Abstract
Introduction
Neuroendocrine tumors (NETs) frequently metastasize to the liver. Surgical debulking offers symptomatic relief and improved survival. However, the frequent presence of multifocal, bilobar disease and high recurrence rates introduce doubt regarding their optimal management. Parenchyma-sparing debulking (PSD) procedures (ablation, enucleation, wedge resections) may offer similar survival improvements as resection, while minimizing morbidity and preserving functional liver tissue.
Methods
Clinicopathologic variables from 228 patients with small bowel (SBNETs) or pancreatic NETs (PNETs) managed surgically at one institution were collected. Liver-directed surgery (LDS) was carried out when significant debulking was deemed feasible. Survival was assessed using the Kaplan-Meier method.
Results
108 PNET and SBNET patients underwent LDS with primarily PSD procedures. Nearly two-thirds of patients achieved 70% cytoreduction and 84% had concurrent resection of their primary. The median number of lesions treated was 6 (range 1–36). There were no 30-day operative mortalities. The 30-day major complication rate was 13.0%. Patients that achieved 70% cytoreduction enjoyed improved progression free (median 3.2 years) and overall survival (median not reached).
Conclusion
PSD procedures are safe and can achieve significant cytoreduction, which is associated with improved survival. Lowering the debulking target threshold to 70% may benefit NET patients by increasing eligibility for cytoreduction.
Introduction
As many as 60% of patients with NETs will present with disseminated disease.1 These tumors commonly metastasize to the liver, and the most common cause of death in these patients is liver failure secondary to replacement by tumor.2 In contrast to many cancer types, the presence of metastatic disease does not preclude surgical treatment, and in the case of gastroenteropancreatic (GEP) NETs, quality of life and survival can be improved when hepatic metastases are treated aggressively.3 However, aggressive resection of liver tumors rarely translates into cure, as even when R0 resection is achieved, 5-year recurrence rates of up to 94% have been reported.4
Surgical debulking of hepatic metastases in NET patients has been associated with improved survival compared to historical controls in a number of studies.5,6 The median survival of SBNET and PNET patients with M1 disease is 56 and 24 months, respectively, in the Surveillance, Epidemiology and End Results program (SEER) database.7 Early surgical series employing resection for cytoreduction of liver metastases reported median survival times of 80 to 90 months.5,6 Debulking has traditionally been approached using hepatic resection, and while potentially curative, may come at the cost of high morbidity and mortality. Careful patient selection for these procedures is required, and many NET patients will be excluded because most have numerous, bilobar metastases not amenable to complete resection. Incorporation of additional techniques such as ablation into standard debulking protocols has expanded the number of patients manageable by surgery, as scattered metastases in both lobes can be treated safely in one operation.8 These combination approaches to cytoreduction have demonstrated excellent efficacy and similar survival rates as those that use resection only. Recent reports of hepatic debulking using combination cytoreduction demonstrated 5-year survival of around 75%,1,9 which is on par with patients debulked with resection only.4
It is not clear what the optimal target should be for cytoreduction in patients with NET liver metastases, but a reasonable endpoint would be one in which both symptoms and survival could be improved. An often quoted objective for optimal cytoreduction has been 90%,5,10 but these criteria may leave fewer than 10% of patients with NET liver metastases suitable for debulking procedures.5,11 Recent studies have proposed relaxing cytoreduction criteria to 70%, citing improved symptoms and progression free survival (PFS) in patients who were treated in this manner.1,12 In this report, we describe our experience treating a large cohort of patients with metastatic SBNET and PNETs using PSD methods. We study factors associated with patient survival, including preoperative hepatic tumor burden and biochemical response to debulking, and the effects of different levels of cytoreduction on postoperative outcomes are explored.
Methods
Clinical data for patients undergoing surgery for SBNETs and PNETs at a single center between 1999 and 2015 were retrospectively reviewed in a prospectively maintained surgical database under an Institutional Review Board-approved protocol. Clinicopathologic patient data included sex, age, tumor grade, multifocality of the primary tumor, TNM stage, postoperative symptoms, postoperative octreotide treatment status, nonsurgical liver treatments, and presence of extrahepatic metastases. Tumor grade was determined by assessment of the Ki-67 proliferation index in whole sections from primary tumors. Pre and postoperative levels of chromogranin A (CgA), pancreastatin (PST), and neurokinin A (NKA) were recorded (where available), and biochemical response to liver debulking was assessed in patients with elevated preoperative levels. A biochemical response was defined as a decline of any of these hormone levels ≥ 50% from preoperative levels. Patients were considered nonresponders if the postoperative biomarker level increased, remained stably elevated, or the reduction was < 50%.13 Operative details examined were the surgical approach, types of liver-directed procedures used, number of hepatic lesions treated, and whether the primary tumor was also resected at the time of LDS. Postoperative 30-day complication rates, 30-day mortality rates, length of stay, and reoperation rate due to postoperative complications were analyzed. Postoperative complications were classified according to the system proposed by Dindo et al.14
Patients were generally operated upon for cure or palliation of their disease, with the objective of removing the primary tumor, regional lymph nodes, and debulking their metastatic disease. The senior surgeon determined the extent of surgery using a combination of preoperative and intraoperative data. Surgical treatment of hepatic metastases was performed using a combination of enucleation, wedge resection, radiofrequency or microwave ablation, and anatomic resection. In general, surface lesions were treated by enucleation or wedge resection, whereas deeper lesions were ablated. When possible, primary tumor resection and hepatic cytoreduction were performed concurrently. When patients had multiple liver surgeries (n=6), only data from the first procedure were included. For ablation cases, the surgeon carefully mapped out the location of the lesions identified on preoperative imaging and then localized these with intraoperative ultrasound (employing the help of a radiologist in early patients). Lesions were targeted using a needle guide and a single ablation probe. Radiofrequency ablation (Angiodynamics, Latham, NY) was the primary method employed until the last several years, where microwave ablation (Acculis, Angiodynamics, Latham, NY) was favored. Given their similarity, enucleation and wedge resection were combined into one category for the analyses.
