Abstract
Alpha-synuclein is highly expressed in the central nervous system and plays an important role in pathogenesis of neurodegenerative disorders such as Parkinson's disease and Lewy body dementia. Previous studies have demonstrated the expression of α-synuclein in hematopoietic elements and peripheral blood mononuclear cells, although its roles in hematopoiesis and adaptive immunity are not studied. Using an α-synuclein knock out (KO) mouse model, we have recently shown that α-synuclein deficiency is associated with a mild defect in late stages of hematopoiesis. More importantly, we demonstrated a marked defect in B lymphocyte development and IgG, but not IgM production in these mice. Here we show a marked defect in development of T lymphocytes in α-synuclein KO mice demonstrated by a significant increase in the number of CD4 and CD8 double negative thymocytes and significant decreases in the number of CD4 single positive and CD8 single positive T cells. This resulted in markedly reduced peripheral T lymphocytes. Interestingly, splenic CD4+ and CD8+ T cells that developed in α-synuclein KO mice had a hyperactivated state with higher expression of early activation markers and increased IL-2 production. Moreover, splenic CD4+ T cells from α-synuclein KO mice produced lower levels of IL-4 upon antigenic stimulation suggesting a defective Th2 differentiation. Our data demonstrate an important role for α-synuclein in development of T lymphocytes and regulation of their phenotype and function.
Keywords: α-synuclein, CD4, CD8, T cells, Treg, lymphopoiesis
Introduction
Synucleins (including α-, β- and γ-synucleins) are a group of small proteins (14-17kDa) that are expressed at high levels in the central nervous system (Lavedan, 1998). They have been described only in vertebrates and are characterized by very conserved amino-terminal and less conserved caboxy-terminal sequences (George, 2002). A peptide derived from α-synuclein was first identified in the purified amyloid plaques of the brains of Alzheimer's patients (Ueda et al., 1993), and α-synuclein was later identified as a major component of Lewy bodies in Parkinson's disease (PD) and dementia with Lewy bodies (Irizarry et al., 1998). Point mutations in α-synuclein are known to cause familial Parkinson's disease (Zarranz et al., 2004). However, the physiologic function(s) of α-synuclein remain poorly understood, although its presynaptic localization (Iwai et al., 1995), and membrane interaction suggest a role for neurotransmitter release (Bendor et al., 2013). In fact, nigrostriatal nerve terminals of α-synuclein knock-out mice have shown increased dopamine release in response to paired stimuli suggesting an inhibitory role for α-synuclein in regulation of neurotransmitter release (Abeliovich et al., 2000).
The role of α-synuclein in the development of the hematopoietic system has been the subject of a few studies. The expression of α-synuclein was shown in erythroid precursors and megakaryocytes in bone marrow, as well as erythrocytes and platelets in peripheral blood (Hashimoto et al., 1997; Nakai et al., 2007). Maturation of megakaryocytes leads to increased expression of α-synuclein with a concomitant down-regulation of β-synuclein (Hashimoto et al., 1997). Consistent with these findings, α-synuclein has been shown to be expressed by blasts of acute erythroid leukemia and acute megakaryoblastic leukemia, whereas its expression is reduced in megakaryocytes of myeloproliferative disorders (Maitta et al., 2011). Expression of β-synuclein, on the other hand, is noted only in the blasts of acute megakaryoblastic leukemia (Maitta et al., 2011).
Some reports have suggested a role for α-synuclein in the development and function of the immune system. Human peripheral blood mononuclear cells (PBMC), including B and T lymphocytes, NK cells and monocytes express α-synuclein (Kim et al., 2004; Shin et al., 2000), and α-synuclein expression is higher in PBMCs of individuals with PD compared to those of healthy controls (Gardai et al., 2013; Kim et al., 2004). In fact, monocytes from familial and sporadic PD patients have defective phagocytosis compared to age-matched controls (Gardai et al., 2013). Moreover, PD patients have decreased number of peripheral blood B and T lymphocytes, with a more prominent reduction in the number of CD4+ T cells than CD8+ T cells (Baba et al., 2005; Stevens et al. 2012). This is associated with a Th1 deviation and elevated ratio of IFN-γ to IL-4 producing T cells (Baba et al., 2005). Macrophages and microglia isolated from transgenic mice over-expressing human α-synuclein under its own promoter are defective in secretion of pro-inflammatory cytokines and phagocytosis (Gardai et al., 2013) In contrast to these findings, α-synuclein KO microglia release higher levels of pro-inflammatory cytokines upon stimulation (Austin et al., 2011). Together, these findings are suggestive of a role for α-synuclein in regulation of the innate and adaptive immune responses.
