Abstract
Extremity trauma, spinal cord injuries, head injuries and burn injuries place patients at high risk of pathologic extraskeletal bone formation. This heterotopic bone causes severe pain, deformities and joint contractures. The immune system has been increasingly implicated in this debilitating condition. This review summarizes the various roles immune cells and inflammation play in the formation of ectopic bone, and highlights potential areas of future investigation and treatment. Cell types in both the innate and adaptive immune system such as neutrophils, macrophages, mast cells, B cells and T cells have all been implicated as having a role in ectopic bone formation through various mechanisms. Many of these cell types are promising areas of therapeutic investigation for potential treatment. The immune system has also been known to also influence osteoclastogenesis, which is heavily involved in ectopic bone formation. Chronic inflammation is also known to have an inhibitory role in the formation of ectopic bone, whereas acute inflammation is necessary for ectopic bone formation.
Keywords: Heterotopic Ossification, Fibrodysplasia Ossificans Progressiva, Immune system, Ectopic bone, B cells, T cells, Macrophages
1. Introduction
Heterotopic ossification (HO) is the extraskeletal formation of lamellar bone, which largely occurs following traumatic injury, burn injury, spinal cord injury, traumatic burn injury, and soft tissue damage. HO also frequently occurs after joint arthroplasty, and in soldiers with blast wounds(1,2). In one study of 243 combat-wounded patients, 64.6% of patients eventually developed HO(3). The development of HO was found to be correlated with presence and severity of traumatic brain injury, amputation, and injury severity(3). Another study identified that in patients with lower-limb amputations, both traumatic and non-traumatic, 22.8% of patients developed HO(4). Given these statistics it is evident that the trauma and critical care surgeon will treat heterotopic ossification frequently during their career.
HO is also a significant consideration in patient with burn wounds. In one study of 5,031 burn injured patients, the incidence of HO was found to be 1.2%(5). Another more recent study found the incidence in burn injured patients to be 5.6%(6). It was also noted in this study that percent of total body surface area burned, mechanical ventilation, number of procedures, sepsis, and time to active movement were predictive of HO formation(6). It has also been shown that in burn injured patients that have already developed HO, early surgical excision of heterotopic ossification results in improved range of motion(7).
HO formation is largely thought to be related to the inflammatory response to these inciting injuries, which in turn causes ectopic bone formation through the up-regulation of pro-osteogenic genes and activation of osteopotent progenitor cells(1,8,9). Interestingly, the steps involved in HO formation mimic that of limb development with early hypoxia followed by hypoxia inducible 1 alpha signaling and up-regulation of SOX-9 (central chondrogenic transcription factor) and chondrogensis. This early cartilage is subsequently invaded by blood vessels initiating final endochondral bone formation. Severe HO formation can be extremely debilitating by causing joint contractures, pain, and limiting extremity prosthetic use(10). Because it is so destructive once it begins, prevention has become an important focus of research, particularly with regards to pre-operative and post-operative prophylaxis. These efforts have largely been through immune system modulation(11). To date, there has not been comprehensive review of the role of the immune system in HO, which is the goal of this article.
Importantly, HO can also occur as the result of a genetic mutation, as in the case of fibrodysplasia ossificans progressiva (FOP). FOP is a progressive, debilitating disease of ectopic bone formation first described in 1736 by John Freke, which eventually leads to early demise of those affected(12,13). However, it has not been until much more recently that the details behind the pathophysiology began to be identified. Shore et al were the first ones to identify the specific mutation involved in FOP in 2006, linking it to the mutation R206H in the ACVR1 gene, a bone morphogenic protein (BMP) type 1 receptor(14). Kan et al had also identified the role of BMPs in FOP, creating mice that overexpressed BMP-4, creating an FOP-like disease state(15). Children with FOP also develop HO lesions after minor injuries similar to those seen in large trauma patients(16). Discoveries such as these have led to a large increase in the study and understanding of trauma induced HO, with the hope of better understanding the pathophysiology and identifying therapeutic targets for preventing trauma induced HO(14,15).
The root causes of HO have long been elusive, but new research implicating specific genes, progenitor cells and immune signaling pathways, has given hope to the idea of eventually identifying the pathophysiology and a means of treatment for those affected(17–20). More recently, the role of the immune system has begun to emerge as a contributing factor to the formation and progression of ectopic bone formation. Several studies have shown how the inflammatory response is necessary for the formation of HO, and several specific cell populations, such as macrophages and mast cells, and the adaptive immune system, have been particularly implicated in HO development via interactions with osteoprogenitor cells and the release of osteogenic growth factors(19–21).
2. The Inflammatory Response
2.1 Overview
The immune system is largely divided into two main classifications: the innate immune system and the adaptive immune system. The innate immune system is the “first defense” against pathogens, with immune cells such as neutrophils, macrophages, mast cells, eosinophils, basophils and dendritic cells serving this purpose. The innate immune cells recognize pathogens generically, and respond similarly each time a pathogen is encountered. The adaptive immune system, on the other hand, is largely composed of B and T cells and their subtypes. These cells have “memory” once a pathogen is encountered, and create pathogen specific immune responses. Through this mechanism they are able to respond much more rapidly once a pathogen is subsequently encountered.
In addition to pathogen eradication, the immune system is also known to have a large effect on bone remodeling and repair. Bone remodeling is a balance between the activity of osteoblasts and osteoclasts. Osteoblasts originate from bone marrow stromal cells, whereas osteoclasts largely originate from macrophage fusion(22). Osteoclastogenesis can be induced through many inflammatory cytokines which also affect the immune system, such as the predominant cytokine ligand of receptor activator of NF-kB (RANKL), macrophage colony stimulating factor (M-CSF), tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, IL-6, IL-7, IL-17, IL-23, transforming growth factor (TGF)-beta, and interferon (IFN)-gamma(22). Osteoblasts are able to regulate osteoclastogenesis through the release of osteoprogerin (OPG), a RANKL decoy receptor. Additionally T cells are also known to affect osteoblastogenesis and osteoclastogenesis(23).
