Abstract
Background
As a comprehensive stroke center (CSC), we accept transfer patients with intracerebral hemorrhage (ICH) in our region. CSC guidelines mandate receipt of patients with ICH for higher level of care. We determined resource utilization of patients accepted from outside hospitals compared with patients directly arriving to our center.
Methods
From our stroke registry, we compared patients with primary ICH transferred to those directly arriving to our CSC from March 2011–March 2012. We compared the proportion of patients who utilized at least one of these resources: neurointensive care unit (NICU), neurosurgical intervention, or clinical trial enrollment.
Results
Among the 362 patients, 210 (58%) were transfers. Transferred patients were older, had higher median Glasgow Coma Scale scores, and lower National Institutes of Health Stroke Scale scores than directly admitted patients. Transfers had smaller median ICH volumes (20.5 cc versus 15.2 cc; P = .04) and lower ICH scores (2.1 ± 1.4 versus 1.6 ± 1.3; P < .01). A smaller proportion of transfers utilized CSC-specific resources compared with direct admits (P = .02). Fewer transferred patients required neurosurgical intervention or were enrolled in trials. No significant difference was found in the proportion of patients who used NICU resources, although transferred patients had a significantly lower length of stay in the NICU. Average hospital stay costs were less for transferred patients than for direct admits.
Conclusions
Patients with ICH transferred to our CSC underwent fewer neurosurgical procedures and had a shorter stay in the NICU. These results were reflected in the lower per-patient costs in the transferred group. Our results raise the need to analyze cost–benefits and resource utilization of transferring patients with milder ICH.
Keywords: Stroke systems, comprehensive stroke center, hemorrhagic stroke, intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a neurological emergency comprising about 10%–15% of all strokes in the United States.1–6 The proportion is higher at tertiary referral centers, reaching up to 33% at our institution (unpublished data). In fact, as the only Joint Commission-certified comprehensive stroke center (CSC) in the region, transfers from primary stroke centers comprise the fastest growing percentage of admissions, and patients with ICH are transferred far more frequently than patients with ischemic stroke.7 Patients with ICH are frequently transferred to a CSC because of the perceived need for advanced clinical resources and sophisticated neurosurgical capability, and in fact it has been shown that availability of technology and expertise has been associated with better patient outcomes.8,9 Patients with intracranial injury benefit from neurocritical care or neurosurgical resources that are not available at all hospitals.10–12 CSCs are by definition regional facilities that are supposed to accept patients from primary hospitals who request higher level of care.13 However, the extent to which patients with primary spontaneous ICH transferred to CSCs actually utilize these advanced services is not fully known. At our center, where we have had an open-door policy to accept all patients from outside facilities, we retrospectively reviewed all cases of primary ICH and compared the characteristics, hospital course, resource utilization, and total costs accrued for patients directly admitted versus transferred from an outside facility to our center.
Research Design and Methods
Overview
From our prospectively collected data registry,14 we identified all patients admitted to our hospital between March 2011 and March 2012 with spontaneous ICH who were at least 18 years old, designating them as either directly admitted to our hospital or transferred from another health-care facility. Patients with arteriovenous malformation (AVM), aneurysm, subdural hematoma (SDHs), or sub-arachnoid hemorrhage (SAH) were excluded because the primary objective was to compare patients with primary ICH so that we could explore potential differences in clinical characteristics and resource utilization between transferred and directly admitted patients. We collected baseline demographics, including age, sex, race, National Institutes of Health Stroke Scale (NIHSS) score on admission, premorbid modified Rankin Scale (mRS) score, history of hypertension, diabetes, hyperlipidemia, smoking, congestive heart failure, coronary artery disease, and discharge mRS. We also collected the patient’s mode of transportation, surgical interventions, Glasgow Coma Scale (GCS),15 ICH location (whether supra- or infratentorial), and participation in clinical trials at our institution. We also performed an assessment of total hospital costs between the 2 groups. We received approval for this study from our local institutional review board.