We have recently shown that α-synuclein deficiency impairs late stages of the development of B lymphocytes and abrogates their function. Alpha-synuclein KO mice had a marked reduction in the number of mature B cells that was associated with loss of normal architecture of secondary lymphoid organs. Moreover, while total serum IgM levels were similar between α-synuclein KO and wild-type (WT) mice, total serum IgG levels were markedly reduced in α-synuclein KO animals. Furthermore, in contrast to WT mice, α-synuclein KO animals failed to produce IgG in response to immunization with a T cell-dependent antigen (Xiao et al., 2014). This led to the possibility that (in addition to a B cell maturation defect) abnormal T cell development and/or function might be at least partly involved in the defective antibody response. Based on these findings, we sought to study the effect of α-synuclein deficiency on T lymphocyte development and function.
Materials and Methods
Mice
Alpha-synuclein−/− (KO) mice (B6;129X1-Sncatm1Ros1/J) and age and sex-matched (8-10 weeks old) Alpha-synuclein+/+ B6;129X1/J wild type (WT) mice were purchased from Jackson laboratory (Bar Harbor, ME) as previously described (Xiao et al., 2014). Mice were housed at Case Western Reserve University in a pathogen-free facility at room temperature with a standard light/night cycle, and fed a standard diet with water intake ad libitum. All animal protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Flow cytometry
Thymi, spleens, lymph nodes and bone marrow (from femur and tibia) were harvested from α-synuclein KO and WT animals, processed and stained at 4°C for 30 minutes. Cells were washed extensively and fixed in 1% paraformaldehyde and kept in dark until analysis. Three-color flow-cytometry was performed using the following monoclonal antibodies: anti-mouse/rat CD61-PE (2C9.G3), anti-mouse CD41-PE (MWReg30), anti-mouse TER-119-FITC (TER-119), anti-mouse Ly-6G (Gr-1)-FITC (RB6-8C5), anti-mouse Ly-6A/E (Sca-1)-PE (D7), anti-mouse CD11b-PE (M1/70), anti-mouse CD117-APC (2B8), anti-mouse CD3e-APC (145-2C11), anti-mouse CD4-FITC (GK1.5), anti-mouse CD8a-APC (53-6.7), anti-human/mouse CD44-PE-Cyanine5 (IM7), anti-mouse CD62L-PE (MEL-14), anti-mouse CD49d-PE (R1-2), anti-mouse CD69-PE (H1.2F3) (eBiosciences, San Diego, CA) and anti-mouse CD5-PE (53-7.3) (BioLegend Inc., San Diego, CA). Regulatory T cells were analyzed from thymic and splenic cell suspensions after surface staining, followed by fixation/permeabilization and Foxp3 staining using anti-mouse Foxp3-PE (MF-14) according to manufacturer's protocol (BioLegend Inc., San Diego, CA).
Cytokine assay
Splenocytes from α-synuclein KO or WT mice were incubated with anti-CD8 or anti-CD4 mAb-coated magnetic beads (Miltenyi Biotec, Auburn, CA). Purified CD4+ and CD8+ T cells (8 × 104 ) were incubated with Dynabeads® Mouse T-Activator CD3/CD28 at 1:1 ratio in 96 well plates at 37°C and 5% CO2. Supernatants were collected after 48 hours and assayed for IL-2, IL-4 and IFN-γ using eBiosciences Mouse Ready-SET-Go! Reagent® sets (eBiosciences, San Diego, CA) according to manufacturer's protocol.
Histology and Immunohistochemistry
Tissue specimens were fixed in 10% formalin, embedded in paraffin and sectioned at 4 μm and stained with hematoxylin and eosin (H&E). Immunohistochemistry for CD3 and B220 was performed by the Immunohistochemistry Laboratory of the University Hospitals Case Medical Center as previously described (Xiao et al., 2014). Briefly, unstained 4 μm-sections of paraffin blocks from lymphoid organs were baked for 30 min at 60°C in a Boekel Lab oven (Boekel Scientific, Feasterville, PA). Slides were then processed using a BenchMark XT (Ventana) automated immunostainer (Ventana Medical Systems, AZ), deparaffinised, antigen-retrieved with standard cell conditioning 1, a tris-based buffer PH 8.3 solution added for 30 min at 100°C, followed by incubation at 37°C with the primary antibody anti-human/mouse/rat CD3 mAb (SP7) and anti-human/mouse B220 mAb (RA3-6B2) (Abcam, Cambridge, MA), and subsequently counterstained.
Statistical analysis
All statistical analysis was performed using Prism 6 (GraphPad software Inc., La Jolla, CA). Results are presented as mean ± standard deviation (SD) and analyzed using two-tailed student t test. Statistical significance was considered at p<0.05.