The number of preoperative hepatic lesions, the amount of hepatic tumor replacement, and the degree of hepatic debulking achieved were estimated retrospectively by the senior operating surgeon (J.R.H.) using a combination of intraoperative data (ultrasound and operative notes), pathology reports, and imaging studies. Pre and postoperative (usually at 3-6 months) contrast-enhanced computed tomographic scans (CT) or magnetic resonance imaging (MRI) studies were examined side-by-side and slice-by-slice to determine the number of preoperative lesions that were treated and to what effect. Estimation of the amount of hepatic tumor debulked was made based upon these comparisons, taking into account the number of lesions treated/removed versus the total number of lesions and size of each lesion relative to the overall tumor volume. Categories of < 50%, ≥ 50%, ≥ 70%, and ≥ 90% were used based upon the estimated percentage of tumor debulked.
Median event times for progression free and overall survival (OS) were determined from the time of surgery using the Kaplan-Meier method, and p values were calculated using the log rank test. Median follow up was estimated by the reverse Kaplan-Meier method. Factors predictive of successful 70% or 90% cytoreduction and a biochemical response were examined with logistic regression. Clinicopathologic and surgical characteristics were compared using Fisher's exact test, chi-square test or Welch's t-test. All analyses were performed in R v 3.1.2 (Vienna, Austria).
Results
Clinicopathologic characteristics
Between 1999 and December 2014, 228 patients with SBNETs or PNETs were operated upon at our institution. Of these, 108 (28 PNETs and 80 SBNETs) underwent LDS for their hepatic metastases. The demographic and clinicopathologic characteristics of the cohort are displayed in Table 1. The proportions of males and females in each group were not significantly different. The median age at surgery in the PNET group was 54.7 years old, and in SBNETs was 60.3 years old. In both groups, the majority of NETs were well-differentiated and of low or intermediate grade. Substantially more SBNET patients had multifocal primary tumors (48.8%) compared to PNET patients (3.6%, p < .001). Seventy-eight percent of SBNETs and 14.2% of PNETs undergoing LDS had T3 or T4 tumors. Nodal metastases were found in 75.0% of PNET versus 87.5% of SBNET patients. A greater proportion of SBNET patients had distant (non-liver) metastatic disease than PNET (50 vs. 10.7%; p < .001).
Table 1. Demographics and clinicopathologic characteristics of the NET cohort who underwent liver directed surgery.
The single PNET patient with a familial syndrome had MEN1.
| PNET (n=28) | SBNET (n=80) | p value | |
|---|---|---|---|
| Sex | |||
| M | 13 (46.4%) | 49 (61.3%) | .25 |
| F | 15 (53.6%) | 31 (38.8%) | .25 |
| Median age at surgery | 54.7 (22.68-79.26) | 60.3 (15-80.5) | .01 |
| Grade | |||
| G1/G2 | 25 (89.3%) | 74 (92.5%) | .89 |
| G3 | 2 (7.1%) | 2 (2.5%) | .59 |
| Not graded | 1 (3.6%) | 4 (5.0%) | 1 |
| Multifocal primary | 1 (3.6%) | 39 (48.8%) | < .001 |
| Functional primary tumor | 3 (10.7%) | 56 (70.0%) | < .001 |
| T stage | |||
| T1 | 8 (28.6%) | 2 (2.5%) | < .001 |
| T2 | 16 (57.1%) | 12 (15.0%) | < .001 |
| T3 | 2 (7.1%) | 29 (36.3%) | < .001 |
| T4 | 2 (7.1%) | 34 (42.5%) | < .001 |
| N stage | |||
| N0 | 4 (14.3%) | 9 (11.3%) | .93 |
| N1 | 21 (75.0%) | 70 (87.5%) | .21 |
| Distant metastases (non-liver, non-LN) | 3 (10.7%) | 40 (50.0%) | < .001 |
| Familial syndrome | 1 (3.6%) | NA | |
| Preoperative symptoms | |||
| Abdominal pain only | 8 (30%) | 15 (19%) | .37 |
| Abdominal pain + diarrhea and/or flushing | 7 (26%) | 36 (46%) | .12 |
| Diarrhea and/or flushing only | 5 (19%) | 20 (25%) | .65 |
| No symptoms | 7 (26%) | 8 (10%) | .50 |
| Non-surgical therapies | |||
| Octreotide | 25 (89.3%) | 66 (82.5%) | .58 |
| Hepatic arterial embolization | 6 (21.4%) | 14 (17.5%) | .86 |
| Systemic chemotherapy | 14 (50.0%) | 6 (7.5%) | < .001 |
| Peptide receptor radionuclide therapy | 2 (7.1%) | 13 (16.3%) | .38 |
The majority of patients were symptomatic, experiencing either abdominal pain, flushing, diarrhea, or some combination of those symptoms. Interestingly, PNET patients also frequently experienced symptoms of diarrhea and/or flushing. There was no significant difference in the proportion of patients who received octreotide postoperatively. Chemotherapy was the most common liver-directed nonsurgical treatment in PNETs, whereas SBNET patients were more likely to be treated with hepatic arterial embolization or peptide receptor radionuclide therapy.