Transfer Process
Memorial Hermann Hospital at the Texas Medical Center in Houston, Texas, is a Joint Commission-certified CSC, which has an open-door policy to accept transfer requests from surrounding hospitals. Requests for transfer were received by a physician, who would use the clinical details to determine the destination unit (emergency department, intensive care unit [ICU], stroke unit, or floor). Those patients who were already admitted (i.e., not from the emergency department) at their originating hospitals required insurance approval before transfer, while those transferred from the emergency department did not. All patients in the ED of outside facilities that were requested by the outside hospital as emergent were immediately transferred to our facility. Transferred patients arrived by either air or ground transport. Patients presenting directly to our center arrived by either air, ground, or private transport.
Imaging
Initial computed tomography scans for all patients were reviewed by 2 neurologists blinded to their presentation group (transfer or direct) or outcomes. Hematoma volumes were measured by the ABCD2 method.16 ICH location was also designated as either supra- or infratentorial, and categorized for location to either basal ganglia, thalamus, lobar, brain stem, cerebellar, or pure intraventricular hemorrhage (IVH). ICH score was calculated for all patients.17
CSC Utilization
We assessed the proportion of patients who were placed in the neurointensive care unit (NICU) stay, underwent neurosurgical interventions (including hemicraniectomy, external ventricular drain, and hematoma evacuation) in each group, and were enrolled in clinical trials (3 interventional trials—CLEAR-IVH, MISTIE, and SHRINC—and 3 observational trials).
Cost Analysis
An analysis of hospitalization costs was also performed, comparing those of directly presenting and transfer patients. This involved all hospitalization costs excluding transportation. Both patient groups were further divided into 2 subgroups according to the following ICH volumes: less than 5 mL and greater than 5 mL; such an ICH volume limit was considered an adequate threshold to compare stroke severity, allowing general comparisons of resource usage by subgroup. The analysis was conducted from the provider perspective, which does not consider direct costs to patients, payers, or society.
Statistical Methods
Descriptive analyses for demographic factors, pre-existing comorbidities, hospital presentation, and clinical characteristics are presented as means (standard deviation) or median (interquartile range) for continuous variables and as proportions for categorical variables. Univariable differences between transferred and directly admitted patients were analyzed using t-test, chi-squared test, and Wilcoxon’s rank sum test. All analyses were performed using Stata version 13.0 (StataCorp LP, College Station, TX).
Results
Clinical Characteristics
Over a 12-month period, we admitted 362 total patients with spontaneous ICH, 210 (58%) of whom were transfers. We found a number of significant differences between the 2 groups presented in Table 1. Transferred patients were older and had a higher proportion of cardiac disease, including atrial fibrillation, compared with those who presented directly to our center. Fewer transferred patients had a history of substance abuse. Transferred patients had a higher symptom onset-to-arrival time than those patients who directly presented to our hospital. Transferred patients had higher median GCS scores and lower NIHSS scores on arrival at our center.
