and Table S3.
Genomewide colocalization of BRD4, NSD3, and CHD8 at active promoters and enhancers across the AML genome
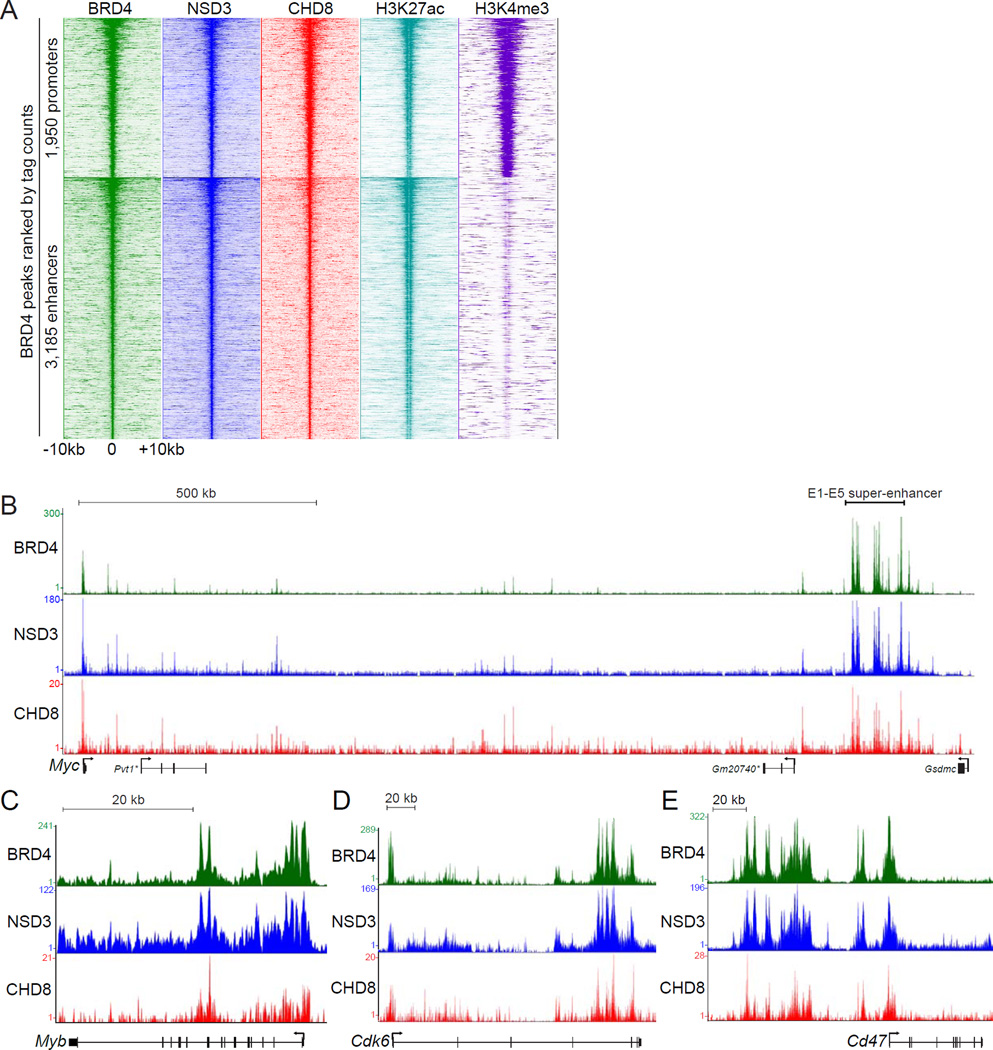
To further corroborate the presence of BRD4-NSD3-CHD8 complexes in AML, we compared the genomic localization of all three factors in RN2 cells using ChIP-seq. A density plot analysis of genomic intervals surrounding 5,135 high-confidence BRD4-occupied promoter and enhancers revealed a similar enrichment pattern of NSD3 and CHD8 across these locations (Figure 5A) (Roe et al., 2015). BRD4 has been shown previously to regulate Myc expression in AML cells via a super-enhancer (with individual enhancer constituents E1 to E5) located 1.7 megabases downstream of the Myc promoter (Shi et al., 2013b). The E1-E5 super-enhancer and the Myc promoter were found to exhibit high levels of BRD4, NSD3, and CHD8 in an overlapping pattern of enrichment, whereas the intervening regions exhibited lower occupancy (Figure 5B). As additional examples, we observed similarity between the enrichment of BRD4, NSD3, and CHD8 at Myb, Cdk6, Cd47, and Bcl2 loci (Figure 5C-5E, Figure S5A). ChIP analysis revealed that H3K36 di-methylation, but not H3K36 mono- and tri-methylation, broadly correlated with NSD3-short occupancy at the Myc E1-E5 super-enhancer (Figure S5B–S5C and data not shown). Collectively, these experiments show that BRD4, NSD3, and CHD8 occupy similar locations across the genome of leukemia cells.