Although mainly incriminated in host-pathogen interactions, inflammation and wound healing, the immune system also plays a central role in fracture repair. The immune system’s role has been largely related to the inflammatory response following bone injury(24). The inflammatory response induces a cascade of cytokines, which in turn promote angiogenesis and induce osteoprogenitor cells to release bone morphogenic proteins (BMPs) and promote osteogenic differentiation(24). Progenitor cells differentiate into osteoblasts, which in turn release cytokines such as IL-1, IL-6 and IL-11 to promote osteoclastogenesis(24). Additionally, it has been demonstrated that the adaptive immune system plays a role in osteoblastogenesis for new bone formation following injury(25). The competing actions of osteoblasts and osteoclasts following differentiation allow for the initial remodeling of fractured bone(24). Much research has been done to examine the relationship between osteoblasts, osteoclasts, progenitor cells and the immune system, and these relationships will be further reviewed later in this paper.
2.2 Inflammation and Heterotopic Ossification
The immune system has been a major target of HO research in recent years as its role in ectopic bone formation has become clearer. Forsberg et al were able to demonstrate that the inflammatory markers IL-3, IL 12p70, effluent IL-3 and effluent IL-13 were associated with HO, implicating the inflammatory response in general as important in the formation of HO(18). Evans et al also showed that HO was associated with more severe injuries, and the inflammatory markers IL-6, IL-10, MCP-1, effluent IL-10 and MIP-1-alpha, further implicating the inflammatory response and Th1 and Th2 cytokine production in HO evolution. Finally, Mitchell et al identified single nucleotide polymorphisms (SNPs), particularly toll-like receptor 4 and complement factor H, as being associated with a decrease in HO, directly connecting the immune system and alternative complement pathway as being involved in HO(26). These studies help solidify the role of the inflammatory response as being essential for HO.
Other papers have identified lymphocytes specifically as being involved in HO and FOP(27,28). Fiori et al identified that the BMP-p38 MAPK pathway is dysregulated in patients with FOP, with increased activation of p38 MAPK, and higher expression of ID1 and ID3(27). Shafritz et al were able to specifically identify that BMP-4 overexpression in lymphocytes was associated with ectopic bone formation in patients with FOP(28). These studies helped to clarify the role of the immune system and some of the candidate signaling mechanisms implicated in HO and FOP by identifying the major signaling pathways involved.
Another series of papers also highlights the complex role the immune system plays in HO and FOP. Spruce et al describe a patient with FOP who underwent bone marrow transplantation for the treatment of aplastic anemia, which successfully took with the second graft(29). Twenty-five years later, Kaplan et al noted that transplantation alone was not sufficient to inhibit FOP, but immunosuppression was successful in inhibiting FOP(30). This was demonstrated by the patient not having any flares while immunosuppressed, but having a recurrence of the FOP once the immunosuppression was weaned. These papers highlight the intriguing role of the immune system in HO formation, although the mechanism by which these processes occur remains elusive.
3. Cell Specific Responses
3.1 Neutrophils
Neutrophils have been largely considered to be mainly responsible for mounting an initial and nonspecific immune inflammatory response. However that mantra began to change two decades ago with the discovery of the specific mechanisms by which neutrophils govern and regulate the inflammatory and immune responses(31). Regardless, the role of neutrophils has not been extensively studied in the setting of HO, but they have been described as being involved(20,32). However, some information is known regarding their participation in bone remodeling. It has been shown that RANKL is expressed by inflammatory and normal neutrophils, while RANK is expressed in inflammatory neutrophils(33). Additionally, Chakravarti et al showed that neutrophils can activate osteoclasts via up regulation of RANKL in an inflammatory setting(34). They demonstrated that cell-cell interaction can induce osteoclastogenesis via surface RANKL(34). Allaeys et al also demonstrated that neutrophils retract osteoblasts from the surface of bone through surface elastase in the setting of chronic gout, permitting osteoclasts to cause bone erosion(35).
With regards to HO, Chakkalakal et al identified that neutrophils were present in the initial inflammatory response that precedes heterotopic bone formation(20). O’Brien et al showed that prostaglandin E2 (PGE2) exposure in tendon-derived stem cells led to a dose dependent increase in BMP-2 and osteoblast differentiation(32). They hypothesized that neutrophils may be involved in this early step of HO formation because neutrophils are major sources of PGE2(36). It is likely that neutrophils play a role in the formation of ectopic bone, but the literature describes their role as concomitant with the assistance of other immune cells.
3.2 Macrophages
If neutrophils are the purest of the innate immune cells, then monocytes and macrophages are considered the cross-talkers of immunity, interacting both with the adaptive and the innate immune systems. In addition, macrophages have both an inflammatory and anti-inflammatory phenotype that serves to regulate the early and late phases of the inflammatory response. The ability of the macrophage to orchestrate immunity strongly implicates their role as immune effector cells in ectopic bone formation(19–21). Chakkalakal et al demonstrated that macrophages were present in the tissue remodeling stage following injury in knock-in mice that clinically mimic FOP, which implicated them in the formation of HO(20). Other studies have further elucidated the effects of macrophages on HO. Kan et al and Aro et al identified that macrophages in mice forming HO express high levels of osteogenic growth factors, including BMP4(19,21). They also demonstrated the critical nature of macrophages in the formation of HO, particularly in initiation of HO formation, with selective destruction of macrophages in genetically prone mice significantly reducing the amount of HO formation.
Macrophages have a relatively well-identified role in normal endochondral bone formation, particularly in fracture repair(37–40). It is well known that macrophages can differentiate into osteoclasts in the proper microenvironment, but recent studies have shown other interactions with bone development and formation as well(41). Several studies have identified macrophages in the initial inflammatory stages of bone repair, and that they may stimulate proliferation of fibroblasts(37–39). Raggatt et al identified that macrophages were present early in chondrogenic centers, and persisted as the callus was formed(40). They also identified the important role of macrophages by monitoring callus formation following macrophage elimination. If eliminated early, callus formation never occurred. When eliminated late, callus formation was significantly reduced(40). These studies help demonstrate the critical role that macrophages play in the initiation and prolongation of endochondral bone formation and provide future avenues of research as to how macrophages may initiate and propagate HO evolution.
Although the literature is still sparse with regards to the macrophage specific role in HO and FOP, what has been demonstrated strongly points to a critical role of macrophages in the induction of HO following tissue injury and the initial inflammatory response. Although one could easily consider the homology between vessel injury and atherosclerotic plaque formation with peripheral tissue injury and HO formation, the reality is that little evidence exists to correlate the two inflammatory processes. The underlying mechanism behind macrophage induced HO formation requires more investigation, particularly in the realm of potential therapeutic targets affecting the ability of macrophages to induce ectopic bone formation, as this could be a very promising area of study for future research.
