Abstract
Background
Achalasia has three distinct manometric phenotypes. This study aimed to determine if there were corresponding histopathologic patterns.
Methods
We retrospectively examined surgical muscularis propria biopsies obtained from 46 patients during laparoscopic esophagomyotomy. Pre-operative (conventional) manometry tracings were reviewed by 2 expert gastroenterologists who categorized patients into Chicago Classification subtypes. Pathology specimens were graded on degree of neuronal loss, inflammation, fibrosis, and muscle changes.
Key Results
Manometry studies were categorized as follows: type I (n=20), type II (n=20), type III (n=3), and esophagogastric junction outflow obstruction (EGJOO) (n=3). On histopathology, complete ganglion cell loss occurred in 74% of specimens, inflammation in 17%, fibrosis in 11%, and muscle atrophy in 2%. Comparing type I and type II specimens, there was a statistically significant greater proportion of type I specimens with aganglionosis (19/20 vs. 13/20, p=0.044) and a statistically significant greater degree of ganglion cell loss in type I specimens (Wilcoxon Rank-Sum, p=0.016). CD3+/CD8+ cytotoxic T cells represented the predominant inflammatory infiltrate on immunohistochemistry. Three patients had completely normal appearing tissue (1 each in type II, type III, EGJOO).
Conclusions and Inferences
The greater degree, but similar pattern, of ganglion cell loss observed in type I compared to type II achalasia specimens suggests that type I achalasia represents a progression from type II achalasia. The spectrum of histopathologic findings – from complete neuronal loss to lymphocytic inflammation to apparently normal histopathology – emphasizes that ‘achalasia’ represents a pathogenically heterogeneous patient group with the commonality being EGJ outflow obstruction.
Keywords: Achalasia, Esophagus, Histopathology, Immunohistochemistry, Motility
INTRODUCTION
Achalasia is characterized by impaired lower esophageal sphincter (LES) relaxation and failed peristalsis. Previous histopathologic studies done with esophagectomy or surgical myotomy specimens have demonstrated absence or reduction in ganglion cells as well as varying degrees of cytotoxic T-cell inflammation in the myenteric plexus of patients with achalasia, prompting the hypothesis that achalasia is a neurodegenerative disease resulting from an inflammatory and/or autoimmune process ultimately leading to aganglionosis [1,2,3,4,5]. Studies demonstrating an association between achalasia and certain HLA phenotypes [6,7,8,9], and an increased immunologic response to Herpes Simplex Virus-1 (HSV-1) in the serum [10] and LES myenteric plexus [11] of achalasia patients further support the notion of inflammation playing a central role in pathogenesis.
Recently, achalasia has been recognized as a heterogeneous disease with 3 distinct manometric patterns that differ in their responsiveness to treatment [12]. Type I achalasia is characterized by minimal contractility in the esophageal body, type II by intermittent periods of pan-esophageal pressurization, and type III by premature (spastic) distal esophageal contractions [13]. In addition to unique patterns exhibited in the esophageal body on manometry, it also appears that differing LES responses to multiple rapid swallows exist among the subtypes. Kushnir et al. described a gradient in the degree of LES pressure response to multiple rapid swallows (five 2 ml water swallows 2–3 seconds apart), with type I patients exhibiting no LES response, type II patients exhibiting a more pronounced decline in LES pressure, and type III patients exhibiting the most pronounced decline in LES pressure, almost to a normal level of relaxation [14]. This pattern is perhaps suggestive of close to complete absence of excitatory and inhibitory postganglionic function in type I achalasia, contrasted with some degree of preserved postganglionic function in type II and type III achalasia.
In addition to the three manometric subtypes of achalasia, the Chicago Classification also recognizes the entity of esophagogastric junction outflow obstruction (EGJOO), defined by impaired EGJ relaxation along with sufficient evidence of peristalsis such that criteria for types I, II, or III achalasia are not met [15]. This too proves to be a heterogeneous entity encompassing some patients with mechanical causes of outflow obstruction and some atypical or, perhaps early cases of achalasia.
