Abstract
Background
Oropharyngeal dysphagia is prevalent in individuals with amyotrophic lateral sclerosis (ALS) leading to malnutrition, aspiration pneumonia and death. These factors necessitate early detection of at risk patients to prolong maintenance of safe oral intake and pulmonary function. This study aimed to evaluate the discriminant ability of the Eating Assessment Tool (EAT-10) to identify ALS patients with unsafe airway protection during swallowing.
Methods
70 ALS patients completed the EAT-10 survey and underwent a standardized videofluoroscopic evaluation of swallowing (VFES). Two blinded raters determined airway safety using the Penetration Aspiration Scale (PAS). A between groups ANOVA (safe vs. penetrators vs. aspirators) was conducted and sensitivity, specificity, area under the curve (AUC) and likelihood ratios calculated.
Key Results
Mean EAT-10 scores for safe swallowers, penetrators and aspirators (SEM) were: 4.28 (0.79) vs. 7.10 (1.79) vs. 20.50 (3.19) respectively with significant differences noted for aspirators vs. safe swallowers and aspirators vs. penetrators (p<0.001). The EAT-10 demonstrated good discriminant ability to accurately identify ALS penetrator/aspirators (PAS≥3) with a cut off score of 3 (AUC:0.77, sensitivity: 88%, specificity: 57%). The EAT-10 demonstrated excellent accuracy at identifying aspirators (PAS≥6) utilizing a cut off score of 8 (AUC:0.88, sensitivity: 86%, specificity: 72%, likelihood ratio: 3.1, negative predictive value: 95.5%).
Conclusions and Inferences
The EAT-10 differentiated safe vs. unsafe swallowing in ALS patients. This patient self-report scale could represent a quick and meaningful aide to dysphagia screening in busy ALS clinics for the identification and referral of dysphagic patients for further instrumental evaluation.
Keywords: Eating Assessment Tool, EAT-10, Amyotrophic Lateral Sclerosis, Screen, Aspiration, dysphagia
Graphical Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal, neurodegenerative disease causing both upper and lower motor neuron degeneration. Progressive weakness and/or spasticity of the muscles of mastication, tongue, lips, pharynx, larynx and esophagus lead to dysphagia in approximately 85% of ALS patients (1). Degeneration of respiratory musculature further contributes to impaired airway protection with impairments in the ability to generate adequate expiratory pressures to produce an effective and protective cough (2, 3). Sequalae of dysphagia in ALS includes: malnutrition; compromised pulmonary status; pneumonia; increased risk of death; as well as reports of reductions in quality of life and mental wellbeing (1, 4–7). Indeed, aspiration and malnutrition have been noted to increase the risk of death by 7.7 times in this patient population (5). These factors necessitate the early identification of at risk individuals to ensure optimal medical management and to prolong safe oral intake and pulmonary function in this challenging patient population.
Although there have been limited advances in the management and treatment for individuals with ALS, attendance at specialized multidisciplinary (MTD) clinics has been noted to improve quality of life, extend survival and reduce hospitalizations and mortality (8–11). Current practice recommendations of both the American Academy of Neurology (12) and the European Federation of Neurological Societies (13) include a MTD model of care where patients are seen by a comprehensive team of health care professionals who each focus on the evaluation and management of specific health domains that include: walking, breathing, speaking, eating, activities of daily living and psychosocial needs during one clinical visit. This presents a challenge to the Speech Language Pathologist (SLP) who is tasked with examining and providing education on communication and swallowing function in a short time frame (fifteen minutes at our MTD clinic). The high incidence of silent aspirators recently noted in individuals with ALS (14) further compromises the ability to easily detect at risk patients in a clinical evaluation without the aid of instrumental visualization tools. There is therefore a critical need for practical screening tools that are quick, efficient and accurate to detect dysphagia and aspiration risk in this patient population.
The Eating Assessment Tool 10 (EAT-10) is a validated, self-administered, symptom-specific dysphagia outcome tool that has been implemented in clinics worldwide and validated in English (15), Italian (16), Spanish (17) and Brazilian (18). The EAT-10 is fast and easy to administer and therefore meets the practical requirements of a screener in the ALS MTD clinical setting that could be implemented by nursing staff or SLPs for identification of at risk individuals who require further more exhaustive instrumental evaluation of swallowing function. Although the EAT-10 has been documented to demonstrate good discriminant ability for identifying individuals who aspirate in two general patient populations in the United States (19) and Spain (20); to date no group has examined its ability to identify aspiration risk in individuals with ALS. Therefore, the aim of the current investigation was to evaluate the discriminant ability of the Eating Assessment Tool to identify aspiration risk in individuals with ALS.