3.3 Mast Cells
Mast cells are another cell type that strongly implicated in the formation of HO. Chakkalakal et al demonstrated in their study that mast cells were present throughout the inflammatory process and with ectopic bone formation(20). Gannon et al also demonstrated the strong presence of mast cells in HO formation, documenting mast cells being present in much greater concentrations than normal in all stages of HO formation(42). The role of mast cells has more recently been further elucidated beyond their simple presence in increased numbers during HO formation. Salisbury et al identified the role that sensory neurons play in causing neurogenic inflammation following injury(43). The sensory neurons release signaling factors such as substance P and calcitonin gene related peptide, which recruit mast cells. The mast cells then undergo degranulation following recruitment. Salisbury et al were able to demonstrate that HO formation was reduced in animals that lacked sensory neurons, thus preventing signaling factor release and impairing mast cell recruitment. Studies have shown that prevention of mast cell degranulation using cromolyn mitigated HO formation, confirming their role in HO formation as well as identifying a potential human therapeutic intervention(43).
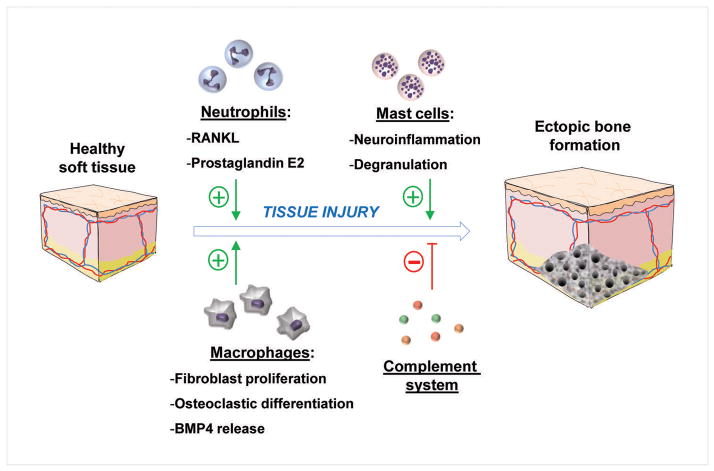
Inhibiting mast cell degranulation could be a highly useful therapeutic target for the prevention of ectopic bone formation. However, pure inhibition is not the only means of disrupting mast cell involvement in ectopic bone formation to prevent HO. Radiation therapy has long been known to be an effective means of prophylaxis for HO, particularly prior to surgery(11). Hoff et al demonstrated that irradiation prior to surgery increases inflammatory markers, and basic FGF, which is released by macrophages(44). They hypothesize that increased levels of these markers following radiation therapy are the result of early mast cell degranulation, induced by the radiation. Therefore, the mast cell functions are disrupted prior to the injury and they are prevented from performing their critical role in HO formation. Further studies are warranted on this topic, but what has been found so far has been a promising avenue for therapeutic intervention and prophylaxis of HO. Additionally, in HO resulting from spinal cord injury, burns, or traumatic brain injury, where the location of the resulting HO is unknown, one should target mast cells. (Figure 1).
Figure 1.
Effects of innate immune system cells following soft tissue injury which result in ectopic bone formation.
3.4 Adaptive Immune System
The adaptive immune system, namely B and T cells, have been identified in HO formation as well, although their role has not been characterized quite as extensively as other cell types. Chakkalakal et al demonstrated in their study that CD 45+ T cells invaded the soft tissue following muscle destruction in cases of HO, but did not characterize their involvement further(20). Furthermore, Kan et al were able to further elucidate the role of B and T cells in ectopic bone formation. They demonstrated that in mice without mature B and T cells, HO still formed immediately upon injury. However, at all time points it was noted that the rate of spread and overall quantity of HO formed was significantly less compared to mice with an intact adaptive immune system(19). This indicated the varying role of different aspects of the immune system in HO formation, with macrophages being more critical to the induction of HO formation, and B and T cells being necessary for the propagation of ectopic bone formation.
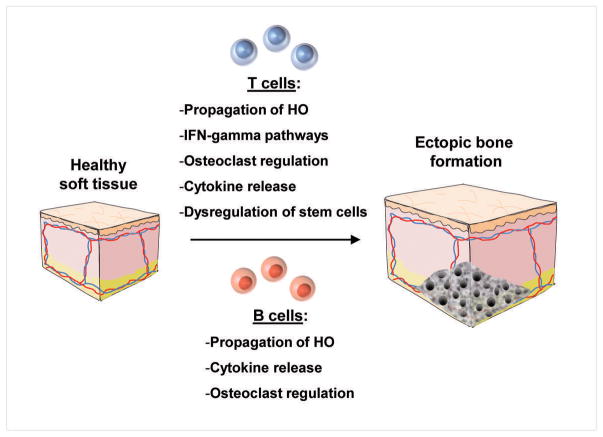
However, the role of the adaptive immune system has not been completely detailed. Hoff et al showed as part of their study on prophylactic radiation therapy in the prevention of HO that radiation induced B cell maturation, as well as led to an increase in CD 8+ T cells and release of IFN-gamma(44). They hypothesized that increased levels of CD 8+ T cells helped prevent HO formation. This hypothesis is strengthened by the findings of Dighe et al which showed that T cells and IFN-gamma release interfere with mesenchymal stem cell bone repair(45). Mesenchymal stem cells have been implicated as critical in HO, strengthening the theory that T cells prevent HO formation(46). However, it is clear that the role of T cells has not fully been elucidated in HO, and further studies are needed to analyze different T cell types and characterize their specific roles in HO formation (Figure 2).
Figure 2.
Effects of B and T cells and the adaptive immune system which result in ectopic bone formation.
4. Specific Cell Types in Bone
4.1 CD4+ T Cells
CD4+ T cells have classically been characterized into Th1 (inflammatory) and Th2 (immune modulatory) type cells. However, smaller subsets, such as Th17 cells and Treg cells, also exist under this category. Their effects are largely related to the immune system modulation and regulation in the elimination of infectious processes. However, it has been shown by Sato et al that these cells are able to inhibit osteoclast formation, via IFN-gamma in Th1 cells, and IL-4 in Th2 cells(47). Sato et al also identified that Th17 cells, which will be described further in the next paragraph, exhibit the opposite capability, and are osteoclastogenic via the release of inflammatory factors such as IL-17(47).