Figure 5. Genomewide colocalization of BRD4, NSD3, and CHD8 at active promoters and enhancers across the AML genome.
(A) Density plot analysis comparing ChIP-seq datasets obtained using the indicated antibodies. Indicated is a 20 kilobase interval surrounding 1,950 BRD4-occupied promoters and 3,185 BRD4-occupied enhancers, identified previously as high-confidence BRD4 occupied sites (Roe et al., 2015). H3K27ac and H3K4me3 datasets from RN2 were described previously (Shi et al., 2013a). Each row represents a single peak. (B–E) ChIP-seq occupancy profiles with the indicated antibodies at various loci. The y-axis reflects the number of cumulative tag counts in the vicinity of each region. Validated transcript models from the mm9 genome assembly are depicted below. The asterisks indicate non-coding RNAs. See also Figure S5.
BRD4 recruits NSD3 and CHD8 to super-enhancer regions at oncogene loci
We next performed ChIP-qPCR experiments to evaluate whether BRD4, NSD3, and CHD8 associate with chromatin in an interdependent manner. Using the ectopically expressed FLAG-tagged NSD3-short, we confirmed the association of this isoform with the Myc E1-E5 super-enhancer using ChIP with anti-FLAG antibodies (Figure 6A). Deletion of the BRD4 interacting region of NSD3-short (100-263) led to a complete loss of its genomic occupancy while deletion of the CHD8 interacting region (384-645) or deletion of the TAD (1–100) had no effect (Figure 6B–C, S6A–B). Unexpectedly, we observed that the W284A mutation of the PWWP domain failed to disrupt NSD3-short chromatin occupancy at the E1-E5 Myc super-enhancer (Figure S6C). This raises the possibility of a post-recruitment function of the NSD3-short PWWP domain. Collectively, these results indicate that the interaction with BRD4 is the principal means by which NSD3-short is recruited to chromatin.
Figure 6. BRD4 recruits NSD3 and CHD8 to the Myc +1.7 Mb super-enhancer region.
(A–C) ChIP-qPCR analysis at the E1-E5 Myc super-enhancer region evaluating the occupancy of the indicated FLAG-NSD3-short constructs using anti-FLAG antibody or control IgG. (D–F) ChIP-qPCR analysis with the indicated antibodies in RN2 cells treated with DMSO vehicle or 500 nM JQ1 for 6 hours. (G–L) ChIP-qPCR analysis with the indicated antibodies in RN2 cells transduced with the indicated TRMPV-Neo shRNA constructs and treated with dox for 48 hours. All error bars represent the SEM of three independent biological replicates. *p<0.05, **p<0.01, two-tailed Student’s t-test. See also Figure S6 and Table S4.
To further evaluate the existence of BRD4-NSD3-CHD8 complexes on chromatin, we performed ChIP-qPCR analysis following the exposure of RN2 cells to 500 nM JQ1 for 6 hours. As expected, exposure to JQ1 led to the rapid release of BRD4 from the Myc E1-E5 super-enhancer and from super-enhancers at other oncogene loci (Figure 6D, Figure S6D). Importantly, under these conditions JQ1 also caused the eviction of NSD3 and CHD8 from these same regions (Figure 6E-6F, Figure S6E–S6F). These effects were not limited to RN2 cells, as we found that JQ1 also released BRD4, NSD3, and CHD8 from the MYC super-enhancer in human AML cells (NOMO-1 line) and from the Myc super-enhancer in murine B cell acute lymphoblastic leukemia cells (Figure S6G–L). To evaluate the specific contribution of BRD4 to these effects, we performed ChIP-qPCR in RN2 cells following conditional BRD4 knockdown using a doxycycline regulated shRNA, which confirmed a BRD4 requirement for NSD3 and CHD8 chromatin occupancy (Figure 6G-6I). Knockdown of NSD3 also led to significant reductions in CHD8 occupancy, but no effect on BRD4 (Figure 6J–L). Taken together, these findings suggest that BRD4 tethers NSD3 to chromatin, which in turn facilitates recruitment of the CHD8 chromatin remodeler.