MATERIALS AND METHODS
Subjects
Seventy individuals with a diagnosis of probable or definite ALS by the Revised El-Escorial Criteria (21) were included in this study. Mean age was 62.1 years (range: 30–83, SD: 10.3), 61.3% were males (n=46), mean ALS Functional Rating Scale-Revised (ALSFRS-R) scores were 34.8 (range: 16–47, SD: 7.9), and mean disease duration from symptom onset was 17.9 months (range: 2–72 months, SD: 13.0). Diagnosis was confirmed by the neuromuscular neurology specialist at a university ALS Center. Patients were enrolled if they had: 1) preserved cognition as evidenced by a score of >24 points on the Mini Mental Status Exam (22); 2) no allergies to barium; 3) no tracheotomy or mechanical ventilation; 4) absence of diaphragmatic pacer; and 5) no significant concurrent respiratory disease. This study was approved by the University Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All patients met the inclusion criteria and provided written consent to participate.
Testing Procedures
The Eating Assessment Tool (EAT-10)
ALS patients attended a single testing session. Upon arrival individuals were brought into an evaluation room and comfortably positioned (either seated or in their wheelchair) and provided a pen and the EAT-10 survey (see Appendix) that they were asked to complete. For patients who could not hold a pen due to severe limb paralysis, the research therapist circled the appropriate response given by the patients (either verbally, by pointing or a head nod) to assist completion.
Videofluoroscopic Evaluation of Swallowing (VFES)
Participants underwent a standardized videofluoroscopic evaluation of swallowing. Patients were seated upright in a lateral viewing plane using a properly collimated Phillips BV Endura fluoroscopic C-arm unit (GE OEC 8800 Digital Mobile C-Arm system type 718074). A Swallowing Signals Lab unit (Kay Pentax, Lincoln Park, NJ) digitally recorded the fluoroscopic images at 29.97 frames per second using a scan converter. A video counter was used to imprint a time code and the participants’ identification code on each examination to aid subsequent analysis. In accordance with a standard protocol, a penny (U.S. currency, 1.9 centimeters) was taped to the chin or neck of the patient for measurement calibration. Lateral views were obtained while the patient, seated in an examination chair, was administered liquid barium measured with a syringe or graduated medicine cup. A standardized bolus presentation protocol was used and consisted of: two 1-cc boluses of liquid contrast agent (Varibar thin barium sulfate suspension, EZ-EM, Inc., Westbury, NY), one 3-cc of thin liquid contrast, one 3-cc of pudding (EZ-pudding, EZ-EM, Inc.), one 20-cc bolus of liquid contrast and 90cc sequential swallows of thin liquid contrast. In anterior-posterior view, the patient was administered a 20-cc bolus of liquid contrast.
Data and Statistical Analysis
Total EAT-10 scores were calculated with scores ranging from 0 (no impairment) to 40 (severe impairment). Digital recordings of swallowing studies were played back using QuickTime (7.7.4). Airway protection during swallowing was measured using the validated Penetration-Aspiration Scale (PAS) (23), an eight-point ordinal scale of airway safety describing the degree of airway invasion, the participant’s response and whether the invasive material is successfully ejected from the airway (see Appendix B). Two raters independently performed the PAS analysis in a blinded fashion across all bolus trials. For statistical analysis the worst PAS score was utilized as has been previously performed (24). When agreement was not reached on a PAS score, a third expert rater was used to determine the final PAS score. This occurred in one subject. To determine EAT-10 profiles across ALS airway safety groups (safe vs. penetrators vs. aspirators), a between groups ANOVA was performed with LSD post-hoc testing. To determine the diagnostic accuracy of the EAT-10, a receiver operating characteristic (ROC) curve was created with area under the curve (AUC), sensitivity, specificity, optimal cut point, positive predictive value, negative predictive value and likelihood ratios calculated. This analysis was performed under two different binary classifications. The first was the ability of the EAT-10 to identify penetrator/aspirators (PAS≥3) vs. non-penetrator/aspirators (PAS≤2) similar to the methods of Rofe et al (20). The second analyzed the ability of the EAT-10 to distinguish aspirators (PAS≥6) from non-aspirators (PAS≤5) similar to the methods of Clafin and colleagues (19). Means, standard error and 95% confidence intervals are reported. Statistical comparisons were performed using SPSS (Version 22) with alpha set at 0.05.