Table 1.
Baseline clinical and ICH characteristics for patients transferred to our CSC compared to those presenting directly to our CSC
| Characteristics | Direct (N = 152) | Transfer (N = 210) | P value |
|---|---|---|---|
| Demographics | |||
| Age (mean ± SD) | 59.8 ± 13.8 | 64 ± 15.2 | <.01 |
| Males (%) | 58.5 | 55.2 | .53 |
| Hypertension (%) | 80.2 | 80.9 | .87 |
| Diabetes (%) | 23.0 | 31.4 | .08 |
| Coagulation disorder (%) | 2.6 | 4.7 | .30 |
| Atrial fibrillation (%) | 4.6 | 10.9 | .03 |
| Coronary disease/MI (%) | 9.2 | 15.7 | .07 |
| Past history of ICH (%) | 9.9 | 9.1 | .79 |
| History of substance abuse (%) | 17.1 | 10.0 | .05 |
| Onset to CSC arrival time (h, median/IQR) | 1.7/.8–4.3 | 6.2/4.3–11.7 | <.01 |
| Onset-to-arrival categories (%) | |||
| < 12 h | 61.8 | 37.1 | <.01 |
| 12–24 h | 4.6 | 10.5 | .04 |
| > 24 h or unknown | 33.6 | 52.4 | <.01 |
| Mode of transportation (%) | |||
| Ambulance | 67.6 | 67 | .37 |
| Air | 26.5 | 32.5 | |
| GCS score at CSC arrival (median/IQR) | 10/4.5–14 | 13/7–15 | .02 |
| NIHSS score at CSC arrival (median/IQR) | 18/12–32 | 12/3–25 | <.01 |
| Platelets (median/IQR) | 207/161.5–252 | 204.5/168–254 | .31 |
| INR (median/IQR) | 1.01/.9–1.1 | 1.05/1.0–1.1 | .13 |
| PTT (median/IQR) | 28.9/26–32 | 29.6/27.2–33.2 | .40 |
| ICH characteristics | |||
| ICH score (median/IQR) | 2/1–3 | 1/1–3 | <.01 |
| ICH volume (median/IQR) | 20.5/8.7–47.9 | 15.2/3.6–39.1 | <.01 |
| ICH location (%) | |||
| Basal ganglia | 28.9 | 20.5 | .06 |
| Thalamus | 34.8 | 25.7 | .06 |
| Lobar | 26.3 | 39.1 | .01 |
| Brain stem | 5.9 | 4.3 | .48 |
| Cerebellar | 3.3 | 7.1 | .12 |
| Pure IVH | .6 | 3.3 | .12 |
| IVH present (%) | 60.5 | 45.2 | <.01 |
| Supratentorial ICH < 5 cc (%) | 8.6 | 20.5 | <.01 |
| Neuroworsening | 32.9 | 22.4 | .03 |
Abbreviations: CSC, comprehensive stroke center; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; INR, international normalized ratio; IQR, interquartile range; IVH, intraventricular hemorrhage; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; PTT, partial thromboplastin time; SD, standard deviation.
Arrival time refers to the time each patient arrived at the CSC.
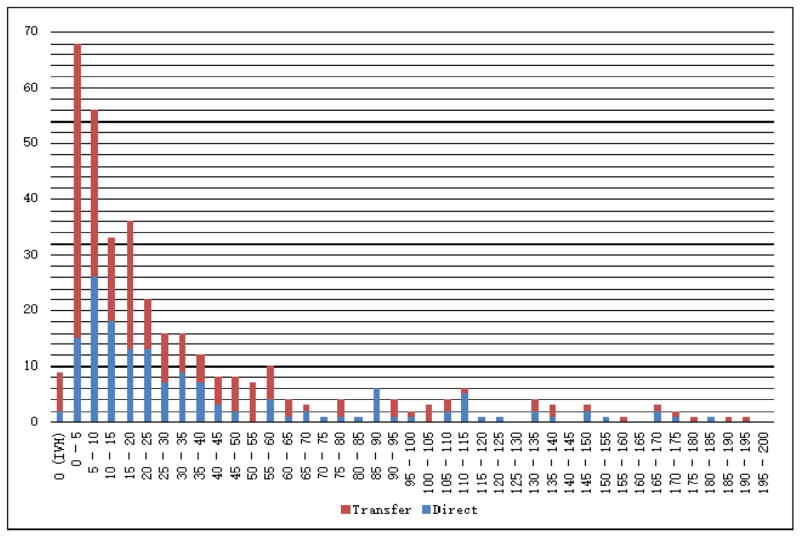
In terms of ICH characteristics, transferred patients had lower ICH scores and ICH volumes upon arrival at our CSC, and tended to be lobar in anatomical location (Table 1) compared to the directly arriving group. Transferred patients had fewer IVH than directly arriving patients. We compared volume distributions between the transferred and directly arriving group (Fig 1), which revealed a disproportionate number of hemorrhages measuring 0–5 mL in the transferred group compared to the directly arriving group. Given that supratentorial hemorrhages generally have more favorable outcomes than infratentorial hemorrhages, we calculated the proportion of patients with supratentorial ICH with volumes less than 5 mL, finding a significantly higher proportion of these patients in the transfer group (20.5% compared to 8.6%; P < .01; Table 1).
Figure 1.
Stacked frequency chart of ICH volumes for patients transferred to our CSC compared to those presenting directly to our CSC. Abbreviations: CSC, comprehensive stroke center; ICH, intracerebral hemorrhage.