Discussion
Our investigation into the mechanisms underlying the therapeutic effects of BET inhibitors in leukemia has revealed several features of BRD4-mediated transcriptional activation, which we have shown occurs, at least in part, via ET domain-mediated recruitment of NSD3-short and CHD8. The reliance of BRD4 on NSD3-short, and not NSD3-long, for transcriptional activation was unexpected, since the short isoform lacks the catalytic SET domain and six of the chromatin reader domains found on the long isoform. To explain this observation, we have shown that NSD3-short utilizes four discrete surfaces to maintain the proliferative state of leukemia cells. This includes an interaction with BRD4, an interaction with CHD8, a PWWP-mediated interaction with H3K36 methylation, and an acidic transactivation domain. While NSD3-long is likely to have important roles in other contexts (Jacques-Fricke and Gammill, 2014; Zhou et al., 2010), our findings demonstrate that NSD3-short can function as an adaptor protein that coordinates multiple regulatory machineries on chromatin to allow transcriptional activation.
Prior studies performed in non-hematopoietic cell types have suggested that the demethylase protein JMJD6 can also interact with the ET domain of BRD4 to allow transcriptional activation (Liu et al., 2013; Rahman et al., 2011). We have performed extensive shRNA and CRISPR-based targeting of JMJD6 in leukemia cells, which has failed to reveal an effect on cell viability or proliferation (Shi et al., 2015). Hence, in leukemia cells it appears that NSD3-short is the relevant ET domain-binding partner that supports BRD4-dependent transcriptional activation. This then raises the possibility that different cell types utilize distinct ET-interacting partners of BRD4 to promote transcription. The presence of cell type-specific BRD4 effector proteins may underlie to the well-described context-specific gene expression changes induced by BET inhibitors (Shi and Vakoc, 2014). Since our prior CRISPR-scan of Brd4 has implicated the ET and CTD regions as essential for leukemia maintenance (Shi et al., 2015), it is likely that BRD4 employs both NSD3-short/CHD8 and P-TEFb as distinct effectors to activate its downstream target genes.
While the reader function of the NSD3-short PWWP domain is essential to support leukemia proliferation, it was surprising to find that this domain was dispensable for NSD3-short recruitment to the Myc super-enhancer region, which instead is dependent on the BRD4 interaction. This result implies a post-recruitment function for this chromatin reader module. It is possible that the PWWP module interacts with additional non-histone ligands to promote gene activation, however our IP-MS analysis comparing wild-type and W284A NSD3-short failed to identify PWWP-dependent interacting proteins (Table S1). Alternatively, it is also possible that the PWWP interaction with H3K36-methyl allosterically regulates the NSD3-short adaptor function. A recent study has demonstrated that the interaction of the Rpd3S complex with the nucleosome results in a conformational change that modulates its H3K36-methyl recognition function (Ruan et al., 2015). It will be worthwhile in future studies to evaluate whether the PWWP-mediated interaction with H3K36-methylated nucleosomes, or other binding interactions of NSD3, alter the conformation of NSD3-short and the functional output of its interacting partners.
Our study provides evidence that links the CHD8 chromatin remodeler to the cancer maintenance functions of BRD4 and NSD3. CHD8 is best known for its role in neurodevelopment and as one of the most commonly mutated genes in autism spectrum disorders, with our study now suggesting a widespread role for CHD8 as a cancer dependency (Bernier et al., 2014). CHD8 has been shown previously to interact with the androgen receptor and with c-Myc, which are also TFs known to interact with BRD4 (Asangani et al., 2014; Dingar et al., 2015; Menon et al., 2010; Wu et al., 2013). CHD8 is also known to promote Wnt signaling by directly activating β-catenin target genes Thompson et al., 2008). Collectively, these results imply that targeting of CHD8, potentially via chemical inhibition of its chromodomains or ATPase activity, would suppress cancer-promoting transcriptional pathways in various malignancies.