4.2 Th17 Cells
Th17 cells have been implicated in a plethora of inflammatory and immune modulatory processes and possess phenotype capable of having a profound effect on bone and HO evolution. Th17 cells release inflammatory cytokines such as IL-17, IL-21, IL-22, and IL-6. They can also secrete IFN-gamma, which has the ability to induce osteoclastogenesis in monocytes(48,49). IL-17A can induce RANKL expression in fibroblasts and osteoblasts, but Th17 cells can also express their own RANKL and TNF to stimulate osteoclastic precursors(50). Inhibition of IL-17A has been shown to decrease bone erosion in collagen-induced arthritis models, indicating the potential therapeutic value in further study of Th17 cells(50).
4.3 CD4+ T Reg Cells
Treg cells are known to inhibit osteoclast formation(51). Kelchtermans et al demonstrated not only the osteoclastogenic inhibition, but that activation of Treg cells can improve clinical symptoms of collagen-induced arthritis(52). Kim et al demonstrated that Treg cells inhibited osteoclast differentiation in a cytokine-dependent manner, and that TGF-beta and IL-4 were key cytokines in this process(53). Axmann et al also were able to demonstrate that CTLA-4, an inflammatory modulating molecule released by Treg cells which binds to CD80/86, can directly bind osteoclast precursor cells and inhibit their differentiation(54).
4.4 CD8+ T Cells
CD8+ T cells are known to inhibit osteoclasts; they inhibit osteoclastogenesis via proteins such as osteoprotegerin (OPG) and RANKL. However, inhibition of OPG released by CD8+ T cells does not prevent the osteoclastogenesis inhibition, suggesting that other mechanisms are also involved(55). CD8+ T cells have also been shown to have pro-osteoblastic properties. Terauchi et al demonstrated that intermittent PTH administration increased the production of Wnt10b in CD8+ T cells, and this activated the Wnt signaling cascade in pre-osteoblasts(56). They also showed that inhibition of Wnt10b or T cell null mice have no bone anabolism in response to intermittent PTH administration, further solidifying their role in bone strengthening(56).
4.5 CD8+ T Reg Cells
CD8+ Treg cells have been documented in humans, but have not been extensively studied due to their rarity(22,57). However, Buchwald et al identified that CD8+ Treg cells can inhibit osteoclast bone resorption, as well as inhibit cytoskeletal reorganization in mature osteoclasts(58). They demonstrated that osteoclasts can induce CD8+ Treg cells in an antigen-dependent manner, but CD8+ Treg cell inhibition of osteoclasts can be achieved without antigens or direct contact(58). It is hypothesized that CD8+ Treg cells may have a role via this identified regulatory feedback loop in skeletal homeostasis(58).
4.6 Natural Killer (NK) T Cells
NK T cells have not been implicated in the formation of HO specifically, but their effects on bone raises questions towards the possibility. NK T cells have been shown to produce inflammatory cytokines such as IFN-gamma and TNF-beta, which have been shown to modulate other immune cells, and could have an impact on HO formation(59). Invariant NK T cells have also been shown to promote osteoclast development, and enhance their resorptive capabilities(60). Additionally, NK T cells were identified as being highly activated in the synovium of patients with chronic joint inflammation, largely rheumatoid arthritis, and to produce IFN-gamma and TNF-alpha(61).
4.7 Gamma-Delta T Cells
Gamma-delta T cells are a minority of T cells, but they can play an important role in the immune system’s influence on skeletal injury. They are considered an intermediary between the innate and the adaptive immune system, and have been shown to be involved in multiple inflammatory responses such as carcinogenesis resistance and infection control(62,63). Additionally, Kaylan et al demonstrated that bone-increasing nitrogen-bisphosphonate therapy correlated with a decrease in gamma-delta T cells, and that patients who experienced osteonecrosis of the jaw were significantly deficient in gamma-delta T cells(64). Their role has not been described explicitly in HO formation, but research should be done to investigate their possible role given their deleterious effects on the skeletal system in other circumstances.
4.8 B Cells
B cells have a well-documented involvement in bone metabolism. In multiple myeloma, B-cell derived plasma cells have been shown to support osteoclastogenesis, likely through multiple signaling molecules such as RANKL, Decoy receptor 3, and IL-7(22,65). B cells have also been proposed to be involved in osteoclast formation, causing increased bone resorption following ovariectomy(66). However, Weitzmann et al demonstrated that B cells in peripheral blood inhibit the formation and reduce the lifespan of osteoclasts via the secretion of TGF-beta, which can stimulate OPG production(67,68). Bone loss has also shown to be increased in B-cell deficient rats with periodontal disease, further demonstrating their possible role in bone protection(69). Conversely, Marusic and colleagues unearthed evidence suggesting that a genetic lack of B lymphocytes may create a change in the immunological milieu at the site of new bone induction, which stimulates the initial accumulation and proliferation of mesenchymal progenitor cells implicated in bone formation(70). Moreover Li and coauthors, have shown that B-cell null mice are consistently osteoporotic and deficient in BM osteoprotegerin which was reversed by B cell reconstitution(71).
Onal et al further studied the role of B cells in ovariectomy-induced bone loss, particularly with relation to RANKL. They demonstrated that mice lacking RANKL in B cells were partially protected from bone loss(72). Human immunodeficiency virus infection has also been linked to osteoporosis and bone fracture, and HIV-1 transgenic rats were demonstrated by Vikulina et al to undergo osteoclastic bone resorption(73). This bone resorption is due to decreased B cells production of OPG and increased production of RANKL(73). Finally, B cells have been shown by Choi et al have a positive effect on osteoclastogenesis in response to stimulation from Th2 cell cytokines, and a negative effect in response to Th1 cell cytokines(74). This demonstrates the complex, interconnected signaling mechanisms responsible for immune cell effects on bone, particularly with regards to bone loss and osteoclastogenesis.