Utilization of CSC Resources
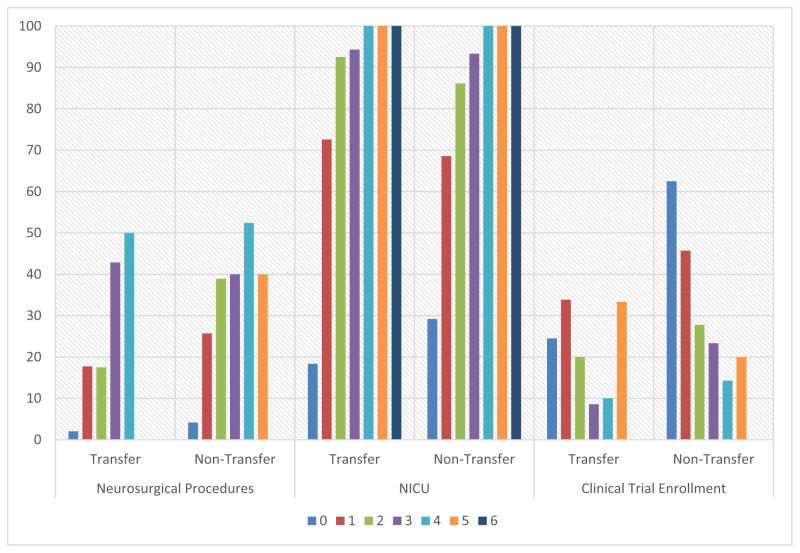
While similar proportions spent at least 1 day in the NICU, we observed that ICH patients transferred into our center underwent fewer neurosurgical procedures. Further, a smaller proportion of transfer patients were enrolled into interventional clinical trials. Although no statistically significant difference was found in the proportion of patients admitted to the NICU between the 2 groups, transferred patients had significantly shorter NICU length of stay (LOS) (Table 2). We also analyzed differences in CSC resource utilization by ICH score categories and found similar trends in surgical intervention, neurointensive care utilization, and clinical trial enrollments for both groups (Fig 2). Those with lower ICH scores used fewer CSC resources. In a sub-hoc analysis (Table 3), Q6 we found that among patients with ICH volumes less than 5 mL, transfer patients utilized fewer CSC resources compared with directly presenting patients.
Table 2.
Utilization of services unique to CSCs for ICH patients transferred to our CSC compared to those presenting directly to our CSC
| Direct (N = 152) | Transfer (N = 210) | P value | |
|---|---|---|---|
| Overview | |||
| % using at least 1 CSC service (NICU, neurosurgery, or clinical trial enrollment) | 89.5 | 80.0 | .015 |
| Neurointensive care | |||
| % who spent at least 1 day in NICU | 76.9 | 70.5 | .17 |
| Length of stay—days in NICU (median [IQR]) | 3 (1–8) | 2 (0–5) | .02 |
| Neurosurgical procedures (%) | |||
| Received at least 1 procedure | 32.2 | 21.0 | .015 |
| Hemicraniectomy | 7.2 | 2.9 | .05 |
| External ventricular drain | 28.3 | 18.1 | .02 |
| Clot evacuation | 5.3 | 1.4 | .036 |
| Clinical trial enrolment (%) | |||
| Enrolled in at least 1 clinical trial | 30.9 | 20.5 | .013 |
| Interventional trials | 15.1 | 4.8 | <.01 |
| Observational trials | 31.6 | 21.4 | .11 |
Abbreviations: CSC, comprehensive stroke center; ICH, intracerebral hemorrhage; IQR, interquartile range; NICU, neurointensive care unit.
Figure 2.
Comparison of comprehensive stroke care service utilization in patients transferred to our CSC and patients presenting directly to our CSC by ICH score on presentation. Abbreviations: CSC, comprehensive stroke center; ICH, intracerebral hemorrhage; NICU, neurointensive care unit.
Table 3.