While our study establishes a role for NSD3-short in maintaining the growth of AML, it is interesting to note that NSD3 is a putative oncoprotein in other forms of cancer. The most common genetic mechanism of NSD3/WHSC1L1 deregulation is via 8p11–12 genomic amplifications, which occur in breast and lung cancers (Tonon et al., 2005; Yang et al., 2010). This raises the interesting possibility that the adaptor model of NSD3-short defined here in AML will be relevant to the pathogenesis of 8p11–12-amplified epithelial cancers (Yang et al., 2010). This provides a rationale to consider targeting the adaptor functionalities of NSD3, instead of its methyltransferase activity, as a therapeutic approach in cancer. Since other chromatin reader domains (e.g. bromodomains and MBT domains) have proven to be amenable to direct chemical inhibition, the PWWP module of NSD3-short provides an attractive target for future drug development (Filippakopoulos et al., 2010; James et al., 2013).
Experimental Procedures
Cell lines and plasmids
The Tet-ON competent murine MLL-AF9/NrasG12D AML cell line (RN2) used in this study was developed and characterized previously (Zuber et al., 2011a). For shRNA-based competition assays in murine cells, the LMN-GFP or LMN-mCherry shRNA retroviral vectors were used (MSCV-miR30-shRNA-PGKp-NeoR-IRES-GFP/mCherry). For shRNA-based competition assays in human leukemia cells, MLS-GFP shRNA retroviral vectors were used (MSCV-miR30-shRNA-SV40p-GFP). TRMPV-Neo constructs were used for dox inducible shRNA expression in RN2 cells (Zuber et al., 2011a). Cells were treated with 1 µg/ml dox wherever indicated. For CRISPR-Cas9 based targeting of CHD8 and NSD3, the LRG lentiviral vector was used to express the sgRNA (U6-sgRNA-EFS-GFP) in either RN2 or MOLM-13 cells that stably express Cas9 (Shi et al., 2015).
For c-Myc cDNA rescue experiments, the murine Myc cDNA was cloned into the PIG vector (MSCV-PGKp-Puro-IRES-GFP). For all retroviral and transfection-based expression of NSD3-short, a PIG vector was used containing the human NSD3-short cDNA (#31357; Addgene) with a C-terminal 3XFLAG. FLAG-tagged human BRD4 1-495 and 608-699 fragments were expressed using the pcDNA3 vector. FLAG-tagged human BRD4 700-1362, short, and long fragments were expressed using PIG. For GAL4-fusion experiments, human NSD3 or BRD4 ET domain fragments were cloned in-frame and C-terminal to the GAL4 DNA binding domain in the pFN26A (BIND) hRluc-neo Flexi Vector (Promega). Constructs with point mutations were generated by overlap PCR. For bacterial expression of GST-NSD3 fragment, NSD3-short cDNA sequences were PCR cloned into a pGEX-4T1 vector (#28-9545-49; GE Healthcare). For baculoviral expression, the BRD4 ET domain (608-699) and NSD3 100-263 coding sequences were cloned with an N-terminal Strep2-SUMO tag into the vector pFL. Untagged NSD3 100-263 coding sequence was cloned into the vector pSPL. All of the cloning procedures were performed using the In-Fusion cloning system (#638909; Clontech) or using SLIC (Sequence- and Ligation-Independent Cloning).
Competition assay to measure cell proliferation
For shRNA-based competition assays, RN2 cells were retrovirally transduced with the indicated LMN shRNA vectors (which express the shRNA and GFP from constitutive promoters), followed by tracking of GFP percentages using a Guava Easycyte HT instrument (Millipore) over time in culture. shRNA-induced proliferation arrest was monitored by GFP-negative cells outcompeting GFP-positive cells, which is represented in several plots as fold depletion [%GFP+(d2)/%GFP+(d12)]. For evaluating effects of specific cDNAs on shRNA-induced phenotypes in leukemia cells, RN2 or HL60 cells were first retrovirally transduced with PIG (empty or with a cDNA), followed by puromycin (1 µg/ml) selection for 3–7 days. Subsequently, LMN-shRNAs-mCherry vectors were retrovirally transduced and the GFP+mCherry+ double positive population of cells were tracked over time using a BD LSR II flow cytometer. Complete shRNA sequences are provided in Table S5.