Previous histopathologic studies of achalasia have largely been done on patients with advanced disease being treated with surgical myotomy or esophagectomy. As such, they may not be representative of the full disease spectrum. Given the observed physiological differences among achalasia subtypes and EGJOO that have subsequently come to light, we aimed to examine if corresponding unique histopathologic patterns in the muscularis propria also existed. Specifically, we sought to evaluate the hypothesis that type I achalasia progresses from type II achalasia by means of a T-cell mediated inflammatory neurodegenerative process targeted at ganglion cells of the myenteric plexus [16].
METHODS
Study design and population
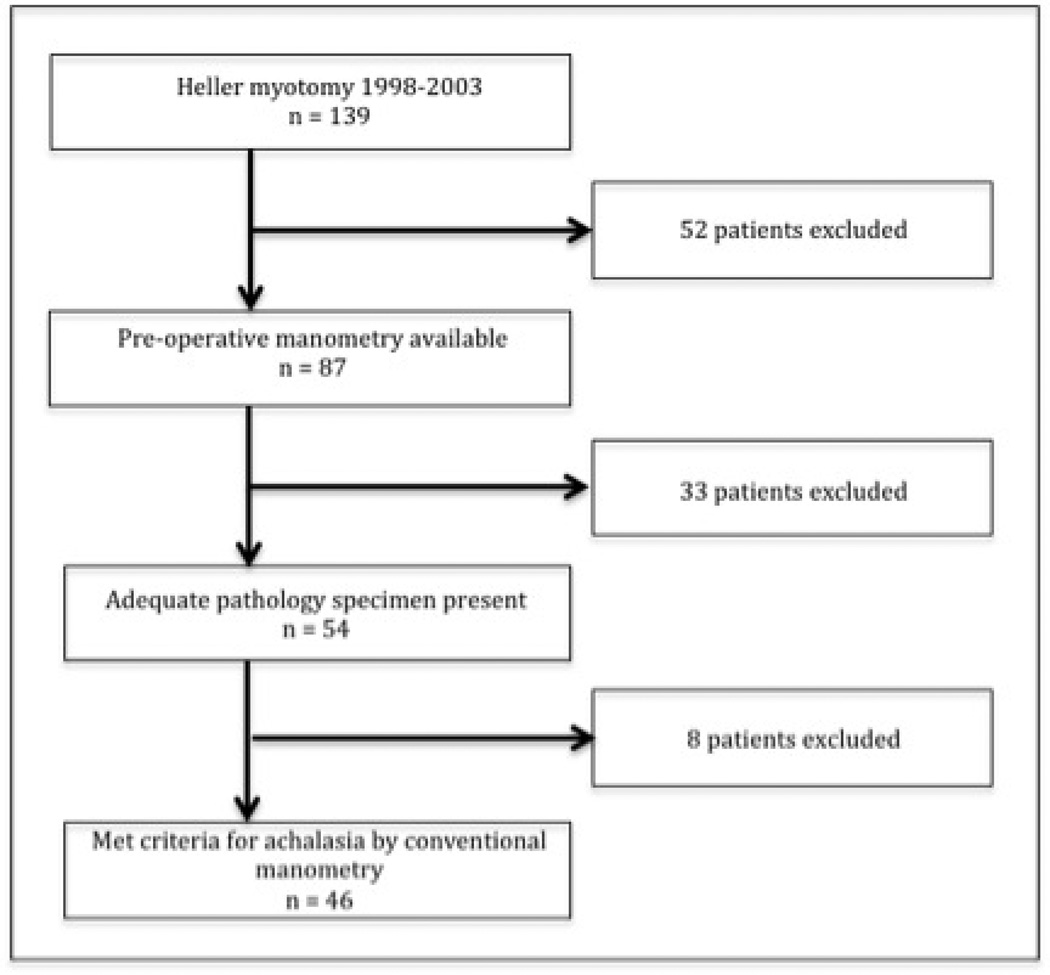
This was a retrospective study of a consecutive series of adult patients who underwent laparoscopic esophagomyotomy and muscularis propria biopsy between 1997 and 2003 at Northwestern Memorial Hospital. The Institutional Review Board of Northwestern University approved the study. Patients were included if they had both a preoperative (conventional) manometry study and an adequate esophageal muscularis propria biopsy available. A consort diagram (Figure 1) illustrates the patients examined.