RESULTS
1. EAT-10 Scores Across Airway Safety Groups
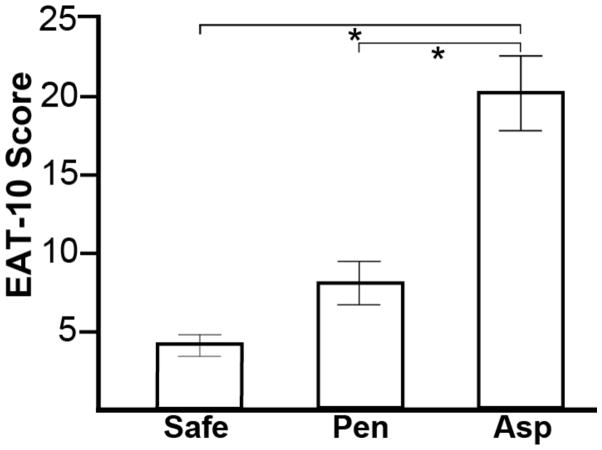
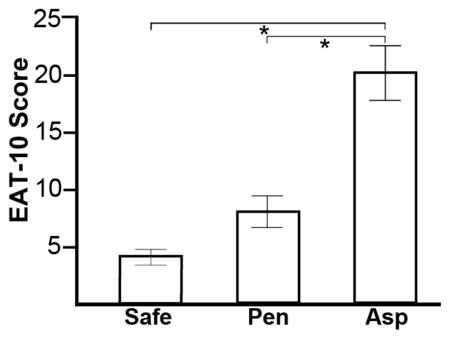
Mean EAT-10 scores for safe swallowers, penetrators and aspirators (SEM) were: 4.28 (0.79) vs. 7.10 (1.79) vs. 20.50 (3.19) respectively. A significant main effect was observed for patient-rated EAT-10 scores across airway safety groups, F(2,68)=27.93, p<0.001. Post- hoc analysis revealed that EAT-10 scores were higher (worse) in aspirators vs. safe swallowers (mean difference=16.31, SEM=2.18, 95% CI=11.95, 20.67, p<0.001) and aspirators vs. penetrators (mean difference=13.05, SEM=3.07, 95% CI=6.93, 19.18 p<0.001). Figure 1 displays mean EAT-10 scores across airway safety groups.
Figure 1.
Mean (SEM) Eating Assessment Tool (EAT-10) scores for ALS patients with safe swallowing (PAS≤2), penetrators (PAS:3–5) and aspirators (PAS≥6). * Indicates a significant difference (p<0.001).
2. Discriminant ability of the EAT-10 to predict penetration and aspiration in ALS patients (PAS ≥3)
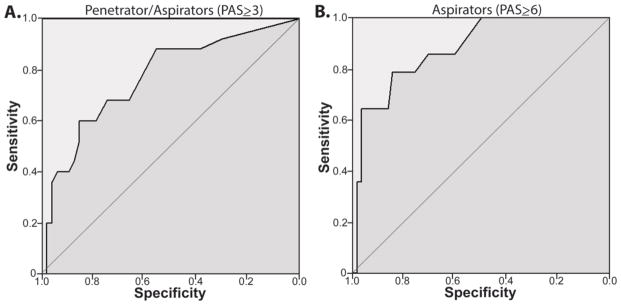
The EAT-10 was significant to detect ALS penetrator/aspirators (PAS≥3) (AUC:0.77, p<0.001). Table 1 summarizes ROC analysis data and Figure 2A displays ROC graphs. An EAT-10 cut value of 3 demonstrated a sensitivity of 88.0%, specificity of 56.7%, a positive predictive value of 52.4%, negative predictive value of 89.7% and a likelihood ratio of 2.0.
Table 1.