Accession numbers
The accession number for the raw and processed sequencing data reported in this paper is GEO: GSE71186, with the subseries accession numbers GEO: GSE71183 for ChIP-seq and GEO: GSE71185 forRNAseq, respectively.
Supplementary Material
Acknowledgements
We thank Christof Fellmann and Johannes Zuber for shRNA sequence designs; Osama El Demerdash, Ying Jin, Yuan Hao, and Molly Hammell for assistance with genomic analysis; James Bradner and Jun Qi for providing JQ1; Zihua Wang and Michael Wigler for assistance with deep sequencing. This work was supported by Cold Spring Harbor Laboratory NCI Cancer Center Support grant CA455087 for developmental funds and shared resource support. Additional funding was provided by the Alex’s Lemonade Stand Foundation and the V Foundation. J.S.R. is supported by the Martin Sass Foundation and the Lauri Strauss Leukemia Foundation. L.J. is supported by the Cold Spring Harbor Laboratory Women in Science Award and as an Investigator of the Howard Hughes Medical Institute. C.R.V. is supported by a Burroughs-Wellcome Fund Career Award, National Institutes of Health grant NCI RO1 CA174793, and a Leukemia & Lymphoma Society Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
C.S., J.J.I., J.S., C.R.V designed experiments and analyzed results; C.S., J.J.I., E.W., and J.A.M. carried out experiments; J.S.R. assisted with the analysis of genomic data; D.J.P., Y.S., L.J.T., J.S., and C.R.V. supervised the research; C.S. and C.R.V wrote the manuscript.
References
- Angrand PO, Apiou F, Stewart AF, Dutrillaux B, Losson R, Chambon P. NSD3, a new SET domain-containing gene, maps to 8p12 and is amplified in human breast cancer cell lines. Genomics. 2001;74:79–88. doi: 10.1006/geno.2001.6524. [DOI] [PubMed] [Google Scholar]
- Asangani LA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke L, Wilder-Romans K, Dhanireddy S, Engelke C, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts L, Baker C, Vulto-van Silfhout AT, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Lin CY, Duan Q, Griffin G, Federation AL, Paranal RM, Bair S, Newton G, Lichtman AH, Kung AL, et al. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Molecular cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Gudgin EJ, Horton SJ, Giotopoulos G, Meduri E, Robson S, Cannizzaro E, Osaki H, Wiese M, Putwain S, et al. Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia. Leukemia. 2014;28:311–320. doi: 10.1038/leu.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi L, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi L, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8758–8763. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingar D, Kalkat M, Chan PK, Srikumar T, Bailey SD, Tu WB, Coyaud E, Ponzielli R, Kolyar M, Jurisica I, et al. BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors. Journal of proteomics. 2015;118:95–111. doi: 10.1016/j.jprot.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi L, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CA, Rahman S, Walsh EM, Kuhnle S, Grayson AR, Lemieux ME, Grunfeld N, Rubin BP, Antonescu CR, Zhang S, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer discovery. 2014;4:928–941. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Molecular and cellular biology. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Gammill LS. Neural crest specification and migration independently require NSD3-related lysine methyltransferase activity. Molecular biology of the cell. 2014;25:4174–4186. doi: 10.1091/mbc.E13-12-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LI, Barsyte-Lovejoy D, Zhong N, Krichevsky L, Korboukh VK, Herold JM, MacNevin CJ, Norris JL, Sagum CA, Tempel W, et al. Discovery of a chemical probe for the L3MBTL3 methyllysine reader domain. Nature chemical biology. 2013;9:184–191. doi: 10.1038/nchembio.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Molecular cell. 2005;79:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Kim SM, Kee HJ, Eom GH, Choe NW, Kim JY, Kim YS, Kim SK, Kook H, Kook H, Seo SB. Characterization of a novel WHSC1-associated SET domain protein with H3K4 and H3K27 methyltransferase activity. Biochemical and biophysical research communications. 2006;345:318–323. doi: 10.1016/j.bbrc.2006.04.095. [DOI] [PubMed] [Google Scholar]