Analysis of patients with supratentorial ICH with volumes less than 5 mL
| Supratentorial <5 cc
|
All others
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | Transfer | All | Direct | Transfer | All | |||||||
|
|
|
|
|
|
|
|
||||||
| Total (N) | 12.0 | 44.0 | 56.0 | 140.0 | 166.0 | 306.0 | ||||||
| Total patients in subgroup | 152.0 | 7.9% | 210.0 | 21.0% | 362.0 | 15.5% | 152.0 | 92.1% | 210.0 | 79.0% | 362.0 | 84.5% |
| ICH score (mean) | .8 | .5 | .6 | 2.1 | 2.0 | 2.1 | ||||||
| SD | 1.0 | .7 | .8 | 1.4 | 1.3 | 1.4 | ||||||
| ICH volume (median/IQR) | 2.7 | 1.6 | 1.7 | 21.8 | 19.5 | 20.9 | ||||||
| ICH location | ||||||||||||
| Basal ganglia | 1.0 | 8.3% | 7.0 | 15.9% | 8.0 | 14.3% | 43.0 | 30.7% | 36.0 | 21.7% | 79.0 | 25.8% |
| Thalamus | 9.0 | 75.0% | 26.0 | 59.1% | 35.0 | 62.5% | 43.0 | 30.7% | 28.0 | 16.9% | 71.0 | 23.2% |
| Lobar | 2.0 | 16.7% | 11.0 | 25.0% | 13.0 | 23.2% | 38.0 | 27.1% | 71.0 | 42.8% | 109.0 | 35.6% |
| Brain stem | .0 | .0% | .0 | .0% | .0 | .0% | 9.0 | 6.4% | 9.0 | 5.4% | 18.0 | 5.9% |
| Cerebellar | .0 | .0% | .0 | .0% | .0 | .0% | 5.0 | 3.6% | 15.0 | 9.0% | 20.0 | 6.5% |
| Pure IVH | .0 | .0% | .0 | .0% | .0 | .0% | 2.0 | 1.4% | 7.0 | 4.2% | 9.0 | 2.9% |
| IVH present (%) | 4.0 | 33.3% | 9.0 | 20.5% | 13.0 | 23.2% | 88.0 | 62.9% | 86.0 | 51.8% | 174.0 | 56.9% |
| Hematoma expansion (%) | 3.0 | 25.0% | 9.0 | 20.5% | 12.0 | 21.4% | 20.0 | 14.3% | 38.0 | 22.9% | 58.0 | 19.0% |
| ICU needed (%) | 4.0 | 33.3% | 13.0 | 29.5% | 17.0 | 30.4% | 113.0 | 80.7% | 135.0 | 81.3% | 248.0 | 81.0% |
| Received neurosurgery (unique) | 3.0 | 25.0% | .0 | .0% | 3.0 | 5.4% | 46.0 | 32.9% | 44.0 | 26.5% | 90.0 | 29.4% |
| Hemicraniectomy | .0 | .0 | .0 | 11.0 | 6.0 | 17.0 | ||||||
| EVD | 3.0 | .0 | 3.0 | 40.0 | 38.0 | 78.0 | ||||||
| Clot evacuation | 1.0 | .0 | 1.0 | 7.0 | 3.0 | 10.0 | ||||||
| Clinical trials | ||||||||||||
| Unique | 6.0 | 50.0% | 9.0 | 20.5% | 15.0 | 26.8% | 36.0 | 25.7% | 31.0 | 18.7% | 67.0 | 21.9% |
| CLEAR | 1.0 | .0 | 1.0 | 8.0 | 1.0 | 9.0 | ||||||
| MISTIE | .0 | .0 | .0 | 2.0 | .0 | 2.0 | ||||||
| SHRINC | .0 | .0 | .0 | 12.0 | 9.0 | 21.0 | ||||||
| ASSIST | .0 | 1.0 | 1.0 | 6.0 | 1.0 | 7.0 | ||||||
| ERICH | 6.0 | 9.0 | 15.0 | 24.0 | 31.0 | 55.0 | ||||||
| TEG | .0 | .0 | .0 | 12.0 | 3.0 | 15.0 | ||||||
| Interventional trials (CLEAR + MISTIE + SHRINC) | 1.0 | 8.3% | .0 | .0% | 1.0 | 1.8% | 22.0 | 15.7% | 10.0 | 6.0% | 32.0 | 10.5% |
| Observational trials (ASSIST + ERICH + TEG) | 6.0 | 50.0% | 10.0 | 22.7% | 16.0 | 28.6% | 42.0 | 30.0% | 35.0 | 21.1% | 77.0 | 25.2% |