Figure 1.
Esophageal manometry
Esophageal manometry was performed using a 9-lumen silicone manometric assembly (Dentsleeve Pty. Ltd, Bowden, South Australia) incorporating a 6-cm sleeve sensor, 7 side-hole recording sites (distal sleeve, proximal sleeve, and 3, 6, 9, 12, 15, and 29 cm proximally), and a large central channel. Recordings were made with a Neomedix recording system and processed using Gastromac software (Noemedix Systems Pty. Ltd, Warriewood, NSW, Australia). The manometric catheter was passed transnasally and positioned with the sleeve sensor straddled the lower esophageal sphincter. Patients were studied in a supine position. The side hole at 29 cm in the pharynx was used as a swallow marker. A 10-minute baseline recording was obtained during which patients were instructed to swallow as infrequently as possible. Next, 10 consecutive 5-ml water swallows were obtained, performed at least 30 seconds apart.
Manometric data analysis and interpretation
Manometry tracings were reviewed by 2 expert gastroenterologists (PJK, JEP) who categorized patients into subtypes of achalasia using the Chicago Classification diagnostic scheme based on the esophageal pressurization patterns observed. Conventional manometric definitions of achalasia subtypes were adapted from the study by Salvador et al.[17]. EGJ pressure was referenced to gastric pressure. EGJ basal pressure was defined as the mean EGJ pressure during a 5- to 10-s artifact-free period before each swallow. EGJ relaxation pressure was defined as the mean Post-deglutitive EGJ pressure after which no further decrease occurred.
Achalasia was defined as a mean EGJ relaxation pressure >10 mm Hg and aperistalsis. Type I achalasia was defined as having no distal esophageal pressurization >30 mm Hg in at least 8 of 10 swallows. Type II achalasia was defined as having at least 2 swallows with >30 mmHg pan-esophageal pressurization. Type III achalasia was defined as having 2 or more spastic contractions with an amplitude >70 mm Hg, a duration >6 seconds, and a latency <4.5 seconds (judged from the time of the swallow to the time of the pressure upstroke at the recording site 3 cm proximal to the sleeve sensor). EGJOO was defined as a mean EGJ relaxation pressure >10 mmHg with some instances of intact or weak peristalsis such that it did not meet criteria for type I, type II, or type III achalasia. Cases that did not meet manometric criteria for type I, type II, type III, or EGJOO (n=8) were excluded, these included absent peristalsis (n=6), weak peristalsis (n=1), and hypercontractile LES (n=1).
Biopsy procedure and histopathology
Biopsies of the LES muscularis propria were obtained during laparoscopic myotomy surgery. Pathology slides were reviewed by three collaborating pathologists who were blinded to achalasia subtype. Specimens were graded on degree of neuronal loss, muscular damage, inflammation, and fibrosis.
Neuronal loss was assessed by comparing ganglion cells per nerve bundle in all specimens examined. LES specimens from 5 patients who underwent distal esophagectomy for gastric tumors were used as controls. Degree of fibrosis was assessed for each case and graded according to no fibrosis (score = 0), mild fibrosis (score = 1), moderate fibrosis (score = 2) and severe fibrosis (score = 3). Myopathy was scored in a categorical fashion, with muscle tissue graded as normal, atrophic, or hypertrophic. The presence, absence, and type of inflammation were recorded for all cases. In specimens with inflammation and adequate tissue, the pattern of lymphocytic inflammation was analyzed immunohistochemically with antibodies against total T lymphocytes (CD3), total B lymphocytes (CD20), regulatory T cells (FOXP3), helper T cells (CD4), and cytotoxic T cells (CD8). All three pathologists reviewed each of the specimens together and arrived at a consensus result when describing each of the findings of inflammation, myopathy, and fibrosis.
Antibodies and Immunohistochemistry
Immunohistochemical staining for CD3, CD20, FOXP3, CD4 and CD8 were performed on formalin-fixed, paraffin-embedded, 5-µm-thick tissue sections. Tissue sections were deparaffinized in xylene for a total of 15 min and subsequently rehydrated. Immunostaining was performed on a Leica Bond-Max machine for CD3 (Leica, clone LN10), CD4 (Leica, clone 4B12), CD8 (Leica, clone 4B11), CD20 (Leica, clone MJ1) and FOXP-3 (Abcam, clone 236A/E7).