Results
Normal morphology and composition of hematopoietic elements in α-synuclein KO mice
In order to study the role of α-synuclein in development of different hematopoietic elements, we compared bone marrow, peripheral blood and lymphoid organs of age and sex-matched α-synuclein KO mice and WT animals of the same genetic background (n=10). In our previous publication, we have reported a unique complete blood count profile in α-synuclein KO mice. We showed that these mice have a relative decrease in red blood cell count, hemoglobin and hematocrit compared to α-synuclein WT animal. While white blood cell counts were not different, significant decrease in the percentage of lymphocytes and increase in the percentages of neutrophils and monocytes was noted in α-synuclein KO mice. Interestingly, while platelet counts were similar between α-synuclein KO and WT mice, mean platelet volume was lower in KO animals.(Xiao et al., 2014)
To study the effect of α-synuclein deficiency on hematopoiesis, we studied the morphology and composition of different hematopoietic element in the bone marrow. Sections of bone marrow showed no apparent differences in the morphology of erythroid, myeloid and megakaryocytic lineages (Figure 1). Flow cytometric analysis of bone marrow demonstrated similar percentages and numbers of hematopoietic stem cells, myeloid, erythroid and megarkaryocytic lineage precursors between α-synuclein KO and WT mice (Table 1). As previously described, a marked decrease in the absolute number of B220hi population was noted in the bone marrow of α-synuclein KO mice (Table 1 and (Xiao et al., 2014)). Interestingly, a significant 40% reduction in the number of mature circulating CD3+ T cells was also noted in the bone marrow of α-synuclein KO mice (p=0.0323) (Table 1).
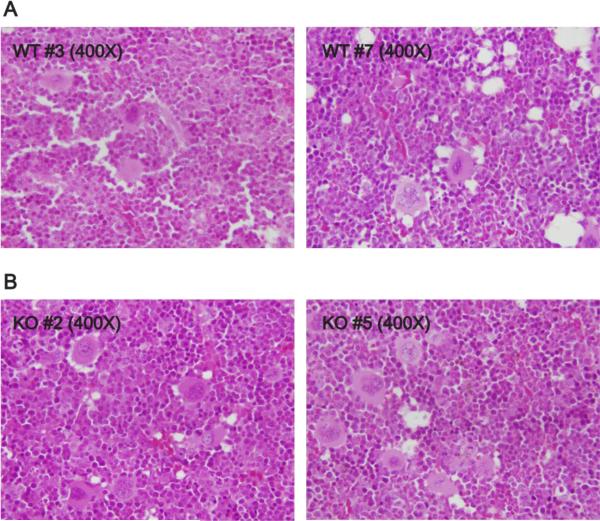
Figure 1.
Alpha-synuclein deficiency does not affect the morphology hematopoietic elements. Representative sections of bone marrow from WT (A) and α-synuclein KO (B) mice were examined by H&E staining at 400X magnification (bone marrow sections from 10 mice of each strain were examined).
Table 1.
Bone marrow lineage analysis of WT and KO mice (n=10 for each strain, SD: Standard deviation)
| Wild type | Knock out | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p value | |
| Percentage | |||
| Myeloid lineage | 31.1 (8.5) | 36.3 (5.9) | 0.1343 |
| Erythroid lineage | 24.4 (7.2) | 24.8 (5.6) | 0.8940 |
| Megakaryocytic lineage | 29.4 (8.5) | 34.1 (5.5) | 0.1581 |
| Hematopoietic stem cells | 0.269 (0.109) | 0.221 (0.057) | 0.2348 |
| B cells (B220lo) | 24.6 (5.2) | 21.1 (4.6) | 0.1334 |
| B cells (B220hi) | 4.2 (1.4) | 2.0 (0.9) | 0.00047 |
| T cells | 2.9 (1.1) | 2.1 (0.9) | 0.1460 |
| Absolute count (×106/μL) | |||
| Myeloid lineage | 12.9 (4.8) | 13.5 (7.9) | 0.8492 |
| Erythroid lineage | 9.9 (3.2) | 8.6 (3.0) | 0.3625 |
| Megakaryocytic lineage | 12.2 (4.7) | 12.4 (6.5) | 0.9579 |
| Hematopoietic stem cells | 0.107 (0.036) | 0.082 (0.042) | 0.1792 |
| B cells (B220lo) | 10.2 (3.3) | 7.6 (3.5) | 0.0981 |
| B cells (B220hi) | 1.9 (1.1) | 0.83 (0.69) | 0.0154 |
| T cells | 1.2 (0.4) | 0.7 (0.4) | 0.0323 |