| Used any CSC resource? (ICU/NSG/clinical trials) | 8.0 | 66.7% | 21.0 | 47.7% | 29.0 | 51.8% | 126.0 | 90.0% | 145.0 | 87.3% | 271.0 | 88.6% |
| Did not use any CSC resource? | 4.0 | 33.3% | 23.0 | 52.3% | 27.0 | 48.2% | 14.0 | 10.0% | 21.0 | 12.7% | 35.0 | 11.4% |
| Used only (ICU/NSG/interventional trials) | 4.0 | 33.3% | 13.0 | 29.5% | 17.0 | 30.4% | 119.0 | 85.0% | 138.0 | 83.1% | 257.0 | 84.0% |
| Did not use any CSC interventional resource? | 8.0 | 66.7% | 31.0 | 70.5% | 39.0 | 69.6% | 21.0 | 15.0% | 28.0 | 16.9% | 49.0 | 87.5% |
| Discharge disposition | ||||||||||||
| Home with/without outpatient rehabilitation | 6.0 | 50.0% | 17.0 | 38.6% | 23.0 | 41.1% | 5.0 | 3.6% | 24.0 | 14.5% | 29.0 | 9.5% |
| Inpatient rehabilitation at MHH | 1.0 | 8.3% | 3.0 | 6.8% | 4.0 | 7.1% | 16.0 | 11.4% | 12.0 | 7.2% | 28.0 | 9.2% |
| Inpatient rehabilitation (other facility) | 1.0 | 8.3% | .0 | .0% | 1.0 | 1.8% | 1.0 | .7% | 1.0 | .6% | 2.0 | .7% |
| Skilled nursing facility with rehabilitation | .0 | .0% | 1.0 | 2.3% | 1.0 | 1.8% | 1.0 | .7% | 6.0 | 3.6% | 7.0 | 2.3% |
| Subacute unit | .0 | .0% | .0 | .0% | .0 | .0% | 4.0 | 2.9% | 4.0 | 2.4% | 8.0 | 2.6% |
| Nursing home | .0 | .0% | .0 | .0% | .0 | .0% | .0 | .0% | 1.0 | .6% | 1.0 | .3% |
| Transfer other service | 4.0 | 33.3% | 21.0 | 47.7% | 25.0 | 44.6% | 62.0 | 44.3% | 76.0 | 45.8% | 138.0 | 45.1% |
| Transfer neurosurgery | .0 | .0% | .0 | .0% | .0 | .0% | 1.0 | .7% | .0 | .0% | 1.0 | .3% |
| Home for custodial/hospice care | .0 | .0% | 2.0 | 4.5% | 2.0 | 3.6% | 14.0 | 10.0% | 9.0 | 5.4% | 23.0 | 7.5% |
| Death | .0 | .0% | .0 | .0% | .0 | .0% | 36.0 | 25.7% | 33.0 | 19.9% | 69.0 | 22.5% |
| Neuroworsening | 2.0 | 16.7% | 3.0 | 6.8% | 5.0 | 8.9% | 48.0 | 34.3% | 44.0 | 26.5% | 92.0 | 30.1% |
| mRS score on discharge | ||||||||||||
| 0 | 1.0 | 3.0 | 4.0 | 1.0 | 1.0 | 2.0 | ||||||
| 1 | .0 | 6.0 | 6.0 | .0 | 5.0 | 5.0 | ||||||
| 2 | 1.0 | 2.0 | 3.0 | 2.0 | 5.0 | 7.0 | ||||||
| 3 | 4.0 | 9.0 | 13.0 | 6.0 | 13.0 | 19.0 | ||||||
| 4 | 3.0 | 18.0 | 21.0 | 27.0 | 36.0 | 63.0 | ||||||
| 5 | 3.0 | 6.0 | 9.0 | 68.0 | 73.0 | 141.0 | ||||||
| 6 | .0 | .0 | .0 | 36.0 | 33.0 | 69.0 | ||||||
| mRS score 0–3 on discharge | 6.0 | 50.0% | 20.0 | 45.5% | 26.0 | 46.4% | 9.0 | 6.4% | 24.0 | 14.5% | 33.0 | 10.8% |
| mRS score 4–6 on discharge | 6.0 | 50.0% | 24.0 | 54.5% | 30.0 | 53.6% | 131.0 | 93.6% | 142.0 | 85.5% | 273.0 | 89.2% |
Abbreviations: EVD, external ventricular drain; ICH, intracerebral hemorrhage; ICU, intensive care unit; IQR, interquartile range; IVH, intraventricular hemorrhage; mRS, modified Rankin Scale; SD, standard deviation.