Operative characteristics
Of the 228 PNET and SBNET patients included in the surgical database, 142 had liver metastases at the time of their surgery and 108 (76%) underwent LDS. The majority of patients had bilobar disease. The median number of lesions seen on preoperative imaging was 10 in PNET and 9 in SBNET patients, and the number of lesions ranged from 0-100 (Table 2). The estimated amount of liver parenchyma replaced by tumor at presentation was 19% in PNET and 10% in SBNET patients (p = .08). All procedures were performed in an open fashion. In most cases, a combination of procedures was used to debulk a patient's hepatic disease. Enucleation or wedge resection plus ablation was performed most often in both groups. There were no significant differences between PNET and SBNET patients in the types of procedures used to debulk their hepatic disease, nor was there a difference in the amount of disease debulked (mean of 75% in PNETs and 80% in SBNETs), or the median number of lesions treated (6 in both PNETs and SBNETs). Of all the patients who underwent hepatic cytoreduction, 63.9% achieved a 70% reduction in their tumor burden. Attaining 90% cytoreduction was more difficult, and was achieved in only 38.9% of cases. Most patients also had their primary tumor resected during the same operation as their hepatic debulking (96.4% of PNETs, and 80.0% of SBNETs). The median length of stay in the two groups was approximately 1 week.
Table 2. Radiologic, surgical and biochemical findings.
| PNET (n=28) | SBNET (n=80) | p value | |
|---|---|---|---|
| Multiple hepatic debulking surgeries | 2 (7.1%) | 4 (5.0%) | 1 |
| Median # of hepatic lesions visualized preoperatively on CT or MRI | 10 (0-100) | 9 (0-85) | .68 |
| Median % of liver parenchyma replaced by tumor | 19% (1-50) | 10% (0-55%) | .08 |
| Bilobar hepatic disease | 23 (82.1%) | 63 (78.8%) | .91 |
| Median % of liver disease debulked | 75% (13-100%) | 80% (0-100) | .37 |
| # achieved 70% cytoreduction | 18 (64.3%) | 51 (63.8%) | 1 |
| # achieved 90% cytoreduction | 10 (35.7%) | 32 (40.0%) | .86 |
| Type of liver procedures performed | |||
| Enucleation or wedge only | 6 (21.4%) | 17 (21.3%) | 1 |
| Ablation only | 4 (14.3%) | 6 (7.5%) | .49 |
| Resection only | 0 | 1 (1.3%) | 0 |
| Enucleation/wedge + ablation | 16 (57.1%) | 53 (66.3%) | .53 |
| Enucleation/wedge + ablation + resection | 1 (3.7%) | 0 | .581 |
| Enucleation/wedge + resection | 1 (3.6%) | 3 (3.8%) | 1 |
| Median # of hepatic lesions treated | 6 (1-19) | 6 (0-36) | .96 |
| Primary resected at time of liver surgery | 27 (96.4%) | 64 (80.0%) | .08 |
| Median length of stay (days) | 8 (5-17) | 7 (5-24) | .83 |
| Biochemical response (CgA, PST, NKA) | |||
| Nonresponder | 5 (25%) | 18 (32.1%) | .75 |
| Biochemical response | 15 (75%) | 43 (70.5%) | 1 |
A number of clinicopathologic and disease factors were incorporated into linear regression models to determine predictors of achieving biochemical response to debulking and achieving a 70% or 90% cytoreduction. The factors examined were age at surgery, primary tumor multifocality, T stage, N stage, size of largest liver metastasis identified on preoperative imaging, percentage of hepatic tissue replacement, number of preoperative lesions identified on preoperative imaging, and distribution of disease (bilobar versus unilobar). None of the factors tested were significant in any of the models (data not shown).
Patients tolerated their surgeries well. There were no intraoperative or postoperative deaths within 30 days of surgery (Table 3). Patients were routinely treated with an intraoperative octreotide infusion at 100 mcg/hr, which was weaned off postoperatively over 24-36 hours. Sixty-four percent of PNET and 45% of SBNET patients experienced a complication within 30 days of their procedure. The vast majority of complications were minor (grade I or II), accounting for 70.3% of the complications in PNET and 94.1% of the complications in SBNET patients. The major complications (grade III and IV) were mostly intraabdominal abscesses requiring drain placement (n = 7), though there was one pleural effusion requiring drainage and two cases where reoperation was required. The first reoperation was performed for a small bowel obstruction in a PNET patient. The second reoperation was performed for anastomotic leak in a patient who required transhiatal esophagectomy at the time of locally advanced PNET resection and hepatic cytoreduction. Ten PNET and 9 SBNET patients suffered more than one complication postoperatively.
Table 3. Postoperative complications.