Antigen retrieval was carried out at 97°C for 20 minutes in citrate buffer at pH 9.0 for CD3, CD4, CD8 and CD20 and at pH 6.0 for CD20 and FOXP-3. After blocking the endogenous peroxidase activity with 3% hydrogen peroxidase for 10 minutes, the primary antibody incubation for CD3, CD4, CD8, CD20 (all undiluted, ready to use) and FOXP-3 (1:50 dilution) was carried out for 8 minutes at room temperature. Bound primary antibodies were detected with an Leica Refine Detection System DS 9800 (Leica, Nussloch, Germany) according to the manufacturers' instructions. Staining was considered positive when tumor cells showed cytoplasmic reactivity (CD3, CD4, CD8 and CD20) and nuclear reactivity (FOXP-3). Negative controls (substituting Tris-buffered saline for primary antibody) were run simultaneously. Two blinded pathologists assessed all slides.
Statistical analysis
Descriptive statistics were used to analyze demographic, manometry, and histopathologic data. To compare average number of ganglion cells per nerve bundle between type I and type II achalasia, non-parametric analysis was performed given the large number of specimens with a zero value and consequent non-normal distribution.
Fisher’s Exact test was used to compare the proportion of specimens with zero ganglion cells per nerve bundle between type I and type II achalasia. Wilcoxon Rank-Sum test was used to further compare ganglion cell loss between type I and type II achalasia. Two-tailed T-test was used to compare basal and relaxation LES pressures between cases with zero ganglion cells per nerve bundle and cases with greater than zero ganglion cells per nerve bundle.
RESULTS
46 patients (25 females and 21 males) were included in the study. The mean age at time of esophagomyotomy was 42 years, with a range of 15 to 84. Preoperative manometry studies were categorized as follows: type I achalasia (n=20), type II achalasia (n=20), type III achalasia (n=3), and EGJOO (n=3). Among all patients, the mean resting EGJ pressure was 26.5 mm Hg (SD 10.1) and the mean EGJ relaxation pressure was 18.6 mm Hg (SD 7.4).