Defective T cell maturation in α-synuclein KO mice
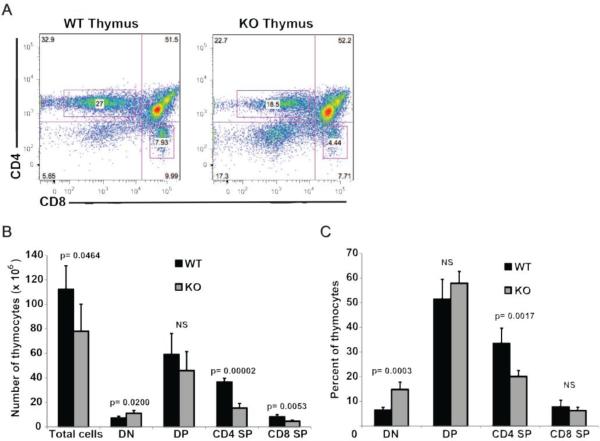
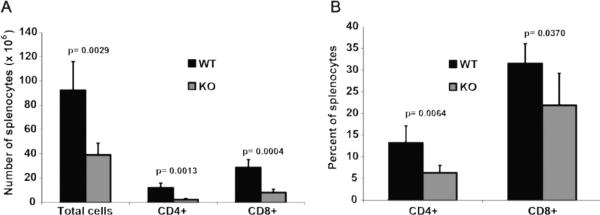
In order to study the development of T lymphocytes, thymi and peripheral lymphoid organs from α-synuclein KO and WT mice were harvested and analyzed by flow cytometry (Figures 2 and 3). Absolute numbers of thymocytes were significantly reduced in α-synuclein KO mice compared to WT animals (78.2 ± 21.9 in KO vs. 112.4 ± 19.1 in WT mice, p=0.046). While CD4 single positive (SP) and CD8 SP T cells in α-synuclein KO mice were reduced to 50% of their numbers in WT animals (p= 0.00002 for differences in CD4 SP cells and p= 0.0053 for differences in CD8 SP cells), number of double negative (DN) thymocytes in α-synuclein KO mice were 1.5 fold higher than their number in WT mice (p= 0.02), suggestive of defective T cell development in α-synuclein KO mice (Figure 2). Strikingly, absolute numbers of mature splenic CD4+ and CD8+ were reduced by 5-fold and 3.5-fold, respectively, in α-synuclein KO mice compared to WT animals (p= 0.0013 for CD4+ and p=0.0004 for CD8+ cells) (Figure 3). Consistent with these findings, the percentage of lymph node CD8+ T cells was significantly reduced in α-synuclein KO mice (43.4 ± 6.7 in WT vs. 34.3 ± 2.9 in KO mice, p=0.023). No difference was noted in the percentage of lymph node CD4+ T cells (data not shown). Taken together, these data show that α-synuclein is required for development of T lymphocytes.
Figure 2.
Alpha-synuclein KO mice have defective thymic maturation. Representative flow cytometry panels of thymocytes stained with anti-CD4 and anti-CD8 antibodies (A). Absolute numbers (B) and percentages (C) of thymocytes from α-synuclein KO and WT mice at different stages of development (DN: double negative, DP: double positive, SP: single positive, n=5 for each strain).
Figure 3.
Alpha-synuclein KO mice have significantly reduced number of mature splenic T lymphocytes. . Absolute numbers (A) and percentages (B) of splenic CD4+ and CD8+ T cells from α-synuclein KO and WT mice (n=5 for each strain).
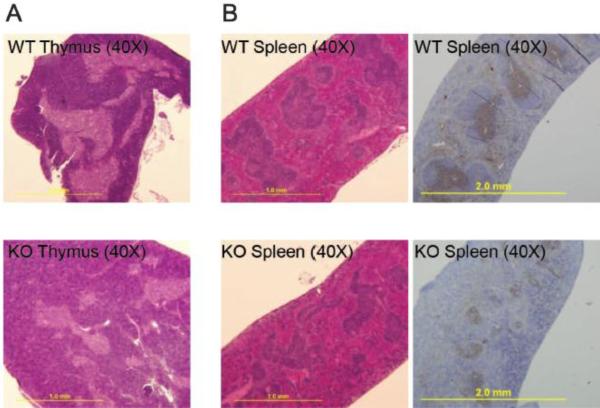
Next, we studied the histology of thymus, spleen and lymph nodes of α-synuclein KO mice in comparison with WT animals. Thymi were smaller in α-synuclein KO mice and harboured fewer cells than their WT counterparts. Moreover, there was a relative increase in the size of the cortex with a corresponding decrease in the size of the medulla in α-synuclein KO mice, consistent with a relative increase in immature (DN and DP) thymocytes and decrease in more mature (SP) thymocytes in α-synuclein KO mice (Figure 4A). Splenic white pulp areas from α-synuclein KO mice had a disorganized architecture, and contained smaller T cell zones as compared to the splenic white pulp areas from WT animals (Figure 4B). This was in addition to decreases in the size of splenic follicles in α-synuclein KO mice.(Xiao et al., 2014) Moreover, while lymph nodes from WT mice had organized follicles separated by paracortical zone, lymph nodes from KO animals had markedly fewer follicles (Xiao et al., 2014) and slightly smaller paracortical T cell zones (Not shown).