All complications were classified using the Clavien-Dindo system.
| PNET (n=28) | SBNET (n=80) | |
|---|---|---|
| Total # of 30-day complications | 37 complications in 18 patients | 51 complications in 36 patients |
| Grade of complication (Clavian Dindo classification) | ||
| I | 7 (18.9%) | 20 (39.2%) |
| II | 19 (51.4%) | 28 (54.9%) |
| III | 7 (18.9%) | 3 (5.9%) |
| IV | 4 (10.8%) | 0 |
| Reoperations due to postoperative complications | 2 | 0 |
| 30 day postoperative mortality | 0 | 0 |
Survival analysis
The median PFS for all of the patients who underwent LDS was 2.2 years. Median OS in this cohort was 10.5 years. Five-year PFS was 30.2%, while 5-year OS was 76.1%. The median follow up was 4.1 years. In PNETs, the median PFS for all those undergoing LDS was 1.6 years and median OS was 10.5 years. In SBNETs, the median PFS for patients having LDS was 2.5 years, while the median OS was not reached. In the years these patients were followed, there were only 17 deaths, and thus the small number of events may have limited the power to detect differences in OS.
A number of different disease or surgical factors were analyzed in each group of patients (PNETs, SBNETs, and combined groups; Table 4) to test their effect on survival. The amount of hepatic replacement in PNETs did not correlate with survival when categories of < 5% and ≥5% or < 25% and ≥25% were examined. In SBNETs, both patients with < 5% and < 25% tumor burden had significantly longer PFS than those with > 5% and ≥ 25% replacement, respectively. In the combined group there was a significant improvement in PFS with < 5% and < 25% hepatic replacement. The number of hepatic lesions identified on preoperative imaging did not affect PFS or OS in PNETs. Both lower categories (< 5 lesions versus ≥ 5 lesions and < 10 versus ≥ 10 lesions) were predictive of improved PFS in SBNETs and the combined group, but were not significant for OS. The degree of hepatic debulking was associated with improved patient survival. In PNETs and in the combined group (Figure 1) both PFS and OS were significantly improved in those in whom ≥ 70% cytoreduction was achieved (versus < 70%). Only PFS, but not OS, was prolonged by 70% cytoreduction in the SBNET group. Ninety percent cytoreduction was associated with improved PFS in both groups, though differences in OS remained not significant.
Table 4. Survival analyses in PNETs, SBNETs and the combined group.
| SURVIVAL: PNETs | |||||||
|---|---|---|---|---|---|---|---|
| Hepatic replacement by tumor | n | Events | Median PFS (years) | p value | Events | Median OS (years) | p value |
| < 5% | 3 | 2 | 2.5 | .58 | 2 | 5.8 | .46 |
| ≥ 5% | 21 | 17 | 1.6 | 5 | NA | ||
| Hepatic replacement by tumor | |||||||
| < 25% | 12 | 8 | 3.2 | .09 | 3 | NA | .45 |
| ≥ 25% | 12 | 11 | 1.3 | 4 | 3.0 | ||
| # of preoperative hepatic lesions | |||||||
| < 5 | 7 | 5 | 2.8 | .18 | 2 | NA | .70 |
| ≥ 5 | 18 | 15 | 1.6 | 5 | NA | ||
| # of preoperative | |||||||
| < 10 | 12 | 10 | 3.0 | .31 | 3 | NA | .38 |
| % hepatic tumor burden debulked | |||||||
| < 70% | 8 | 7 | .51 | < .001 | 4 | 1.7 | .001 |
| ≥ 70% | 18 | 15 | 3.0 | 4 | NA | ||
| % hepatic tumor | |||||||
| < 90% | 16 | 14 | 1.3 | .05 | 6 | 6.1 | .14 |
| ≥ 90% | 10 | 8 | 4.4 | 2 | NA | ||
| CgA, PST, NKA response to debulking | |||||||
| No response | 3 | 3 | .27 | .55 | 1 | NA | .51 |
| Biochem resp. | 14 | 11 | 2.7 | 2 | NA | ||
| SURVIVAL: SBNETs | |||||||
| Hepatic replacement by tumor | n | Events | Median PFS (years) | p value | Events | Median OS (years) | p value |
| < 5% | 20 | 5 | 5.3 | .02 | 2 | NA | .28 |
| ≥ 5% | 55 | 35 | 1.9 | 15 | 9.1 | ||
| Hepatic replacement by tumor | |||||||
| < 25% | 50 | 22 | 3.2 | .02 | 9 | NA | .14 |
| ≥ 25% | 25 | 18 | 1.9 | 8 | 9.1 | ||
| # of preoperative hepatic lesions | |||||||
| < 5 | 27 | 13 | 4.2 | .02 | 6 | NA | .28 |
| ≥ 5 | 48 | 27 | 1.6 | 11 | 9.1 | ||
| # of preoperative hepatic lesions | |||||||
| < 10 | 38 | 17 | 4.2 | < .001 | 8 | NA | .20 |
| ≥ 10 | 37 | 23 | 1.6 | 9 | 9.1 | ||
| % hepatic tumor burden debulked | |||||||
| < 70% | 17 | 15 | 1.7 | .005 | 7 | 9.1 | .18 |