Summary of receiver operator characteristic analysis between 1) Safe (PAS≤2) vs. Unsafe (PAS≥3) swallowers (first row) and 2) Non-aspirators (PAS≤5) vs. Aspirators (PAS≥6) (row two) using a cut point of 8.
| AUC | Sensitivity (%) | Specificity (%) | Cut Point | PPV | NPV | LR | |
|---|---|---|---|---|---|---|---|
| Safe vs. Unsafe | 0.77 (0.65, 0.89) | 88.0 (72.0, 100.0) | 56.7 (43.5, 69.6) | 3 | 52.4 (44.4, 61.8) | 89.7 (79.2, 100.0) | 2.0 |
| Non-Aspirators vs. Aspirators | 0.88 (0.79, 0.97) | 85.7 (64.3, 100.0) | 71.9 (59.7, 82.5) | 8 | 42.9 (32.4, 56.5) | 95.5 (89.1, 100.0) | 3.1 |
AUC: area under the curve, PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio.
Figure 2.
Receiver operator curves (ROC) for the ability of the EAT-10 to Identify ALS patients who A) penetrate or aspirate (PAS≥3) and B) aspirate (PAS≥6).
3. Discriminant ability of the EAT-10 to predict aspiration in ALS patients (PAS≥6)
The EAT-10 was significant to detect ALS aspirators (PAS≥6) (AUC:0.88, p<0.001). An EAT-10 cut value of 8 demonstrated a sensitivity of 85.7%, specificity of 71.9%, a positive predictive value of 42.9%, negative predictive value of 95.5% and a likelihood ratio of 3.1. Table 1 summarizes ROC analysis data and Figure 2B displays ROC graphs.
DISCUSSION
In this group of individuals with ALS, self-reported dysphagia symptoms, as indexed by the EAT-10, predicted swallowing airway safety status. On average, EAT-10 scores were five times higher in ALS aspirators than in those who demonstrated safe swallowing. ALS patients who scored greater than a 3 on the EAT-10 were 2 times more likely to be unsafe swallowers (penetrate or aspirate). This value correctly identified 88.0% of ALS individuals with unsafe swallowing and 89.7% of safe swallowers scored below this criterion score. When examining the discriminant ability of the EAT-10 for identifying ALS aspirators (PAS≥6), a cut value of 8 correctly identified 85.7% of aspirators and patients scoring above this value were 3.1 times more likely to aspirate. Further, 95% of ALS patients with safe swallowing had EAT-10 scores below this value (negative predictive value).
The current findings that the EAT-10 offers high discriminant ability in detecting aspiration in ALS patients is consistent with two previous reports in a general dysphagic population. In 320 consecutive patients referred for VFSE for suspected dysphagia in an outpatient university setting; Cheney et al. (19) reported that individuals whose EAT-10 scores were greater then 15 were 2.2 times more likely to aspirate. This value correctly identified 71% of actual aspirators (i.e. sensitivity) and demonstrated a negative predictive value of 89% (19). Rofes et al. (20) studied 120 patients with suspected swallowing impairment in a hospital setting and reported a high sensitivity (89%) and specificity (82%) for the EAT-10 in identifying the presence of oropharyngeal dysphagia utilizing a cut score of 2. These authors recommended the universal application of the EAT-10 in at risk neurologic patients for referral to further instrumental evaluation. It must be noted that the earlier investigation utilized a PAS score of ≥6 (i.e. aspirators) for their analysis while the later utilized a PAS score ≥3 (i.e. penetrators and aspirators). This methodological difference likely explains the differing criterion scores reported by the two studies. In the present study we investigated the ability of the EAT to detect both unsafe swallowing (PAS>3) and aspiration (PAS>6). This was undertaken due to the rapid progressive nature of ALS and the need to detect early signs of impaired airway protection (i.e. penetration) that will most likely lead to aspiration in due course. Identification of early signs of impaired airway protection may be important to maximize pulmonary, swallowing and nutritional domains in this patient population and indeed has been reported to extend survival in ALS (25).
In this group of ALS patients, it was interesting to note that differences existed not only between safe and unsafe swallowers but also amongst unsafe swallowers themselves depending on the degree of airway invasion. Indeed, the average EAT-10 score was almost three times higher in aspirators compared to penetrators. Thus the EAT-10 appeared to demonstrate good sensitivity to differentiate the relative severity of unsafe swallowing.