Figure 4.
Alpha-synuclein KO mice have reduced number of T lymphocytes associated with disorganized thymus and spleen architecture. (A) Representative section of thymus, examined by H&E staining at 40X magnification showing increased size of cortex in relation to medulla in α-synuclein KO as compared to WT animals. (B) Representative sections of spleen from α-synuclein KO and WT animals examined by H&E staining and after immunohistochemical staining with anti-CD3 antibody at 40X magnification (Data are representative of 5 independent experiments).
Hyperactivation of T cells from α-synuclein KO mice
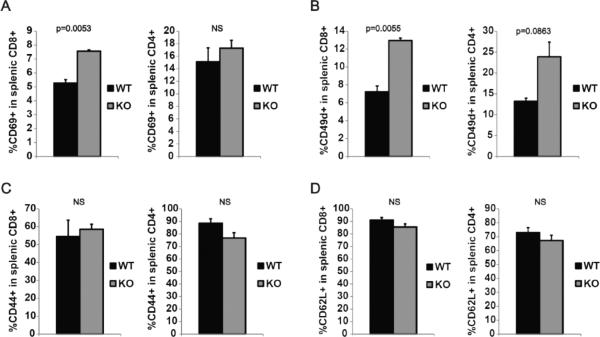
Next, we investigated the lineage differentiation and activation status of T lymphocytes. Mature splenic CD4+ and CD8+ T cells were analyzed for the expression of a panel of early and late activation markers and lymph node homing markers. Flow cytometric analysis of splenic T cells showed increased percentage of CD8+ T cell expressing early activation marker CD69 in α-synuclein KO mice (p=0.0053). No difference in the expression of CD69 was noted between CD4+ T cells from α-synuclein KO and WT mice (Figure 5A). Splenic CD8+ T cell population from α-synuclein KO mice also contained higher percentage of cells expressing early activation marker/lymph node homing marker CD49d (p=0.0055), and a similar trend was present in CD4+ T cell population, although the difference in CD4+ cells did not reach statistical significance (Figure 5B). No difference was noted in the expression of late activation marker CD44 and lymph node homing marker CD62L by CD4+ and CD8+ T cells from α-synuclein KO and WT mice (figure 5C and 5D). Taken together, our data indicate that the small population of T cells that mature in α-synuclein KO mice have an overactivated state.
Figure 5.
Peripheral T cells in α-synuclein KO mice have a hyperactivated state. (A) Naïve splenic T cells from α-synuclein KO and WT mice were analyzed by flow cytometry using antibodies against CD4, CD8 and one of early activation markers CD69 (A), CD49d (B), or late activation marker CD44 (C), or lymph node homing marker CD62L (D).
Defective Th2 differentiation of T cells from α-synuclein KO mice
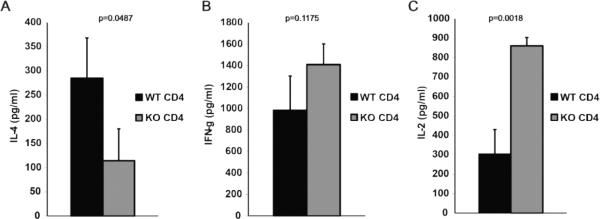
In order to study the T cell function, purified T cells were activated in vitro using anti-CD3/anti-CD28 monoclonal antibody-coated beads and cytokine production was analyzed after 48 hours.. IL-4 production from α-synuclein KO CD4+ T cells was significantly reduced compared to their WT counterparts (p=0.0487) (Figure 6A). IFN-γ production was not different between α-synuclein KO and WT CD8+ T cells (data not shown), while a trend for increased IFN-γ production was noted from α-synuclein KO CD4+ T cells, although it did not reach statistical significance (Figure 6B). Interestingly, CD4+ T cells from α-synuclein KO mice produced higher levels of IL-2 than their WT counterparts (p=0.0022) (Figure 6C). These findings are consistent with a defective Th2 differentiation and deviation towards a Th1 phenotype among CD4+ T cells from α-synuclein KO mice.
Figure 6.
Defective Th2 differentiation in α–synuclein KO mice. Purified naïve splenic CD4+ T cells from α-synuclein KO and WT mice were activated in vitro with anti-CD3/anti-CD28 mAb-coated beads and IL-4 (A), IFN-γ (B) and IL-2 (C) production levels were analyzed after 48 hours.