| ≥ 70% | 51 | 25 | 3.2 | 9 | NA | ||
| % hepatic tumor burden debulked | |||||||
| < 90% | 36 | 25 | 1.6 | .005 | 9 | 9.1 | .46 |
| ≥ 90% | 32 | 15 | 3.8 | 7 | NA | ||
| CgA, PST, NKA response to debulking | |||||||
| No response | 16 | 11 | 1.9 | .59 | 3 | 9.1 | .58 |
| Biochemical resp. | 45 | 26 | 2.6 | 10 | NA | ||
| SURVIVAL: PNET + SBNETs | |||||||
| Hepatic replacement by tumor | n | Events | Median PFS (years) | p value | Events | Median OS (years) | p value |
| < 5% | 23 | 7 52 | 5.3 | .02 | 20 | NA | .49 |
| ≥ 5% | 76 | 1.7 | 4 | 9.4 | |||
| Hepatic replacement | |||||||
| < 25% | 62 | 30 | 3.2 | .001 | 12 | NA | .06 |
| ≥ 25% | 37 | 29 | 1.3 | 12 | 9.1 | ||
| # of preoperative hepatic lesions | |||||||
| < 5 | 34 | 18 | 4.2 | .005 | 8 | NA | .25 |
| ≥ 5 | 66 | 42 | 1.6 | 16 | 9.1 | ||
| # of preoperative hepatic lesions | |||||||
| < 10 | 50 | 27 | 3.2 | < .001 | 11 | NA | .10 |
| ≥ 10 | 50 | 33 | 1.3 | 13 | 6.5 | ||
| % hepatic tumor burden debulked | |||||||
| < 70% | 25 | 22 | 1.3 | < .001 | 11 | 6.5 | .009 |
| ≥ 70% | 69 | 40 | 3.2 | 13 | NA | ||
| % hepatic tumor burden debulked | |||||||
| < 90% | 52 | 39 | 1.5 | < .001 | 15 | 6.5 | .12 |
| ≥ 90% | 42 | 23 | 3.8 | 9 | NA | ||
| CgA, PST, NKA response to debulking | |||||||
| No response | 19 | 14 | 1.9 | .44 | 4 | NA | .79 |
| Biochemical resp. | 59 | 37 | 2.6 | 12 | NA | ||
PFS: progression free survival, OS: overall survival, CgA: chromogranin A, PST: pancreastatin
Figure 1. Kaplan-Meier curve of PFS (a) and OS (b) in all NET patients who were stratified by amount of hepatic disease debulked.
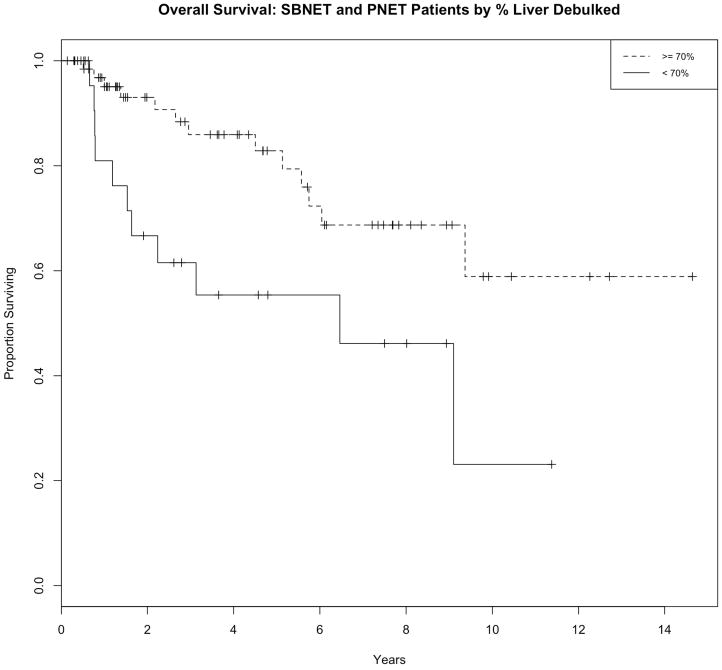
Patients enjoyed longer PFS and OS if 70% reduction of their hepatic tumor burden could be achieved.
Seventy-eight patients had serial biomarker results for at least 1 of the 3 markers analyzed and had elevated preoperative levels, and were therefore available for analysis. Seventy-five percent of patients with PNETs and 70% with SBNETs demonstrated a biochemical response in CgA, PST or NKA postoperatively (Table 2). Achieving a > 50% reduction at the 3 or 6 month follow up visit in any one of these biochemical markers from LDS was not associated with significantly greater PFS or OS. Successful 70% cytoreduction did not correlate with achieving a complete or partial biochemical response (p = 1).
Discussion
The optimal management of hepatic metastases in NETs has not been clearly defined, as prospective, randomized trials have not been performed. Such trials are implausible due to the rarity of this disease, the complexities associated with different primary sites, the highly variable number of lesions, their distribution, and the low likelihood that either patients or surgeons would agree to randomization. Many retrospective studies have been performed, but small, heterogeneous patient populations and application of a variety of surgical approaches hinder definitive conclusions (Table 5). One of the aims of this study was to determine the hepatic tumor burden in patients selected for cytoreduction and identify which factors correlated with improved survival, and thus guide selection of patients for LDS. We found that the majority of patients presented with bilobar hepatic disease, a median of 10-19% hepatic replacement, and approximately 10 lesions seen on imaging. Of these factors, the degree of hepatic replacement may be the best predictor of patient outcome after cytoreduction as patients with < 25% replacement demonstrated significantly improved PFS and OS compared to those with ≥ 25% of their liver replaced by tumor. Although the number of lesions identified preoperatively may be a proxy of the extent of hepatic disease and easier to determine than the degree of hepatic replacement, this was less consistent. In our series, patients had a median of 6 lesions treated during their debulking procedures. Those patients with ≥ 5-10 hepatic lesions progressed sooner than did those with fewer lesions, suggesting that a cutoff of 5-10 lesions may also serve as a useful predictor of which patients are likely to achieve greater benefit from cytoreduction of their hepatic disease.