The current results would support the implementation of the EAT-10 in the clinical evaluation of ALS patients. Given that the EAT-10 is quick and easy to administer and score, it could be included in evaluations at busy MTD ALS clinics and provide additional, useful information regarding the potential risk for aspiration. This could be administered by nursing staff to screen for those who are appropriate for a SLP referral when this is not standard practice, or by SLPs themselves to determine those patients who require further instrumental evaluation of swallowing. At our center, we include the EAT-10 in the mailing packet sent to patients prior to their appointments and ask patients to complete and bring to their clinic visit to maximize efficiency.
Although we report that the EAT-10 offers high clinical utility and excellent discriminant ability to detect aspiration risk in this group of ALS patients, an important caveat to our findings is the fact that such a screening tool cannot and does not replace the need for a thorough clinical evaluation. Therefore, the EAT-10 is recommended to be incorporated in a complete clinical evaluation to provide additional information to aid clinical decision-making processes. This is the first attempt to determine the discriminant ability of the EAT-10 in individuals with ALS with the need for future studies to add to this preliminary dataset in a larger cohort of ALS patients. Future work should incorporate more sensitive screening tools to detect the cognitive dysfunction that can accompany ALS rather then the MMSE utilized in the current study and could also elucidate if any differences exist in the discriminate ability of the EAT-10 in ALS patients with a bulbar onset versus those with a spinal onset in a larger cohort.
In conclusion, our study documents that EAT-10 scores can discriminate safe vs. unsafe ALS swallowers as well as non-aspirators vs. aspirators. This easy to administer validated patient self-report represents a useful screening tool yielding high clinical utility. Therefore we recommend its routine use in ALS MTD clinics to provide further information regarding swallowing and airway safety status in this vulnerable patient population.
Supplementary Material
Key Messages.
Aspiration is prevalent in individuals with amyotrophic lateral sclerosis (ALS) and contributes to malnutrition, aspiration pneumonia and death necessitating accurate and timely identification of at risk individuals.
The Eating Assessment Tool (EAT-10) represents a validated patient self-report scale that is quick and easy to administer in busy multidisciplinary ALS clinics. The aim of this investigation was to determine the discriminant ability of the EAT-10 to identify ALS patients with impaired airway protection during swallowing.
Results demonstrate that the EAT-10 offers high discriminant ability to identify ALS patients who aspirate (sensitivity: 86%, specificity: 76%, negative predictive value: 95%).
The EAT-10 represents a screening tool yielding high clinical utility in this challenging patient population and is recommended in multidisciplinary clinics to determine the need for further, more comprehensive, instrumental evaluation of swallowing function in this high risk patient population.
Acknowledgments
FUNDING:
This study was funded, in part, by grant R21HD075327 from the National Institute of Child Health and Development.
Footnotes
AUTHOR CONTRIBUTION:
EP: Study conceptualization and design, statistical analysis; manuscript write up
LT, RR, JG, SW: Acquisition of data, data analysis
CD: Statistical analysis
TV, CG: Study conceptualization and design.
DISCLOSURE:
EP has been the recipient of federal funding from the National Institutes of Health and the Veterans Affair Hospital.