Abnormal number of regulatory T cells in α-synuclein KO mice
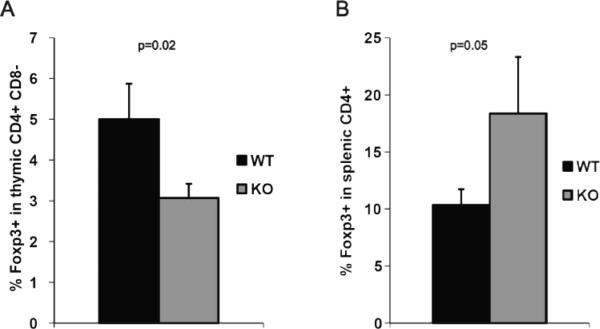
Finally, we asked how α-synuclein deficiency influences the development of Foxp3+ CD4+ regulatory T cells. Flow cytometric analysis of thymocytes revealed a significant reduction in the percentage of Foxp3+ CD4+ regulatory T cells in α-synuclein KO mice compared to WT animals (p=0.02) (Figure 7A). Interestingly, however, the percentage of splenic Foxp3+ CD4+ T cells was increased in α-synuclein KO mice (p=0.05) (Figure 7B).
Figure 7.
Development of Foxp3+ CD4+ regulatory T cells (Tregs) in α-synuclein KO mice. Single cell suspensions were obtained from thymi and spleens of α-synuclein KO and WT mice and stained with antibodies against CD4, CD8 and Foxp3 (intracellular). Percentage of Foxp3+ population was analyzed in CD4+ CD8− population.
Discussion
We recently established the importance of α-synuclein in regulation of one aspect of adaptive immune response: B cell lymphopoiesis and antibody production. We found that late stages of B lymphocyte development is defective in α-synuclein KO mice resulting in marked reduction of transitional and mature circulating B cells. While total serum IgM levels were comparable between naive α-synuclein KO and WT mice, total serum IgG levels were reduced 3-fold in α-synuclein KO mice, suggesting a defect in immunoglobulin class switch. Furthermore, while immunization with a T cell dependent antigen elicited a strong antibody response in WT mice, and similar IgM response between α-synuclein KO and WT mice, almost no IgG1 and IgG2b responses were noted in KO mice (Xiao et al., 2014). These findings suggested a defective helper T cell phenotype in these mice. Our current findings show that the defect mediated by α-synuclein deficiency also extends into the T cell compartment, affecting not only the absolute numbers of peripheral T cells but also disrupting the normal morphology/architecture of lymphoid organs including T cell regions.
T cell development begins by homing of circulating bone marrow-derived progenitors to thymus. These progenitors go through a series of developmental stages that include CD4 and CD8 DN, DP, and SP stages. DP thymocytes that express T cell receptors (TCR) unable to bind to self peptide-major histocompatibility complex (pMHC) die by neglect, whereas those with low to intermediate affinity for self pMHCs are positively selected. DP or SP thymocytes with high affinity for self pMHC undergo clonal deletion. T cell development requires interactions between thymocytes and different thymic stromal cells such as dendritic cells, macrophages and cortical and medullary thymic epithelial cells (Xing and Hogquist, 2012). Thymocytes from α-synuclein KO mice are reduced in total numbers, and contain higher numbers of DN cells and lower numbers of SP cells suggesting a defect in T cell development. Whether this phenomenon is a T cell-intrinsic event or caused by an extrinsic effect mediated by thymic stromal cells is not clear. It has been shown that chemokine receptor CCR7 deficiency leads to defective migration of thymocyte progenitors in the thymus and defective T cell development in a T cell intrinsic manner (Misslitz et al., 2004). On the other hand, lymphotoxin β receptor (LT-βR) deficiency is known to abrogate medullary thymic epithelial cell (mTEC) differentiation leading to abnormal thymus architecture (Boehm et al., 2003). Further experiments utilizing bone marrow chimeric mice from α-synuclein KO and WT mice as well as conditional knock-out mice lacking α-synuclein in single lineages are required to answer these questions.