4.9 Dendritic Cells
Dendritic cells are not thought to be involved in bone biology, with dendritic deficient animals being shown to have no bone defects(22,75). However, dendritic cells have been noted to be present in rheumatoid nodules in rheumatoid arthritis and in tertiary lymphoid tissue propagation, and Santiago-Schwarz et al identified their potential involvement in the pathologic processes of rheumatoid arthritis(76,77). This could be significant given how rheumatoid arthritis and other chronic inflammatory conditions can affect HO formation. Santiago-Schwarz et al demonstrated that the dendritic cells from rheumatoid arthritis synovial fluid were potent stimulators of allogenic T cells and Th1 cells, and that dendritic cell growth was promoted in acellular rheumatoid arthritis synovial fluid, showing that the inflammatory environment contributed to the dendritic cell population growth(77).
Interestingly, Rivollier et al identified that dendritic cells also have the capability to transdifferentiate into osteoclasts in the presence of M-CSF and RANKL, in addition to their traditional immunologic roles(78). They also noted that this transdifferentiation process is greatly enhanced by rheumatoid arthritis synovial fluid with cytokines suck as IL-1 and TNF-alpha(78). Alnaeeli et al demonstrated that, in addition to dendritic cells being able to develop into functional osteoclasts, these dendritic cell-derived osteoclasts induce bone loss after in vivo transfer in mice(79). These studies suggest that dendritic cells may have a much larger role in osteoclastogenesis than previously thought (Table 1).
Table 1. Immune System Effector Cells and HO Impact.
Details the innate and adaptive immune effector cells implicated in augmenting HO formation. The respective known function in regard to HO formation for each effector cell is also listed.
| Innate Immune System | Adaptive Immune system | ||
|---|---|---|---|
| Cell Type | Function | Cell Type | Function |
| Neutrophils |
|
B cells |
|
| Macrophages* |
|
T cells |
|
| Mast Cells |
|
CD4+ T cells |
|
| Dendritic cells* |
|
Th17 cells |
|
| CD4+ T reg cells |
|
||
| CD8+ T cells |
|
||
| CD8+ Treg cells |
|
||
| NK T cells |
|
||
| Gamma-delta T cells |
|
||
Macrophages and Dendritic cell are considered “cross talkers” between the innate and adaptive immune system and some investigators and immunologist consider them as a separate group of cells loosely referred to as adaptive immune system modulators.
5. Osteoclastogenesis
Osteoclasts also play an important role in HO and FOP, with increased osteoclast formation having been seen in HO lesions of FOP-like mice(80). Increased bone breakdown by osteoclasts leads to increased bone formation in HO. The immune system is known to have a wide range of effects on osteoclastogenesis via a wide variety of cells, as described above, which in turn affects HO formation. RANKL is known to have a prominent role in osteoclastogenesis, and also has strong interplay with the immune system(81). T and B cells are known to release RANKL when activated, and neutrophils have also been shown to release RANKL in inflammatory lesions(34,82). Additionally, the role of RANKL on osteoclasts can be induced by TNF alpha, which is an inflammatory marker and mainly produced by activated T cells(22).
Other inflammatory markers are known to modulate osteoclastogenesis via the immune system, such as IL-1, IL-17, IL-23, IL-27, TNF-alpha, IFN-gamma, and TGF-beta. IL-7 promotes osteoclastogenesis through the upregulation of RANKL from B and T cells(83). IL-17 is believed to increase osteoclastogenesis, possibly via synergism with IL-1 and TNF-alpha(84). IL-23 is known to stimulate IL-17, although the exact role that IL-23 plays on osteoclasts has been controversial(85,86). IL-27 has been shown to actually inhibit osteoclastogenesis(87). IFN-gamma has been hypothesized to stimulate bone resorption, and TGF-beta has been shown to stimulate or inhibit osteoclast proliferation depending on multiple other factors, although both IFN-gamma and TGF-beta’s role in osteoclasts is much less well understood compared to the other inflammatory markers described(22).
6. Acute versus chronic inflammation
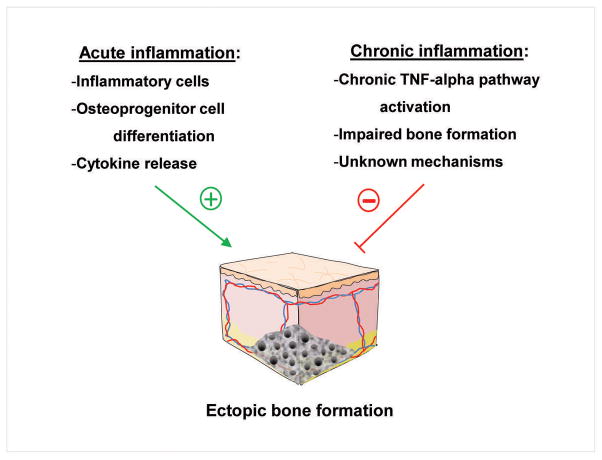
Although it has become clear that inflammation and the immune system are critical to HO formation, its role is even still more complex. Acute inflammation has been shown to lead to events which could trigger HO, but in the setting of chronic inflammation the incidence of HO is severely reduced(46). Roth et al identified that patients with rheumatoid arthritis have a lower incidence of HO compared to other populations undergoing total knee arthroplasty(88). It is known that chronic inflammation has a deleterious effect on bone. Prolonged exposure to TNF-alpha in the setting of chronic inflammation has been shown to initiate a sequence resulting in systemic bone loss(89). Recent studies demonstrate that chronic exposure to TNF-alpha triggered tissue damage similar to other chronic inflammatory diseases such as rheumatoid arthritis(89,90). TNF-alpha also resulted in decreased bone volume and strength(89,90). In a mouse model of type 1 diabetes, researches showed that inflammatory mediators impaired fracture healing, and that TNF-alpha inhibition improved fracture healing in these mice(91). Whether this is the final culmination of chronic inflammation induced cachexia or whether the effects of chronic inflammation on HO formation are purely protective remains to be seen.
Interestingly, it has also been shown that Dickkopf-1 (Dkk-1) signaling, an inhibitor of the Wnt pathway related to bone formation, is altered in diseases such as ankylosing spondylitis, a known bone-forming disease, and related to joint remodeling in rheumatic diseases(92). In one study, it was demonstrated that functionally, Wnt signaling is increased in ankylosing spondylitis, even though Dkk-1 levels were also higher compared to normal subjects or patients with rheumatoid arthritis(93). It was also shown that TNF-alpha treatment in ankylosing spondylitis was found to result in increased Dkk-1 levels, compared to a decreased in those with rheumatoid arthritis(93). Clearly all of the details regarding acute and chronic inflammation and its effects on HO and ectopic bone are not completely understood, however, this is an area that warrants further research for greater understanding of the exact role that the inflammatory response plays throughout ectopic bone formation (Figure 3).