Cost Analysis
Table 4 shows hospitalization costs for both directly arriving and transferred patients. Overall, transfer patients had significantly lower average hospitalization costs than those patients directly admitted, which corresponds with lower average CSC resource utilization—the difference of about $10,000 on cost per patient remains the same even when patients are categorized according to ICH volume. Cost per day of hospitalization, which is total cost divided by total LOS, also illustrates lower resource utilization by transferred patients. However, only mean treatment costs for patients in the directly admitted and transferred subgroups with ICH volume higher than 5 mL were significantly different. The total costs for the transfer group ($7.2 million) were higher than the total costs for the direct group ($6.8 million), mainly because of the larger number of patients.
Table 4.
Hospitalization costs for patients transferred to our CSC compared to those presenting directly to our CSC
| Direct
|
Transfer
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Total costs | Average cost per patient | Cost per in-hospital day | Patients | Total costs | Average cost per patient | Cost per in-hospital day | |
| Total | 152 | $6,719,570 | $44,208 | $6375 | 210 | $7,198,442 | $34,278 | $5754 |
| ICH volume <5 mL | 18 | $699,987 | $38,888 | $5983 | 60 | $1,741,449 | $29,024 | $5710 |
| ICH volume >5 mL | 134 | $6,019,582 | $44,922 | $6424 | 150 | $5,456,993 | $36,380 | $5768 |
Abbreviations: CSC, comprehensive stroke center; ICH, intracerebral hemorrhage.
Discussion
Over the past several years at our center, the number of patients with ICH transferred from outside facilities has steadily increased,7 perhaps as a result of growing our telemedicine and referral systems in the region. As a consequence, we initially suspected that transferred patients to our facility would have more severe and catastrophic hemorrhages, and consequently utilize CSC-specific resources to a higher degree than those presenting directly. Contrary to our expectation, transferred patients tended to have less severe injuries as measured on the NIHSS and ICH scores and utilized CSC-specific resources (namely, NICU, neurosurgical services, and availability of clinical trials) to a lesser degree than directly arriving patients. This situation was also reflected in lower average care costs, and costs per in-hospital day, for the transferred patients. Although no differences were found in the use of the NICU for both groups, our team has a low threshold to transfer patients directly to our ICU given the lack of direct access to objective data such as neuroimaging. This issue may explain in part why the LOS in the ICU was shorter among transfer patients. Overall, patients with lower ICH scores, whether transferred or directly admitted, tended to require less CSC resources.
The higher number of transfer patients with milder injuries admitted to our facility may be explained by the preferences and policies of outside hospitals. Primary stroke centers and community hospitals likely have a low threshold to transfer patients with ICH irrespective of severity. In fact, our finding that the transferred group contained a disproportionately high amount of small ICH volumes less than 5 mL may reflect a general discomfort in caring for ICH patients among the primary centers in our region.
The more favorable ICH characteristics among transferred patients may also explain why less of these patients were enrolled in clinical trials. The interventional trials occurring at our center had minimum volume thresholds (i.e., SHRINC, MISTIE) or required the presence of IVH (i.e., CLEAR-IVH) as part of their inclusion criteria. Their later onset-to-CSC arrival times may also make it more difficult for them to meet the study enrollment time windows. Further, as transferred patients often arrive without surrogate families, study consent is often not possible within the enrollment window.
Our observations bring attention to the issue that not all patients with ICH will necessarily require transfer to a CSC. Given the limited number of beds at any given facility, CSCs want to ensure that their beds are used for patients who need the services of a CSC. Our findings suggest that CSCs may benefit from knowing the ICH score of patients when transfer is requested. At a minimum, CSCs would benefit from directly viewing cranial imaging performed at the hospital requesting transfer, thus allowing them to determine which patients are mild and less likely to require the services available only at a CSC. Keeping patients who may not require the additional resources of a CSC at their hospital of origin would prevent exposure to the unstable rigors of transportation without physician supervision. Reducing the number of transferred patients may also lead to fewer transportation-related costs and unnecessary resource use. This would also imply improved use of healthcare funds at the system level and possible reduction in the overall cost of patient care.