The molecular mechanism(s) through which α-synuclein regulates thymocyte development is/are not known. An intriguing hypothesis is that α-synuclein somehow affects the affinity of TCR-pMHC interactions between developing thymocytes and thymic antigen presenting cells. It is known that higher affinity TCR interactions predispose thymocytes to negative selection (Moran and Hogquist, 2012). It needs to be mentioned that α-synuclein functions as a chaperone for the soluble N-ethylmalemide-sensitive factor attachment protein receptor (SNARE) complex and is needed for high frequency neurotransmitter release through rapid assembly and disassembly of the membrane fusion machinery (Bendor et al., 2013; Diao et al., 2013). To carry out this function, the hydrophilic C-terminus of α-synuclein interacts with SNARE synaptobrevin-2 and promotes SNARE assembly (Burre et al., 2013). On the other hand, overexpression of α-synuclein markedly inhibits neurotransmitter release (Nemani et al., 2010), and large oligomers of α-synuclein are shown to bind to synaptobrevin-2 and prevent SNARE-mediated vesicle lipid mixing (Choi et al., 2013). In T cells, SNARE complex activity is essential to TCR localization to the immunological synapse (Das et al., 2004; Pattu et al., 2012), and a similar membrane fusion machinery involving SNARE is required for granule exocytosis and release of lytic granules from cytotoxic T cells and NK cells (Chiang et al., 2013). It is conceivable that α-synuclein-deficiency leads to enhanced localization of TCR to immunological synapses on developing thymocytes (Hailman et al., 2002) resulting in higher affinity interaction and enhanced negative selection. In fact, thymic CD4 SP cells from α-synuclein KO mice express significantly higher levels of CD5 (mean fluorescent intensity of 68987 ± 968 for WT and 83737 ± 2030 for α-synuclein KO CD4+ T cells, p=0.0083), an inhibitor of TCR signal transduction which its expression on SP cells parallels the avidity of positively selecting TCR-pMHC interactions (Azzam et al., 2001; Wong et al., 2001). This notion may also explain the expression of early activation markers on higher percentage of mature peripheral T cells in α-synuclein KO mice.
Upon cognate TCR-pMHC interactions with Antigen presenting cells (APCs), CD4+ T cells differentiate into different effector subsets such as T helper 1 (Th1), Th2, Th17, follicular helper T (Tfh) and regulatory T (Treg) cells. The differentiation outcome is largely dependent on the nature of cytokines in the microenvironment and the affinity of TCR-pMHC interactions (Zhou et al., 2009; Zhu et al., 2010). In general, stronger TCR signalling favors Th1 differentiation and weaker TCR signalling favors Th2 differentiation (Constant and Bottomly, 1997). For example, while stimulation of naive TCR-transgenic CD4+ T cells with low concentrations of cognate peptide or low-affinity peptide activates GATA3 and leads to Th2 differentiation, high-affinity TCR interactions blocks GATA3 expression and prevents Th2 differentiation (Tao et al., 1997; Yamane et al., 2005). Defective Th2 differentiation and markedly reduced IL-4 production in α-synuclein KO mice might result from alterations in the affinity of TCR interactions in the absence of α-synuclein. Moreover, abrogated IgG1 and IgG2b responses to T cell-dependent antigen in α-synuclein KO mice (Xiao et al., 2014) can be explained by defective Th2 differentiation, as class switching to these immunoglobulin isotypes is highly dependent on Th2 cytokines IL-4 and TGF-β, respectively (Stavnezer, 1996).
Differentiation of regulatory T cells (Treg) in α-synuclein KO mice follows a more complex pathway. While we observed a reduced percentage of thymic Tregs in α-synuclein KO mice compared to WT animals, the percentage of peripheral Tregs was increased. Development of Tregs in the thymus is dependent on multiple factors such as affinity of TCR interactions with pMHC presented by thymic epithelial cells or dendritic cells, interaction of CD28 on T cells with CD80 or CD86 on APCs, and high-affinity IL-2 receptor and other γc cytokine receptor signalling (Josefowicz and Rudensky, 2009). How the combination of these factors leads to reduced thymic Tregs in α-synuclein KO mice is not clear to us at the moment. Reversal of this ratio in splenic Tregs, however, can be at least partly due to increased production of IL-2, a key cytokine in maintenance of peripheral Tregs (Sakaguchi et al., 2008), by mature CD4+ T cells from α-synuclein KO mice.
In summary, our current and previous findings demonstrate the essential role of α-synuclein in development of the adaptive immune responses, including late stages of B and T cell lymphopoiesis, T helper and regulatory T cell development and IgG production. The molecular mechanisms of this regulation and possible role of α-synuclein in human lymphocyte development and function should be subject of future studies.
Acknowledgements
This study was fully supported by grant funding from the Office of Diversity and Inclusion of the University Hospitals Case Medical Center and by the Dr. Harry Taylor Scholar Award also from the University Hospitals Case Medical Center, Cleveland, OH both awarded to RWM. Data included was presented and received Abstract Achievement Awards at both 2013's (Blood 2013 122(21):3490) in New Orleans, LA and 2014's (Blood 2014;124(21):1424) in San Francisco, CA annual meetings of the American Society of Hematology. The authors would like to acknowledge the technological and logistical assistance provided by Ms. Nancy Nagy at CWRU who was supported by NIH R01 AI034343 grant (awarded to CVH), and Mr. Amad Awadallah at UHCMC. In addition, we thank Dr. Hollie M. Reeves for critical review of the manuscript.