Figure 3.
The contrasting effects of acute and chronic inflammation on ectopic bone formation.
7. Conclusions
Trauma induced heterotopic ossification is a complex disease process with potentially significant health consequences. Recent research has shed light on the complex nature of ectopic bone formation, in particular with regards to the role of the immune system in HO formation and propagation. As we have discussed above, several immune cell types have been specifically implicated given their potential roles in HO formation, particularly neutrophils, macrophages, mast cells, and the adaptive immune system. The hope is that with continued research into the participation of these cells in ectopic bone formation, therapeutic targets may be discovered that can alleviate the costly and painful effects of this disease (Table 2).
Table 2. Immune System Effector Cells and References.
Summarizes the cell types associated with HO formation and the references associated with each cell type.
| Cell Type | References |
|---|---|
| Neutrophils | 10, 21–25 |
| Macrophages | 9–12, 26–29 |
| Mast Cells | 10, 30–32 |
| B cells | 9, 12, 23, 32, 53–60, 68, 69 |
| T cells | 9, 10, 12, 13, 23, 32–34, 68, 69 |
| CD4+ T cells | 35 |
| Th17 cells | 36–38 |
| CD4+ T reg cells | 39–42 |
| CD8+ T cells | 32, 43, 44 |
| CD8+ Treg cells | 12, 45, 46 |
| NK T cells | 47–49 |
| Gamma-delta T cells | 50–52 |
| Dendritic cells | 12, 61–65 |
Acknowledgments
Funding provided with the help of 1K08GM109105-01 and Plastic Surgery Foundation National Endowment Award
Footnotes
Conflicts of Interest: None declared.
Author Contributions: All authors contributed significantly to the data acquisition, analysis, interpretation, manuscript writing and critical editing of this paper.
Contributor Information
Casey T. Kraft, Email: caseykr@umich.edu, Medical Student, University of Michigan Medical School, Ann Arbor, Michigan, U.S.A
Shailesh Agarwal, Email: ashailes@umich.edu, Resident Physician, University of Michigan Health System, Department of Surgery, Division of Plastic Surgery, Ann Arbor, Michigan, U.S.A
Kavitha Ranganathan, Email: krangana@umich.edu, Resident Physician, University of Michigan Health System, Department of Surgery, Division of Plastic Surgery, Ann Arbor, Michigan, U.S.A
Victor W. Wong, Email: Vicw.wong@gmail.com, Resident Physician, Johns Hopkins University Health System, Department of Surgery, Division of Plastic Surgery, Baltimore, Maryland, U.S.A
Shawn Loder, Email: shawnjl@umich.edu, Medical Student, University of Michigan Medical School, Ann Arbor, Michigan, U.S.A.
John Li, Email: Jliy2008@gmail.com, University of Michigan, Department of Surgery, Ann Arbor, Michigan, U.S.A
Matthew J. Delano, Email: mjdelano@umich.edu, Assistant Professor of Surgery, University of Michigan Health System, Department of Surgery, Division of Acute Care Surgery, Ann Arbor, Michigan, U.S.A
Benjamin Levi, Email: blevi@umich.edu, Assistant Professor of Plastic Surgery, University of Michigan, Department of Surgery, Division of Plastic Surgery, Ann Arbor, Michigan, U.S.A
References
- 1.Evans KN, Forsberg JA, Potter BK, Hawksworth JS, Brown TS, Andersen R, et al. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012 Nov;26(11):e204–13. doi: 10.1097/BOT.0b013e31825d60a5. [DOI] [PubMed] [Google Scholar]
- 2.Errico TJ, Fetto JF, Waugh TR. Heterotopic ossification. Incidence and relation to trochanteric osteotomy in 100 total hip arthroplasties. Clin Orthop Relat Res. 1984 Nov;(190):138–41. [PubMed] [Google Scholar]
- 3.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009 May;91(5):1084–91. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto ME, Khan M, Jayabalan P, Ziebarth J, Munin MC. Heterotopic ossification in civilians with lower limb amputations. Arch Phys Med Rehabil. 2014 Sep;95(9):1710–3. doi: 10.1016/j.apmr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Elledge ES, Smith AA, McManus WF, Pruitt BA. Heterotopic bone formation in burned patients. J Trauma. 1988 May;28(5):684–7. doi: 10.1097/00005373-198805000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Orchard GR, Paratz JD, Blot S, Roberts JA. Risk Factors in Hospitalized Patients With Burn Injuries for Developing Heterotopic Ossification-A Retrospective Analysis. J Burn Care Res. 2014 Dec 18; doi: 10.1097/BCR.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 7.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004 Apr;86(3):396–403. doi: 10.1302/0301-620x.86b3.14480. [DOI] [PubMed] [Google Scholar]
- 8.Davis TA, Lazdun Y, Potter BK, Forsberg JA. Ectopic bone formation in severely combat-injured orthopedic patients -- a hematopoietic niche. Bone. 2013 Sep;56(1):119–26. doi: 10.1016/j.bone.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Kan L, Kessler JA. Evaluation of the cellular origins of heterotopic ossification. Orthopedics. 2014 May;37(5):329–40. doi: 10.3928/01477447-20140430-07. [DOI] [PubMed] [Google Scholar]
- 10.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005 May;37(3):129–36. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 11.Popovic M, Agarwal A, Zhang L, Yip C, Kreder HJ, Nousiainen MT, et al. Radiotherapy for the prophylaxis of heterotopic ossification: A systematic review and meta-analysis of published data. Radiother Oncol. 2014 Sep 11; doi: 10.1016/j.radonc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Miao J, Zhang C, Wu S, Peng Z, Tania M. Genetic abnormalities in fibrodysplasia ossificans progressiva. Genes Genet Syst. 2012;87(4):213–9. doi: 10.1266/ggs.87.213. [DOI] [PubMed] [Google Scholar]
- 13.Peltier LF. A case of extraordinary exostoses on the back of a boy. 1740. John Freke (1688–1756) Clin Orthop Relat Res. 1998 Jan;(346):5–6. [PubMed] [Google Scholar]