Table 5. Comparison of recent studies detailing surgical management of NET hepatic metastases1,4-6,8,9,12,15,20-26.
| Study design | Surgical selection criteria | Surgical procedures used to treat liver metastases | Survival | Postoperative morbidity* | Postoperative mortality | |
|---|---|---|---|---|---|---|
| Elias, 2003 | Retrospective Analyzed patients with well differentiated NETs with liver metastases who underwent hepatectomy with curative intent (n = 47). |
Complete resection of hepatic disease, primary, regional LN and extrahepatic metastases possible Resection will leave sufficient hepatic reserve Operative risk < 5% |
Minor resection: 34% Major resection: 66% RFA: used along with resection in 10.6% |
5-year OS: 71% | Major and minor: 45% |
5% |
| Sarmiento, 2003 | Retrospective Examined consecutive patients with NET that underwent surgery for their hepatic metastases (n = 170) |
NR | Major resection: 54% | 5-year OS: 61% | Major and minor: 21.1% |
1.2% |
| Boudreaux, 2005 | Retrospective Examined patients with advanced NETs who underwent palliative surgery (not all with liver mets; n = 82) |
NR | Resection: 100% |
4-year OS: No liver mets or unilateral hepatic disease: 89% Bilobar hepatic disease: 52% |
Major and minor: 56% |
2.4% |
| Osborne, 2006 | Retrospective Patients with symptomatic NETs & liver metastases Compared patients who received hepatic embolization (n = 59) to those that received surgical cytoreductive treatment (n = 61) |
Primary and regional disease amenable to complete resection Goal to debulk 90% hepatic disease |
NR |
Median survival: Curative: 50 months Palliative: 32 months Embolization: 24 months |
Surgical cytoreduction: 3.3% |
Surgical cytoreduction: 1.7% |
| Hibi, 2007 | Retrospective Consecutive patients with advanced NETs and liver metastases (n = 21) who underwent liver resection for their hepatic disease |
Goal to resect 100% of metastatic disease | Resection: 100% | 5-year OS: 41% | Major only: 19% |
0% |
| Landry, 2008 | Retrospective Described experience of NET patients (n = 54) with hepatic metastases treated with surgical resection (n = 23) or nonsurgical liver-directed procedures (n = 31) |
NR | Major resection: 70% Wedge resection: 17% Major + wedge: 13% RFA: 17% |
5-year OS: Surgery: 75% No surgery: 62% |
Major only: 26% |
0% |
| Chambers, 2008 | Retrospective Patients with symptomatic, advanced NETs (n = 66) who were selected for hepatic debulking (n = 30) were analyzed for surgical morbidity and survival outcomes |
Goal 70% cytoreduction of hepatic disease | Major resection: 23% Wedge resection: 57% Ablation: 53% |
5-year OS: 74% | Major only: 23% |
0% |
| Elias, 2009 | Retrospective Reported feasibility of using a combination of resection and RFA to treat NET patients with substantial hepatic tumor burden (n = 16) |
Goal to treat 100% of hepatic and extra-hepatic metastases | Major resection: 56% Minor resection: 44% RFA: 100% |
3-year OS: 84% | Major only: 47% |
0% |
| Glazer, 2010 | Retrospective Evaluated patients with advanced NETs who underwent treatment for liver metastases (n = 172) |
Resection will leave sufficient hepatic reserve Adequate functional reserve Lesion correlation between radionuclide scan and conventional imaging All extrahepatic disease can be completely treated |
Resection: 73.3% RFA alone: 10.5% |
5-year OS: 77.4% | Clavien-Dindo grade I – IV: 22.1% |
0% |
| Akyildiz, 2010 | Retrospective Reported 15-year follow up of 89 NET patients treated with laparoscopic RFA |
Progressive disease Max tumor size 10 cm, max # of lesions 15 < 20% tumor volume involved Predominance of mets in the liver |
1 RFA procedure: 73% 2 RFA procedure: 21% 3 RFA procedure: 4.5% 4 RFA procedure: 1.1% |
5-year OS: 57% (after 1st procedure) | 5.6% | 1.1% |
| Mayo, 2010 | Retrospective, Multinstitutional Analyzed patients who underwent surgical treatment (resection, ablation or both) for hepatic NET metastases (n = 339). Included those with multiple hepatic surgeries. |
NR | Resection: 77.6% RFA: 2.9% Resection + RFA: 19.5% |
Median OS 1st operation: 125 mo 2nd operation: 141 mo from 1st operation, 89 mo from 2nd operation |
NR | NR |
| Norlen, 2012 | Retrospective Subset analysis of patients who underwent liver debulking procedures (n = 103) |
NR | Resection: 35.2% RFA: 42% |
5-year OS: Resection: 86% RFA: 94% No debulking: 53% |
Major only: 1.9% | Resection: 1.8% RFA: 2.9% |
| Taner, 2013 | Retrospective Examined patients with metastatic NETs that were debulked using a combination of resection and intraoperative ablation (n= 94). |
NR | Minor resection: 81% Major resection: 19% RFA: 100% |
5-year OS: 80% | Local complication in 1 patient | 0% |
| Boudreaux, 2014 | Retrospective Described experience with hepatic surgical cytoreduction of SBNET patients (n = 189) with stage IV disease. |
NR | Resection: 72% RFA: 31% |
5-year OS: 87% | Clavien-Dindo grade I-II: 57% Clavien-Dindo grade III-IV: 13% |
7% |
| Graf-Baker, 2014 | Retrospective Studied patients with advanced NETs who underwent hepatic debulking of metastases (n = 52) |
Patients evaluated intraoperatively and included in study if estimated that 70% cytoreduction possible | Formal resection: 31% Wedge resection: 98% |
5-year PFS: 64% | NR | NR |
| Current series | Retrospective Analyzed experience using parenchymal-sparing hepatic debulking procedures in SBNETs and PNETs with advanced disease (n = 108) |
Aim of 70% cytoreduction | PSD only: 95% PSD + major resection: 4% Major only: 1% |
5-year OS: 72% | Clavien-Dindo grade III-IV: 13% |
0% |
Complication rates are calculated as the number of reported complications divided by the number of patients included in the study. In most studies, the method used to classify complications was not mentioned, though the nature of complications reported has been clarified based on the author's description in the table, when possible. Major complications would include those that would fall under the Clavien-Dindo class III and IV categories. Minor complications include those that would fall under the Clavien-Dindo class I and II categories. Those papers that classified their complications using Clavien-Dindo have been indicated in the table.