Abbreviations
- KO
knock-out
- WT
wild type
- PD
Parkinson's disease
- APC
antigen presenting cell
- TCR
T cell receptor
- MHC
major histocompatibility complex
- DN
double negative
- DP
double positive
- SP
single positive
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
AS designed and performed the experiments, analyzed the data and wrote the manuscript; WX performed experiments, analyzed data and co-wrote the manuscript; YZ performed experiments and analyzed data; SS and JS performed experiments; HJM and CVH analyzed the data and reviewed the manuscript; RWM designed and performed experiments, analyzed the data, co-wrote and critically revised the manuscript to its final version.
Conflict of interest
The authors declare no competing financial interests in the submission of this article.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Austin SA, Rojanathammanee L, Golovko MY, Murphy EJ, Combs CK. Lack of alpha-synuclein modulates microglial phenotype in vitro. Neurochem Res. 2011;36:994–1004. doi: 10.1007/s11064-011-0439-9. [DOI] [PubMed] [Google Scholar]
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC. Properties of native brain alpha-synuclein. Nature. 2013;498:E4–6. doi: 10.1038/nature12125. discussion E6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SC, Theorell J, Entesarian M, Meeths M, Mastafa M, Al-Herz W, Frisk P, Gilmour KC, Ifversen M, Langenskiold C, Machaczka M, Naqvi A, Payne J, Perez-Martinez A, Sabel M, Unal E, Unal S, Winiarski J, Nordenskjold M, Ljunggren HG, Henter JI, Bryceson YT. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood. 2013;121:1345–1356. doi: 10.1182/blood-2012-07-442558. [DOI] [PubMed] [Google Scholar]
- Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, Lee NK, Shin YK. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci U S A. 2013;110:4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- Diao J, Burre J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT. Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Mao W, Schule B, Babcock M, Schoebel S, Lorenzana C, Alexander J, Kim S, Glick H, Hilton K, Fitzgerald JK, Buttini M, Chiou SS, McConlogue L, Anderson JP, Schenk DB, Bard F, Langston JW, Yednock T, Johnston JA. Elevated alpha-synuclein impairs innate immune cell function and provides a potential peripheral biomarker for Parkinson's disease. PLoS One. 2013;8:e71634. doi: 10.1371/journal.pone.0071634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JM. The synucleins. Genome Biol. 2002;3:REVIEWS3002. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A, Saitoh T, Miller A, Masliah E. NACP, a synaptic protein involved in Alzheimer's disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun. 1997;237:611–616. doi: 10.1006/bbrc.1997.6978. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jeon BS, Heo C, Im PS, Ahn TB, Seo JH, Kim HS, Park CH, Choi SH, Cho SH, Lee WJ, Suh YH. Alpha-synuclein induces apoptosis by altered expression in human peripheral lymphocyte in Parkinson's disease. FASEB J. 2004;18:1615–1617. doi: 10.1096/fj.04-1917fje. [DOI] [PubMed] [Google Scholar]
- Lavedan C. The synuclein family. Genome Res. 1998;8:871–880. doi: 10.1101/gr.8.9.871. [DOI] [PubMed] [Google Scholar]
- Maitta RW, Wolgast LR, Wang Q, Zhang H, Bhattacharyya P, Gong JZ, Sunkara J, Albanese JM, Pizzolo JG, Cannizzaro LA, Ramesh KH, Ratech H. Alpha- and beta-synucleins are new diagnostic tools for acute erythroid leukemia and acute megakaryoblastic leukemia. Am J Hematol. 2011;86:230–234. doi: 10.1002/ajh.21932. [DOI] [PubMed] [Google Scholar]
- Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Hogquist KA. T-cell receptor affinity in thymic development. Immunology. 2012;135:261–267. doi: 10.1111/j.1365-2567.2011.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, Ohtaka-Maruyama C, Okado H, Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson's disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358:104–110. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattu V, Qu B, Schwarz EC, Strauss B, Weins L, Bhat SS, Halimani M, Marshall M, Rettig J, Hoth M. SNARE protein expression and localization in human cytotoxic T lymphocytes. Eur J Immunol. 2012;42:470–475. doi: 10.1002/eji.201141915. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Shin EC, Cho SE, Lee DK, Hur MW, Paik SR, Park JH, Kim J. Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol Cells. 2000;10:65–70. doi: 10.1007/s10059-000-0065-x. [DOI] [PubMed] [Google Scholar]
- Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C, Halliday GM. Reduced T helper and B lymphocytes in Parkinson's disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Shameli A, Harding CV, Meyerson HJ, Maitta RW. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson's disease. Immunobiology. 2014;219:836–844. doi: 10.1016/j.imbio.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]