- 14.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006 May;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 15.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004 Oct;165(4):1107–15. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor JM, Evans DA. Fibrodysplasia ossificans progressiva. The clinical features and natural history of 34 patients. J Bone Joint Surg Br. 1982 Feb 1;64-B(1):76–83. doi: 10.1302/0301-620X.64B1.7068725. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow ECY, Lin AC, et al. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007 Jan 5;282(1):526–33. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014 Sep;472(9):2845–54. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan L, Liu Y, McGuire TL, Berger DMP, Awatramani RB, Dymecki SM, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009 Jan;27(1):150–6. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, et al. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012 Aug;27(8):1746–56. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aro HT, Viljanto J, Aho HJ, Michelsson JE. Macrophages in trauma-induced myositis ossificans. APMIS. 1991 May;99(5):482–6. doi: 10.1111/j.1699-0463.1991.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 22.Mori G, D’Amelio P, Faccio R, Brunetti G. The Interplay between the bone and the immune system. Clin Dev Immunol. 2013;2013:720504. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Amelio P. Interactions between the immune system and bone. World Journal of Orthopedics. 2011;2(3):25. doi: 10.5312/wjo.v2.i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008 Jun;14(2):179–86. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam D, Mau E, Wang Y, Wright D, Silkstone D, Whetstone H, et al. T-Lymphocytes Enable Osteoblast Maturation via IL-17F during the Early Phase of Fracture Repair. In: Frey O, editor. PLoS ONE. 6. Vol. 7. 2012. Jun 29, p. e40044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell EJ, Canter J, Norris P, Jenkins J, Morris J. The genetics of heterotopic ossification: insight into the bone remodeling pathway. J Orthop Trauma. 2010 Sep;24(9):530–3. doi: 10.1097/BOT.0b013e3181ed147b. [DOI] [PubMed] [Google Scholar]
- 27.Fiori JL, Billings PC, de la Peña LS, Kaplan FS, Shore EM. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2006 Jun;21(6):902–9. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 28.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, et al. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996 Aug 22;335(8):555–61. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 29.Spruce WE, Forman SJ, Blume KG, Farbstein MJ, Scott EP, Wolf JL, et al. Successful second bone marrow transplantation in a patient with myositis ossificans progressiva and aplastic anemia. Am J Pediatr Hematol Oncol. 1983;5(4):337–40. doi: 10.1097/00043426-198324000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, et al. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007 Feb;89(2):347–57. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 31.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013 Jul 1;210(7):1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien EJO, Frank CB, Shrive NG, Hallgrímsson B, Hart DA. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012 Oct;93(5):319–31. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poubelle PE, Chakravarti A, Fernandes MJ, Doiron K, Marceau A-A. Differential expression of RANK, RANK-L, and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res Ther. 2007;9(2):R25. doi: 10.1186/ar2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarti A, Raquil M-A, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009 Aug 20;114(8):1633–44. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 35.Allaeys I, Rusu D, Picard S, Pouliot M, Borgeat P, Poubelle PE. Osteoblast retraction induced by adherent neutrophils promotes osteoclast bone resorption: implication for altered bone remodeling in chronic gout. Lab Invest. 2011 Jun;91(6):905–20. doi: 10.1038/labinvest.2011.46. [DOI] [PubMed] [Google Scholar]
- 36.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology. 2010 Sep 1;49(9):1618–31. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 37.Bourque WT, Gross M, Hall BK. Expression of four growth factors during fracture repair. Int J Dev Biol. 1993 Dec;37(4):573–9. [PubMed] [Google Scholar]
- 38.Oni OO. The early stages of the repair of adult human diaphyseal fractures. Injury. 1997 Oct;28(8):521–5. doi: 10.1016/s0020-1383(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 39.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- 40.Raggatt LJ, Wullschleger ME, Alexander KA, Wu ACK, Millard SM, Kaur S, et al. Fracture Healing via Periosteal Callus Formation Requires Macrophages for Both Initiation and Progression of Early Endochondral Ossification. Am J Pathol. 2014 Dec;184(12):3192–204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990 Sep;87(18):7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001 Aug;32(8):842–8. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 43.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011 Oct;112(10):2748–58. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoff P, Rakow A, Gaber T, Hahne M, Sentürk U, Strehl C, et al. Preoperative irradiation for the prevention of heterotopic ossification induces local inflammation in humans. Bone. 2013 Jul;55(1):93–101. doi: 10.1016/j.bone.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Dighe AS, Yang S, Madhu V, Balian G, Cui Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res. 2013 Feb;31(2):227–34. doi: 10.1002/jor.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson ER, Wong VW, Krebsbach PH, Wang SC, Levi B. Heterotopic ossification following burn injury: the role of stem cells. J Burn Care Res. 2012 Aug;33(4):463–70. doi: 10.1097/BCR.0b013e31825af547. [DOI] [PubMed] [Google Scholar]
- 47.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006 Nov 27;203(12):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008 Jun;9(6):650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 49.Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T, et al. IFN-γ-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35(11):3353–63. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 50.Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells. Arthritis Res Ther. 2008;10(5):225. doi: 10.1186/ar2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007 Dec;56(12):4104–12. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 52.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009 May 1;68(5):744–50. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 53.Kim YG, Lee C-K, Nah S-S, Mun SH, Yoo B, Moon H-B. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007 Jun 15;357(4):1046–52. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 54.Axmann R, Herman S, Zaiss M, Franz S, Polzer K, Zwerina J, et al. CTLA-4 directly inhibits osteoclast formation. Ann Rheum Dis. 2008 Nov;67(11):1603–9. doi: 10.1136/ard.2007.080713. [DOI] [PubMed] [Google Scholar]
- 55.Choi Y, Woo KM, Ko SH, Lee YJ, Park SJ, Kim HM, et al. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur J Immunol. 2001 Jul;31(7):2179–88. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 56.Terauchi M, Li J-Y, Bedi B, Baek K-H, Tawfeek H, Galley S, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009 Sep;10(3):229–40. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002 Nov;123(5):1516–26. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 58.Buchwald ZS, Kiesel JR, DiPaolo R, Pagadala MS, Aurora R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLoS ONE. 2012;7(6):e38199. doi: 10.1371/journal.pone.0038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001 May 15;97(10):3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 60.Hu M, Bassett JHD, Danks L, Howell PGT, Xu K, Spanoudakis E, et al. Activated invariant NKT cells regulate osteoclast development and function. J Immunol. 2011 Mar 1;186(5):2910–7. doi: 10.4049/jimmunol.1002353. [DOI] [PubMed] [Google Scholar]