The overall histopathologic findings of neuronal loss, inflammatory infiltrate, fibrosis, and myopathy are summarized in Table 1. Fibrosis was scored as mild (score = 1) in all 5 cases in which it was identified. Inflammatory infiltrates were localized to the myenteric plexus in most cases, but infiltration into surrounding areas was occasionally seen. Three patients had completely normal appearing tissue on histopathologic evaluation compared to control samples (1 each type II, type III, EGJOO).
Table 1.
Summary of histopathologic findings of neuronal loss, inflammatory infiltrate, fibrosis, and myopathy in the 46 achalasia specimens reviewed.
| Histopathologic finding | n (%) |
|---|---|
| Complete aganglionosis | 34/46 (74%) |
| Inflammatory infiltrate | 8/46 (17%) |
| Lymphocytic | 7/8 (88%) |
| Eosinophilic | 1/8 (13%) |
| Fibrosis | 5/46 (11%) |
| Myopathic changes (atrophy) | 1/46 (2%) |
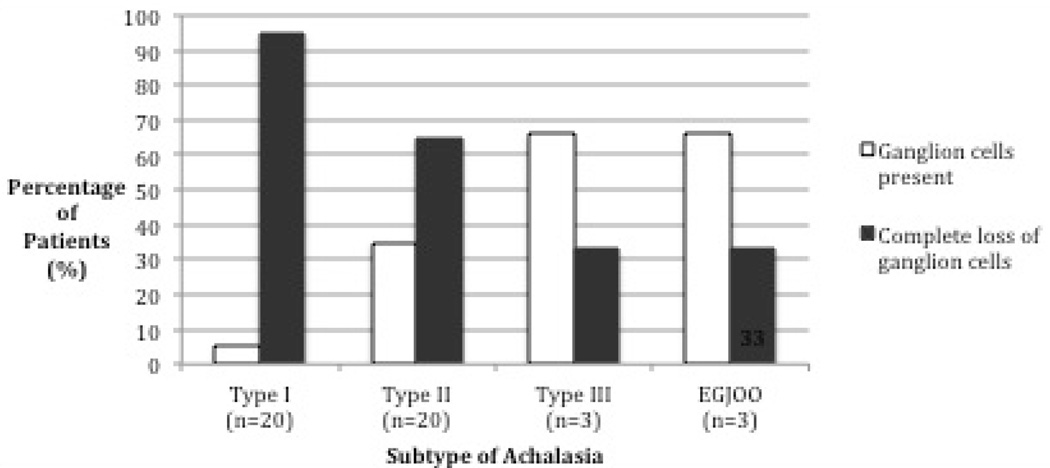
The degree of neuronal loss by achalasia subtype is depicted in Figure 1. Fisher’s Exact Test comparing the proportion of specimens with zero ganglion cells per nerve bundle between type I and type II achalasia showed a statistically significant greater proportion of type I specimens with aganglionosis (19/20 vs. 13/20, p = 0.044). Wilcoxon Rank-Sum Test comparing ganglion cells per nerve bundle between type I and type II achalasia demonstrated a statistically significant greater degree of ganglion cell loss in type I vs. type II achalasia (p = 0.016). The average number of ganglion cells per nerve bundle among the 5 control esophagectomy specimens was 0.86 compared to 0.45 among type II specimens and 0.03 among type I specimens.
Comparing EGJ measures in achalasia specimens with aganglionosis to achalasia specimens with greater than zero ganglion cells per nerve bundle using two-tailed T-test, there was no significant difference in EGJ basal pressure (mean 26.2 mmHg vs. 27.1 mmHg, p = 0.82) and no significant difference in EGJ relaxation pressure (mean 18.1 mmHg vs. 20.3 mmHg, p = 0.55).
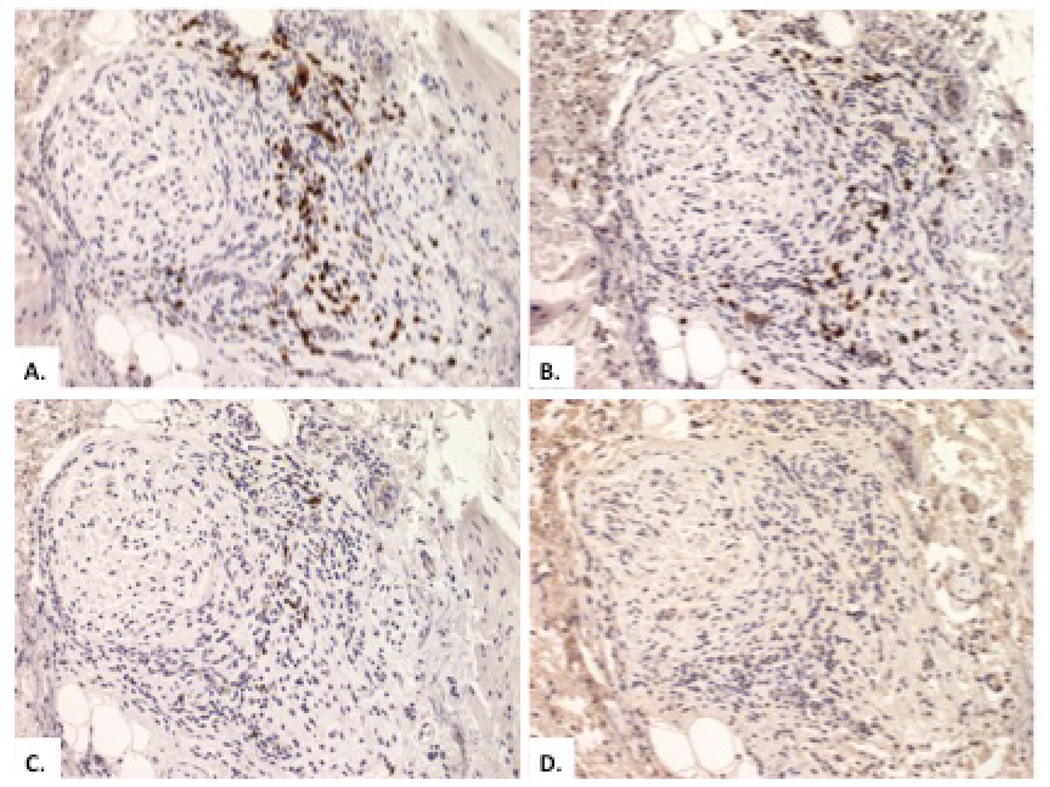