The effect of hepatic debulking on symptomatic improvement has been well-established1,5,15, but its effect on hormone levels has been less well analyzed. In a study by Jensen et al., 18 of 19 patients (95%) had reduction of their CgA levels after hepatic debulking.16 Norlen et al. measured 5-HIAA levels in 103 patients undergoing hepatic debulking with ablation and/or resection and found significant reductions in postoperative levels.17 Nearly 70% of patients in the current study series had reductions in CgA, NKA or PST after undergoing cytoreduction. In many cases, this correlated with reduction or amelioration of symptoms. Interestingly, a reduction in biomarker levels ≥ 50% did not correlate with improved PFS or OS in any group. The results are likely to have been affected by the number of patients excluded due to incomplete data (n=27). The degree of cytoreduction achieved did not have a consistent correlation with postoperative biomarker levels, although this observation would also be influenced by the amount of extrahepatic disease remaining, progression in the interval prior to postoperative biomarker levels being drawn (usually done at 3 months), and the accuracy of the debulking estimates.
The optimal target for hepatic cytoreduction in NETs is still uncertain. McEntee et al.'s early experience with 37 NET patients set the debulking target at 90%,10 although the rationale for choosing this threshold is unclear. Nevertheless, this has continued to be considered the standard, as endorsed by Que et al. in 199518 and Mayo et al. in 2010.4 Unfortunately, this approach may exclude 67-90% of patients with hepatic metastases from surgical consideration.11 An alternative debulking target of 70% has been suggested recently. In 2008, Chambers et al. published their experience with 33 patients in whom a 70% debulking target was used. Utilizing a combination of resection and ablation, 74% 5-year OS was achieved.1 Graff-Baker et al. observed that equal proportions of patients had progression of their liver disease regardless of whether 70-89%, 90-99% or 100% of their hepatic tumor burden was cytoreduced, and therefore also advocated for using a 70% debulking threshold.12 In our series, debulking was attempted in a much higher proportion of patients (76%; 108/142) with hepatic NET metastases, although this denominator did not include patients seen with > 70% liver replacement who were not considered candidates for surgery, and patients known to have aggressive, high-grade tumors preoperatively. This denominator does include patients with diffuse, small metastases in whom we resected their primary tumors, but significant cytoreduction was not considered feasible. McEntee et al. and Glazer et al. noted that only operating upon patients for curative intent or with a goal of debulking 90% of their disease resulted in selection of only 9-25% of patients for surgery.9,19 In this study, we were able to demonstrate significantly improved PFS and OS in patients that achieved 70% cytoreduction. Interestingly, although 90% cytoreduction improved PFS in our patients, it did not significantly impact OS, perhaps due to the relatively small number of death events. These results support previous suggestions that a debulking target of 70% may be reasonable.
Regardless of surgical approach or margin status, nearly all patients will have recurrence of their hepatic disease.4 Thus, hepatic cytoreduction is primarily palliative, even when approached with curative intent, and multiple studies have documented equivalence in patient outcome with R0 versus R1/R2 resections.4,6,9 Adoption of PSD procedures accepts the inevitability of recurrence but benefits the patient by minimizing the amount of healthy liver tissue removed or damaged during the operation. Patients treated with PSD in the current series attained superior 5-year OS (72%) compared to that of Akyildiz et al. (57%) using laparoscopic RFA alone20. Further, PSD is associated with low morbidity and mortality (approximately 20-30% morbidity in most published reports). In this series, only 13% of patients experienced a major complication as a result of their debulking operation. There were no mortalities, pancreatic fistulas requiring drainage, bile duct injuries, nor hepatic abscesses noted. These results suggest these procedures may be performed safely and have the advantage of leaving more functional liver tissue intact.