- Li Y, Trojer P, Xu CF, Cheung P, Kuo A, Drury WJ, 3rd, Qiao Q, Neubert TA, Xu RM, Gozani O, et al. The target of the NSD family of histone lysine methyltransferases depends on the nature of the substrate. The Journal of biological chemistry. 2009;284:34283–34295. doi: 10.1074/jbc.M109.034462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Umehara T, Inoue M, Saito K, Kigawa T, Jang MK, Ozato K, Yokoyama S, Padmanabhan B, Guntert P. Solution structure of the extraterminal domain of the bromodomain-containing protein BRD4. Protein science : a publication of the Protein Society. 2008;17:2174–2179. doi: 10.1110/ps.037580.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma Q, Wong K, Li W, Ohgi K, Zhang J, Aggarwal AK, Rosenfeld MG. Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. Cell. 2013;755:1581–1595. doi: 10.1016/j.cell.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;753:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio-Eterovic AK, Carpenter PB. An open and shut case for the role of NSD proteins as oncogenes. Transcription. 2011;2:158–161. doi: 10.4161/trns.2.4.16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon T, Yates JA, Bochar DA. Regulation of androgen-responsive transcription by the chromatin remodeling factor CHD8. Molecular endocrinology. 2010;24:1165–1174. doi: 10.1210/me.2009-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., 3rd Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proceedings of the National Academy of Sciences of the United States of America. 2011;705:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Min J. Structure and function of the nucleosome-binding PWWP domain. Trends in biochemical sciences. 2014;39:536–547. doi: 10.1016/j.tibs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Molecular and cellular biology. 2011;31:2641–2652. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Molecular cell. 2015 doi: 10.1016/j.molcel.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati R, La Starza R, Veronese A, Aventin A, Schwienbacher C, Vallespi T, Negrini M, Martelli MF, Mecucci C. NUP98 is fused to the NSD3 gene in acute myeloid leukemia associated with t(8;11)(p11.2;p15) Blood. 2002;99:3857–3860. doi: 10.1182/blood.v99.10.3857. [DOI] [PubMed] [Google Scholar]
- Ruan C, Lee CH, Cui H, Li S, Li B. Nucleosome contact triggers conformational changes of Rpd3S driving high-affinity H3K36me nucleosome engagement. Cell reports. 2015;10:204–215. doi: 10.1016/j.celrep.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Molecular cell. 2014;54:728–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nature biotechnology. 2015;33:661–667. doi: 10.1038/nbt.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang E, Zuber J, Rappaport A, Taylor M, Johns C, Lowe SW, Vakoc CR. The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;Nras(G12D) acute myeloid leukemia. Oncogene. 2013a;32:930–938. doi: 10.1038/onc.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, et al. Disrupting the Interaction of BRD4 with Diacetylated Twist Suppresses Tumorigenesis in Basal-like Breast Cancer. Cancer cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, Minder JL, Mercan F, Wang E, Eckersley-Maslin MA, et al. Role of SWLSNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes & development. 2013b;27:2648–2662. doi: 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler PB. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988;333:210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Thompson BA, Tremblay V, Lin G, Bochar DA. CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Molecular and cellular biology. 2008;28:3894–3904. doi: 10.1128/MCB.00322-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, Khatry DB, Protopopov A, You MJ, Aguirre AJ, et al. High-resolution genomic profiles of human lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9625–9630. doi: 10.1073/pnas.0504126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010;142:967–980. doi: 10.1016/j.cell.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Wu H, Zeng H, Lam R, Tempel W, Amaya MF, Xu C, Dombrovski L, Qiu W, Wang Y, Min J. Structural and histone binding ability characterizations of human PWWP domains. PloS one. 2011;6:e18919. doi: 10.1371/journal.pone.0018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. The Journal of biological chemistry. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Molecular cell. 2013;49:843–857. doi: 10.1016/j.molcel.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Molecular cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Liu G, Bollig-Fischer A, Giroux CN, Ethier SP. Transforming properties of 8pl 1–12 amplified genes in human breast cancer. Cancer research. 2010;70:8487–8497. doi: 10.1158/0008-5472.CAN-10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Thomsen R, Kahns S, Nielsen AL. The NSD3L histone methyltransferase regulates cell cycle and cell invasion in breast cancer cells. Biochemical and biophysical research communications. 2010;398:565–570. doi: 10.1016/j.bbrc.2010.06.119. [DOI] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nature biotechnology. 2011a;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011b;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.