There was no difference in the proportion of specimens exhibiting inflammation between type I and type II subgroups using Fisher’s Exact test (3/20 vs. 4/20, p = 1.00). Of the 8 specimens with inflammatory infiltrate, lymphocytes represented the predominant inflammatory cell in 7 cases (88%) with 1 case exhibiting an eosinophil-predominant infiltrate in the myenteric plexus. Immunohistochemistry staining against total T lymphocytes (CD3), total B lymphocytes (CD20), regulatory T cells (FOXP3), helper T cells (CD4), and cytotoxic T cells (CD8) was performed on 7 of the 8 specimens showing inflammation. Of these cases, 1 specimen re-demonstrated an eosinophilic infiltrate and stained negative for all markers, 1 specimen had inadequate tissue and inflammation for staining, and the remaining 5 specimens showed a predominance of CD3+/CD8+ T cells, representing 80–90% of inflammatory cells present in each specimen. Each of these specimens also stained positive for CD3+/CD4+ T cells, but these represented a minority of the inflammatory cells present. CD20 and FOXP3 staining was negative in all specimens, indicating an absence of B lymphocytes and regulatory T cells, respectively.
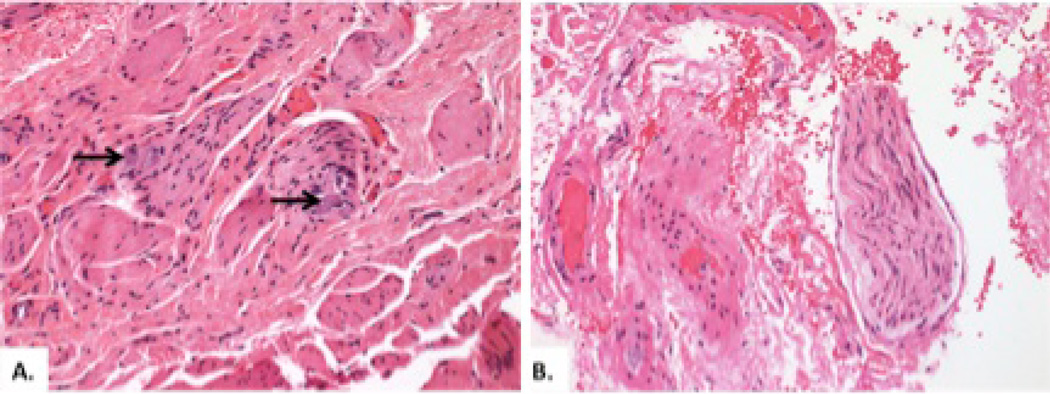
Figure 3 shows the histopathology of a patient with type II achalasia in which ganglion cells were present (A) and the histopathology of a patient with type I achalasia (B) in which there was complete absence of ganglion cells and nerve fibers. Figure 4 demonstrates a CD3+/CD8+ inflammatory infiltrate surrounding ganglion cells in a patient with type II achalasia.
Figure 3.
Figure 4.
DISCUSSION
The aim of this study was to determine if manometric subtypes of achalasia demonstrated corresponding unique histopathologic patterns. Specifically, we sought to determine if there was a greater degree of ganglion cell loss in surgical LES biopsies from type I compared to type II achalasia patients. The major findings of the analysis were of a significantly greater degree of ganglion cell loss in type I compared to type II specimens and a significantly greater proportion of type I specimens with complete absence of ganglion cells in LES biopsy specimens. This supports the hypothesis that type I achalasia represents a progression from type II achalasia.