- 61.De Matos CT, Berg L, Michaëlsson J, Felländer-Tsai L, Kärre K, Söderström K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007 Oct;122(2):291–301. doi: 10.1111/j.1365-2567.2007.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 63.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008 Feb;9(2):146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 64.Kalyan S, Quabius ES, Wiltfang J, Mönig H, Kabelitz D. Can peripheral blood γδ T cells predict osteonecrosis of the jaw? An immunological perspective on the adverse drug effects of aminobisphosphonate therapy. J Bone Miner Res. 2013 Apr;28(4):728–35. doi: 10.1002/jbmr.1769. [DOI] [PubMed] [Google Scholar]
- 65.Oranger A, Carbone C, Izzo M, Grano M. Cellular mechanisms of multiple myeloma bone disease. Clin Dev Immunol. 2013;2013:289458. doi: 10.1155/2013/289458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato T, Shibata T, Ikeda K, Watanabe K. Generation of bone-resorbing osteoclasts from B220+ cells: its role in accelerated osteoclastogenesis due to estrogen deficiency. J Bone Miner Res. 2001 Dec;16(12):2215–21. doi: 10.1359/jbmr.2001.16.12.2215. [DOI] [PubMed] [Google Scholar]
- 67.Thirunavukkarasu K, Miles RR, Halladay DL, Yang X, Galvin RJS, Chandrasekhar S, et al. Stimulation of Osteoprotegerin (OPG) Gene Expression by Transforming Growth Factor-β (TGF-β) MAPPING OF THE OPG PROMOTER REGION THAT MEDIATES TGF-β EFFECTS. J Biol Chem. 2001 Sep 28;276(39):36241–50. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 68.Weitzmann MN, Cenci S, Haug J, Brown C, DiPersio J, Pacifici R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J Cell Biochem. 2000 May;78(2):318–24. doi: 10.1002/(sici)1097-4644(20000801)78:2<318::aid-jcb13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 69.Klausen B, Hougen HP, Fiehn NE. Increased periodontal bone loss in temporarily B lymphocyte-deficient rats. J Periodont Res. 1989 Nov;24(6):384–90. doi: 10.1111/j.1600-0765.1989.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 70.Marusic A, Grcevic D, Katavic V, Kovacic N, Lukic IK, Kalajzic I, et al. Role of B lymphocytes in new bone formation. Lab Invest. 2000 Nov;80(11):1761–74. doi: 10.1038/labinvest.3780186. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian W-P, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007 May 1;109(9):3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, et al. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012 Aug 24;287(35):29851–60. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, et al. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci USA. 2010 Aug 3;107(31):13848–53. doi: 10.1073/pnas.1003020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi Y, Kim JJ. B cells activated in the presence of Th1 cytokines inhibit osteoclastogenesis. Exp Mol Med. 2003 Oct 31;35(5):385–92. doi: 10.1038/emm.2003.51. [DOI] [PubMed] [Google Scholar]
- 75.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000 Jun 1;95(11):3489–97. [PubMed] [Google Scholar]
- 76.JH, AK, Pa H, JT, JR, Dn H. Cells expressing dendritic cell markers are present in the rheumatoid nodule. J Rheumatol. 2000 Feb;27(2):339–46. [PubMed] [Google Scholar]
- 77.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001 Aug 1;167(3):1758–68. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 78.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004 Dec 15;104(13):4029–37. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 79.Alnaeeli M, Penninger JM, Teng Y-TA. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006 Sep 1;177(5):3314–26. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 80.Yano M, Kawao N, Okumoto K, Tamura Y, Okada K, Kaji H. Fibrodysplasia ossificans progressiva-related activated activin-like kinase signaling enhances osteoclast formation during heterotopic ossification in muscle tissues. J Biol Chem. 2014 Jun 13;289(24):16966–77. doi: 10.1074/jbc.M113.526038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001 Jun 1;79(5–6):243–53. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 82.Guerrini MM, Takayanagi H. The immune system, bone and RANKL. Archives of Biochemistry and Biophysics. 2014 Nov 1;561:118–23. doi: 10.1016/j.abb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 83.D’Amelio P, Grimaldi A, Bernabei P, Pescarmona GP, Isaia G. Immune system and bone metabolism: Does thymectomy influence postmenopausal bone loss in humans? Bone. 2006 Sep;39(3):658–65. doi: 10.1016/j.bone.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7(1):29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yago T, Nanke Y, Kawamoto M, Furuya T, Kobashigawa T, Kamatani N, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9(5):R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quinn JMW, Sims NA, Saleh H, Mirosa D, Thompson K, Bouralexis S, et al. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J Immunol. 2008 Oct 15;181(8):5720–9. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- 87.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min K-H, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010 Feb;62(2):402–13. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roth KE, Salzmann G, Maier GS, Schmidtmann I, Rompe JD, Babin K. Risk factors for heterotopic ossification and spur formation after total knee arthroplasty. Arch Orthop Trauma Surg. 2014 Jul;134(7):991–6. doi: 10.1007/s00402-014-1957-0. [DOI] [PubMed] [Google Scholar]
- 89.Guo R, Yamashita M, Zhang Q, Zhou Q, Chen D, Reynolds DG, et al. Ubiquitin ligase Smurf1 mediates tumor necrosis factor-induced systemic bone loss by promoting proteasomal degradation of bone morphogenetic signaling proteins. J Biol Chem. 2008 Aug 22;283(34):23084–92. doi: 10.1074/jbc.M709848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011 Dec;17(6):393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alblowi J, Kayal RA, Siqueira M, Siqueria M, McKenzie E, Krothapalli N, et al. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009 Oct;175(4):1574–85. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daoussis D, Andonopoulos AP. The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. 2011 Oct;41(2):170–7. doi: 10.1016/j.semarthrit.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Daoussis D, Liossis S-NC, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010 Jan;62(1):150–8. doi: 10.1002/art.27231. [DOI] [PubMed] [Google Scholar]