The limitations of this study are the nonblinded fashion in which the percent of hepatic debulking was determined, the error introduced into the liver replacement estimates, and the lack of a control group. The lack of blinding could have introduced bias into the analysis, as the surgeon determining the extent of debulking could have been influenced by his knowledge of each patient's clinical course and status at follow up. Another potential issue is the accuracy of liver replacement and debulking estimates. Although the reviewing surgeon's assessments of preoperative liver replacement were very close to that of the radiologists (in the 27 cases where the radiologists made a call on percent replacement preoperatively), an approximate 10% margin of error should be taken for these estimates. The absence of control group in this study makes it difficult to determine whether the outcomes were a function of the hepatic debulking procedures used, or secondary to other factors. In SEER, the median survival of patients with PNETs and M1 disease is 2 years, and for SBNETs is 4.7 years7, which are significantly shorter than the current median survival of our groups at 10.5 years and survival not reached, respectively. This is not a fair comparison in that SEER patients may not have had their primaries removed, a low fraction probably had debulking surgery, and a lower percentage may have received Octreotide, embolization, or other therapies. However, our results do compare favorably to other series employing debulking (Table 5), many of which were highly selective to include only patients in whom 90% debulking could be achieved.
This series demonstrates that using primarily PSD procedures for NET metastases is safe, allows for treatment of the majority of patients, and gives survival results comparable to series primarily using resection (Table 5). This method is uniquely suited to NET patients, as multiple, bilobar metastases can be treated during a single operation, as well as the primary tumor. Healthy parenchyma is spared, maintaining the option of future cytoreductive procedures, and possibly reducing the risk of hepatic failure. The majority of our patients treated with PSD procedures had ≥ 70% of their hepatic disease debulked, with low rates of morbidity and no mortality. Further study is required to ascertain which patients are most likely to achieve 70% debulking of their disease, as our preliminary analyses failed to identify which patient or disease factors were associated with successful debulking. Finally, based on improvements in PFS and OS when 70% cytoreduction is achieved, our results support lowering the threshold for selecting patients for surgical debulking to 70% in whom this can be accomplished.
Acknowledgments
Funding: Supported by NIH 5T32#CA148062-05 (JEM, SKS)
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–53. doi: 10.1016/j.surg.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Well-Differentiated Neuroendocrine Tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753–66. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 3.Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–63. doi: 10.1016/j.surg.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Annals of Surgical Oncology. 2010;17:3129–36. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver. Journal of the American College of Surgeons. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extra-hepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery. 2003;133:375–82. doi: 10.1067/msy.2003.114. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of Clinical Oncology. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 8.Taner T, Atwell TD, Zhang L, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford) 2013;15:190–5. doi: 10.1111/j.1477-2574.2012.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB. 2010;12:427–33. doi: 10.1111/j.1477-2574.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091–6. [PubMed] [Google Scholar]
- 11.Chamberlain RS, Canes D, Brown KT, et al. Hepatic Neuroendocrine Metastases: Does Intervention Alter Outcomes? Journal of the American College of Surgeons. 2000;190:432–45. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery. 2014;156:1369–77. doi: 10.1016/j.surg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. Journal of Clinical Oncology. 2006;24:401–6. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications. Annals of Surgery. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne DA, Zervos EE, Strosberg J, et al. Improved Outcome with Cytoreduction Versus Embolization for Symptomatic Hepatic Metastases of Carcinoid and Neuroendocrine Tumors. Annals of Surgical Oncology. 2006;13:572–81. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 16.Jensen EH, Kvols L, McLoughlin JM, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Annals of Surgical Oncology. 2007;14:780–5. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 17.Norlen O, Stalberg P, Zedenius J, Hellman P. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours. British Journal of Surgery. 2013;100:1505–14. doi: 10.1002/bjs.9262. [DOI] [PubMed] [Google Scholar]
- 18.Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. American Journal of Surgery. 1995;169:36–42. doi: 10.1016/s0002-9610(99)80107-x. [DOI] [PubMed] [Google Scholar]
- 19.McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091–6. [PubMed] [Google Scholar]
- 20.Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288–93. doi: 10.1016/j.surg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Boudreaux JP, Putty B, Frey DJ, et al. Surgical Treatment of Advanced-Stage Carcinoid Tumors. Annals of Surgery. 2005;241:839–46. doi: 10.1097/01.sla.0000164073.08093.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibi T, Sano T, Sakamoto Y, et al. Surgery for Hepatic Neuroendocrine Tumors: A Single Institutional Experience in Japan. Japanese Journal of Clinical Oncology. 2007;37:102–7. doi: 10.1093/jjco/hyl140. [DOI] [PubMed] [Google Scholar]
- 23.Landry CS, Scoggins CR, McMasters KM, Martin RC., 2nd Management of hepatic metastasis of gastrointestinal carcinoid tumors. Journal of Surgical Oncology. 2008;97:253–8. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 24.Elias D, Goere D, Leroux G, et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. European Journal of Surgical Oncology. 2009;35:1092–7. doi: 10.1016/j.ejso.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Norlen O, Stalberg P, Oberg K, et al. Long-Term Results of Surgery for Small Intestinal Neuroendocrine Tumors at a Tertiary Referral Center. World Journal of Surgery. 2012;36:1419–31. doi: 10.1007/s00268-011-1296-z. [DOI] [PubMed] [Google Scholar]
- 26.Boudreaux JP, Wang Y, Diebold AE, et al. A Single Institution's Experience with Surgical Cytoreduction of Stage IV, Well-Differentiated, Small Bowel Neuroendocrine Tumors. Journal of the American College of Surgeons. 2014;218:837–45. doi: 10.1016/j.jamcollsurg.2013.12.035. [DOI] [PubMed] [Google Scholar]