Relatively little is known of the natural history of achalasia because most patients are treated rather than observed at the time of diagnosis and very few patients have any available histopathology, let alone repetitive samples. With that consideration in mind, it was very interesting that there was no significant difference in the proportion of type I versus type II specimens demonstrating inflammatory infiltrate and only 17% of all achalasia specimens showed ongoing inflammation. Although these findings may reflect limitations of a small LES biopsy, they complicate the notion of a uniformly progressive inflammatory myenteric plexopathy starting with inflammation and ending in aganglionosis. Rather, they suggest heterogeneity among the achalasia population with some patients prone to progression and others not [18]. Our findings suggest that in many cases, the inflammatory process appears to be intermittent or self-limited, ending before progression to end-stage disease has occurred.
The finding of a predominance of CD3+/CD8+ lymphocytes in specimens that had an inflammatory infiltrate confirms findings of previous studies [19, 20]. This subset of patients with an inflammatory infiltrate surrounding ganglion cells and/or neurons represents a subgroup of patients for which future studies of immunotherapy may be attempted, possibly targeted at cytotoxic T cells. Identifying this subset of patients, however, represents a challenge given the inaccessibility of the myenteric plexus to conventional endoscopic biopsy. Similarly, another unexpected finding of our study was the completely normal LES histopathology seen in 3 myotomy specimens. The potential pathophysiology of LES dysfunction in these patients is even more perplexing, highlighting the distinction between manometric diagnoses and histopathologic disease processes. Of these three patients, one each met the manometric criteria for type II achalasia, type III achalasia, and EGJOO. However, apparently that pathology was the result of something other that myenteric plexopathy.
Our study has limitations related to the limited tissue we had available. The examination of LES specimens without specimens from the esophageal body represents a limitation as contractile characteristics of the body of the esophagus distinguish subtypes of achalasia under the Chicago Classification scheme. Histopathologic changes in the LES may reflect only one aspect of disease pathogenesis. Furthermore, because of the retrospective nature of the study, information regarding duration and severity of disease, which likely influenced histopathologic findings, was unavailable. As such, alternative hypotheses, such as the suggestion that type I and type II achalasia represent distinct disease processes with differing disease mechanisms, are difficult to disprove. Additionally, because of the small number of type III and EGJOO specimens available for analysis, it is it difficult to draw conclusions about these subtypes. Nonetheless, this was the largest study to date examining the histopathology of achalasia and the only study specifically devoted to examining the histopathology among Chicago Classification achalasia subtypes. The results highlight how much remains to be discovered about this rare disease.
In conclusion, we retrospectively examined surgically obtained muscularis propria biopsy specimens from the LES of a series of patients undergoing laparoscopic myotomy for clinically-defined achalasia. Their (conventional) manometric studies were reviewed and classified according to the current Chicago classification. Our major finding was of a greater degree, but similar pattern, of myenteric plexus ganglion cell loss in type I compared to type II achalasia specimens suggesting that type I achalasia represents disease progression from type II achalasia. The spectrum of histopathologic findings observed, from complete aganglionosis, to lymphocytic inflammation, to apparently normal histopathology, also emphasizes that ‘achalasia’ as diagnosed by manometry represents a pathogenically heterogeneous patient group with the commonality being EGJ outflow obstruction.
Figure 2.
KEY MESSAGES.
-
-
This study aimed to determine if histopathologic patterns correlated with manometric subtypes of achalasia.
-
-
We retrospectively reviewed manometry tracings of a series of patients who underwent laparoscopic myotomy for treatment of achalasia, classified them using Chicago Classification criteria, and looked for group histopathologic differences in surgical muscularis propria biopsies obtained from the LES.
-
-
The major finding was a greater degree, but similar pattern, of myenteric plexus ganglion cell loss in type I achalasia specimens compared to type II achalasia specimens, suggesting that type I achalasia represents disease progression from type II achalasia.
-
-
A large spectrum of histopathologic patterns was observed, emphasizing that achalasia represents a pathogenically varied patient group.
Acknowledgements
Dr Pandolfino was supported by grants #DK07659 and #DK092217 from the Public Health Service. Dr Kahrilas was supported by grant #DK056033 from the public health service.