However, there are still important reasons to provide close monitoring of patients with milder severity of ICH. Recent evidence suggests that blood pressure (BP) reduction to below a systolic BP less than 140 mmHg may improve outcome.18 For these cases, teleconsultations with hospitalists at community hospitals that include recommendations for BP goals may be a viable alternative to transferring to CSCs for BP management. Another reason to consider transferring milder ICH cases is the opportunity for clinical trial participation. However, the timing of enrollment, patient eligibility, and discussions with family ahead of time are important considerations before transferring to CSCs. Given the advent of using telemedicine to enroll patients at outside hospitals into clinical trials,19 these patients might still be able to be enrolled remotely rather than being transferred, depending on the study.
The policy implications of our study are important. With NICUs and neurosurgical capabilities serving as key components of the CSC designation, the traditional hub-and-spoke distribution of centers is in flux. Growing scrutiny over resource utilization also implies that physicians will need to become more discretionary in their transfer requests. It is not practical or sustainable for any CSC to accept all patients with ICH for presumed higher level of care. Although it has recently been shown that it is cost-effective to transfer patients with severe ICH to centers with NICUs,20 this study did not address the concept of transferring any patient with ICH. Under the current health-care climate, new approaches are needed to identify which patients with ICH require transfer to CSCs. Primary stroke centers may also benefit from more robust intermediate care units that can provide close neurological and BP monitoring without requesting the need to transfer patients to CSCs. Additional study is required to determine the optimal allocation of limited CSC resources.
Acknowledgments
Grant support: This work was supported by National Institutes of Health Training Grant 5 T32 NS007412-12.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Tomsick T, et al. Management of intracerebral hemorrhage in a large metropolitan population. Neurosurgery. 1994;34:882–887. doi: 10.1227/00006123-199405000-00015. discussion 887. [DOI] [PubMed] [Google Scholar]
- 3.Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003;16(Suppl 1):9–13. doi: 10.1159/000069935. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66:1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 5.Fogelholm R, Murros K, Rissanen A, et al. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534–1538. doi: 10.1136/jnnp.2004.055145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Special report from the National Institute of Neurological Disorders and Stroke, Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 7.Albright KC, Boehme AK, Mullen MT, et al. Changing demographics at a comprehensive stroke center amidst the rise in primary stroke centers. Stroke. 2013;44:1117–1123. doi: 10.1161/STROKEAHA.111.666156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardach NS, Zhao S, Gress DR, et al. Association between subarachnoid hemorrhage outcomes and number of cases treated at California hospitals * editorial comment. Stroke. 2002;33:1851–1856. doi: 10.1161/01.str.0000019126.43079.7b. [DOI] [PubMed] [Google Scholar]
- 9.Glance LG, Li Y, Osler TM, et al. Impact of patient volume on the mortality rate of adult intensive care unit patients. Crit Care Med. 2006;34:1925–1934. doi: 10.1097/01.CCM.0000226415.93237.84. [DOI] [PubMed] [Google Scholar]
- 10.Diringer M, Edwards D. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–640. doi: 10.1097/00003246-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Mirski MA, Chang CW, Cowan R. Impact of a neuroscience intensive care unit on neurosurgical patient outcomes and cost of care: evidence-based support for an intensivist-directed specialty ICU model of care. J Neurosurg Anesthesiol. 2001;13:83–92. doi: 10.1097/00008506-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Suarez JI, Zaidat OO, Suri MF, et al. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit Care Med. 2004;32:2311–2317. doi: 10.1097/01.ccm.0000146132.29042.4c. [DOI] [PubMed] [Google Scholar]
- 13.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the brain attack coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar MH, Gonzales NR, Ardjomand-Hessabi M, et al. The University of Texas Houston Stroke Registry (UTHSR): implementation of enhanced data quality assurance procedures improves data quality. BMC Neurol. 2013;13:61. doi: 10.1186/1471-2377-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 16.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 17.Hemphill JC, Bonovich DC, Besmertis L, et al. The ICH Score: a simple, reliable grading scale for intracerebral hemorrhage editorial comment: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CS, Heeley E, Huang Y, et al. INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Sarraj A, Jacobs A, et al. Telemedicine-guided remote enrollment of patients into an acute stroke trial. Ann Clin Transl Neurol. 2015;2:38–42. doi: 10.1002/acn3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher JJ, Kotagal V, Mammoser A, et al. Cost-effectiveness of transfers to centers with neurological intensive care units after intracerebral hemorrhage. Stroke. 2015;46:58–64. doi: 10.1161/STROKEAHA.114.006653. [DOI] [PMC free article] [PubMed] [Google Scholar]