References
- 1.Kuhnlein P, Gdynia HJ, Sperfeld AD, Lindner-Pfleghar B, Ludolph AC, Prosiegel M, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366–74. doi: 10.1038/ncpneuro0853. Epub 2008/06/19. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei medical journal. 2010;51(3):392–7. doi: 10.3349/ymj.2010.51.3.392. Epub 2010/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruoppolo G, Schettino I, Frasca V, Giacomelli E, Prosperini L, Cambieri C, et al. Dysphagia in amyotrophic lateral sclerosis: prevalence and clinical findings. Acta neurologica Scandinavica. 2013;128(6):397–401. doi: 10.1111/ane.12136. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Garrett CG. Otolaryngologic presentations of amyotrophic lateralsclerosis. Otolaryngol Head Neck Surg. 2005;132(3):500–4. doi: 10.1016/j.otohns.2004.09.092. Epub 2005/03/05. [DOI] [PubMed] [Google Scholar]
- 5.Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: A critical review. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2009;10(5–6):310–23. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paris G, Martinaud O, Petit A, Cuvelier A, Hannequin D, Roppeneck P, et al. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. 2013;40(3):199–204. doi: 10.1111/joor.12019. Epub 2013/01/03. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Huang R, Chen D, Song W, Zeng Y, Zhao B, et al. Causes and places of death of patients with amyotrophic lateral sclerosis in south-west China. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2011;12(3):206–9. doi: 10.3109/17482968.2011.572979. Epub 2011/04/22. [DOI] [PubMed] [Google Scholar]
- 8.Aridegbe T, Kandler R, Walters SJ, Walsh T, Shaw PJ, McDermott CJ. The natural history of motor neuron disease: assessing the impact of specialist care. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14(1):13–9. doi: 10.3109/17482968.2012.690419. [DOI] [PubMed] [Google Scholar]
- 9.Chio A, Bottacchi E, Buffa C, Mutani R, Mora G, Parals Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. Journal of neurology, neurosurgery, and psychiatry. 2006;77(8):948–50. doi: 10.1136/jnnp.2005.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooney J, Byrne S, Heverin M, Tobin K, Dick A, Donaghy C, et al. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. Journal of neurology, neurosurgery, and psychiatry. 2015;86(5):496–501. doi: 10.1136/jnnp-2014-309601. [DOI] [PubMed] [Google Scholar]
- 11.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol. 2000;57(8):1171–6. doi: 10.1001/archneur.57.8.1171. Epub 2000/08/06. [DOI] [PubMed] [Google Scholar]
- 12.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1227–33. doi: 10.1212/WNL.0b013e3181bc01a4. Epub 2009/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagnosis ETFo, Management of Amyotrophic Lateral S. Andersen PM, Abrahams S, Borasio GD, de Carvalho M, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)--revised report of an EFNS task force. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2012;19(3):360–75. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaziano J, Tabor L, Richter J, Plowman EK, editors. Prevalence, Timing and Source of Aspiration in Individuals with ALS. Dysphagia Research Society; Chicago, IL: 2015. [Google Scholar]
- 15.Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117(12):919–24. doi: 10.1177/000348940811701210. Epub 2009/01/15. [DOI] [PubMed] [Google Scholar]
- 16.Schindler A, Mozzanica F, Monzani A, Ceriani E, Atac M, Jukic-Peladic N, et al. Reliability and validity of the Italian Eating Assessment Tool. Ann Otol Rhinol Laryngol. 2013;122(11):717–24. doi: 10.1177/000348941312201109. [DOI] [PubMed] [Google Scholar]
- 17.Burgos R, Sarto B, Segurola H, Romagosa A, Puiggros C, Vazquez C, et al. Translation and validation of the Spanish version of the EAT-10 (Eating Assessment Tool-10) for the screening of dysphagia. Nutricion hospitalaria. 2012;27(6):2048–54. doi: 10.3305/nh.2012.27.6.6100. Traduccion y validacion de la version en espanol de la escala EAT-10 (Eating Assessment Tool-10) para el despistaje de la disfagia. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves MI, Remaili CB, Behlau M. Cross-cultural adaptation of the Brazilian version of the Eating Assessment Tool - EAT-10. CoDAS. 2013;25(6):601–4. doi: 10.1590/S2317-17822013.05000012. [DOI] [PubMed] [Google Scholar]
- 19.Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The Ability of the 10-Item Eating Assessment Tool (EAT-10) to Predict Aspiration Risk in Persons With Dysphagia. Ann Otol Rhinol Laryngol. 2015;124(5):351–4. doi: 10.1177/0003489414558107. [DOI] [PubMed] [Google Scholar]
- 20.Rofes L, Arreola V, Mukherjee R, Clave P. Sensitivity and specificity of the Eating Assessment Tool and the Volume-Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(9):1256–65. doi: 10.1111/nmo.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. 2000;1(5):293–9. doi: 10.1080/146608200300079536. Epub 2001/07/24. [DOI] [PubMed] [Google Scholar]
- 22.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. The American journal of psychiatry. 1982;139(9):1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 24.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–8. doi: 10.1007/s00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spataro R, Ficano L, Piccoli F, La Bella V. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: effect on survival. J Neurol Sci. 2011;304(1–2):44–8. doi: 10.1016/j.jns.2011.02.016. Epub 2011/03/05. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.