Abbreviations
- EGJ
esophagogastric junction
- EGJOO
esophagogastric junction outlet obstruction
- GERD
gastroesophageal reflux disease
- HLA
human leukocyte antigen
- HSV-1
Herpes Simplex Virus 1
- LES
lower esophageal sphincter
REFERENCES
- 1.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994 Apr;18(4):327–337. [PubMed] [Google Scholar]
- 2.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–654. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 3.Raymond L, Lach B, Shamji FM. Inflammatory aetiology of primary oesophageal achalasia: an immunohistochemical and ultrastructural study of Auerbach's plexus. Histopathology. 1999 Nov;35(5):445–453. doi: 10.1046/j.1365-2559.1999.035005445.x. [DOI] [PubMed] [Google Scholar]
- 4.Kilic A, Krasinskas AM, Owens SR, Luketich JD, Landreneau RJ, Schuchert MJ. Variations in inflammation and nerve fiber loss reflect different subsets of achalasia patients. J Surg Res. 2007;143:177–182. doi: 10.1016/j.jss.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Csendes A, Smok G, Braghetto I, Ramirez C, Velasco N, Henriquez A. Gastroesophageal sphincter pressure and histological changes in distal esophagus in patients with achalasia of the esophagus. Dig Dis Sci. 1985 Oct;30(10):941–945. doi: 10.1007/BF01308293. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-de-Leon A, Mendoza J, Sevilla-Mantilla C, Fernández AM, Pérez-de-la-Serna J, Gónzalez VA, Rey E, Figueredo A, et al. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci. 2002;47:15–19. doi: 10.1023/a:1013242831900. [DOI] [PubMed] [Google Scholar]
- 7.De la Concha EG, Fernandez-Arquero M, Mendoza JL, Conejero L, Figueredo MA, Perez de la Serna J, Diaz-Rubio M, Ruiz de Leon A. Contribution of HLA class II genes to susceptibility in achalasia. Tissue Antigens. 1998;52:381–384. doi: 10.1111/j.1399-0039.1998.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 8.Verne GN, Hahn AB, Pineau BC, Hoffman BJ, Wojciechowski BW, Wu WC. Association of HLA-DR and -DQ alleles with idiopathic achalasia. Gastroenterology. 1999;117:26–31. doi: 10.1016/s0016-5085(99)70546-9. [DOI] [PubMed] [Google Scholar]
- 9.Wong RK, Maydonovitch CL, Metz SJ, Baker JR., Jr Significant DQw1 association in achalasia. Dig Dis Sci. 1989 Mar;34(3):349–352. doi: 10.1007/BF01536254. [DOI] [PubMed] [Google Scholar]
- 10.Facco M, Brun P, Baesso I, Costantini M, Rizzetto C, Berto A, Baldan N, Palù G, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598–1609. doi: 10.1111/j.1572-0241.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 11.Castagliuolo I, Brun P, Costantini M, Rizzetto C, Palù G, Costantino M, Baldan N, Zaninotto G. Esophageal achalasia: is the herpes simplex virus really innocent? J Gastrointest Surg. 2004;8:24–30. doi: 10.1016/j.gassur.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008 Nov;135(5):1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012 Mar;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushnir V, Sayuk GS, Gyawali CP. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol Motil. 2012 Dec;24(12):1069-e561. doi: 10.1111/j.1365-2982.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015 Feb;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013 Nov;145(5):954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvador R, Costantini M, Zaninotto G, Morbin T, Rizzetto C, Zanatta L, Ceolin M, Finotti E, Nicoletti L, Da Dalt G, Cavallin F, Ancona E. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg. 2010 Nov;14(11):1635–1645. doi: 10.1007/s11605-010-1318-4. [DOI] [PubMed] [Google Scholar]
- 18.Hirano I, Tatum RP, Shi G, Sang Q, Joehl RJ, Kahrilas PJ. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789–798. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]
- 19.Clark SB, Rice TW, Tubbs RR, Richter JE, Goldblum JR. The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am J Surg Pathol. 2000;24:1153–1158. doi: 10.1097/00000478-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Villanacci V, Annese V, Cuttitta A, Fisogni S, Scaramuzzi G, De Santo E, Corazzi N, Bassotti G. An immunohistochemical study of the myenteric plexus in idiopathic achalasia. J Clin Gastroenterol. 2010;44:407–410. doi: 10.1097/MCG.0b013e3181bc9ebf. [DOI] [PubMed] [Google Scholar]