Abstract
The brain might be exposed to irradiation under a variety of situations, including clinical treatments, nuclear accidents, dirty bomb scenarios, and military and space missions. Correctly recalling tasks learned prior to irradiation is important but little is known about post-learning effects of irradiation. It is not clear whether exposure to X-ray irradiation during memory consolidation, a few hours following training, is associated with altered contextual fear conditioning 24 hours after irradiation and which brain region(s) might be involved in these effects. Brain immunoreactivity patterns of the immediately early gene c-Fos, a marker of cellular activity was used to determine which brain areas might be altered in post-training irradiation memory retention tasks. In this study, we show that post-training gamma irradiation exposure (1 Gy) enhanced contextual fear memory 24 hours later and is associated with reduced cellular activation in the infralimbic cortex. Reduced GABA-ergic neurotransmission in parvalbumin-positive cells in the infralimbic cortex might play a role in this post-training radiation-enhanced contextual fear memory.
Keywords: c-Fos, parvalbumin, contextual, fear memory, infralimbic, irradiation
1. Introduction
The brain might be exposed to irradiation under a variety of situations, including clinical treatments, nuclear accidents, dirty bomb scenarios, and military and space missions. Previous studies have investigated short- and long-term effects of pre-training/learning irradiation on learning and memory involving the hippocampus and cortex [1–11] and behaviors mediated by the dopamine reward system [12–15]. Compared to studies assessing cognitive function three months or longer following irradiation [12, 14, 16–18], fewer studies have examined earlier effects of irradiation on brain function. Manda et al showed that 56Fe (1.5 Gy at 500 MeV/n) increased the time male wild-type mice needed to locate the hidden platform in the water maze 30 days following irradiation but not at earlier time points [19, 20]. Recently, we showed early cognitive effects two weeks following 56Fe irradiation in wild-type (0.1 Gy at 500 MeV/n)[21] and human apoE mice (0.5 Gy at 500 MeV/n) [22].
In contrast to the studies described above, little is known about post-learning effects of irradiation. Correctly recalling tasks learned prior to irradiation is important and pertinent to assess. For example it is not clear whether exposure to X-ray irradiation a few hours following training, during memory consolidation, is associated with altered contextual fear conditioning 24 hours after irradiation.
In most studies, there is a bias to study a particular brain region that is pertinent to a specific behavioral or cognitive change seen following radiation exposure. Brain immunoreactivity patterns of the immediately early gene c-Fos, a marker of cellular activity [23–28], can be used to determine which brain areas might be altered in post-training irradiation memory retention tasks. In addition to neurons, glia also expresses c-Fos in brain [24, 29–34]. Cellular activation of c-Fos might be especially important following glutamate activation. C-Fos activation was reported in fibroblasts transfected with the glutamate receptor subunit GluR1 [35] and in glia cells involving activation of the metabotropic glutamate receptor 5 (mGlu5) [36].
GABA-ergic neurons are critical modulators of excitatory neurons in brain and reduced GABA-ergic function can have profound detrimental effects. Dis-inhibition involving reduced GABA-ergic neurotransmission and reduced levels of parvalbumin, a calcium binding albumin protein expressed in fast-spiking GABA-ergic inhibitory inter-neurons [37], is reported following in utero irradiation [38–40]. Contextual fear conditioning depends on enhanced synapses of hippocampal mossy fibers onto parvalbumin-positive neurons, resulting in increased feedforward inhibition connectivity and restriction of the number of c-Fos positive post-synaptic neurons at memory retrieval [41] and increases the percentage of neurons with higher parvalbumin reactivity [42]. The increase in feedforward inhibition connectivity involved a majority of the presynaptic terminals, restricted the numbers of c-Fos-expressing postsynaptic neurons at memory retrieval, and correlated temporally with the quality of the memory [42]. We hypothesized that alterations in the number of c-Fos positive cells and parvalbumin-c-Fos positive cells might be involved in the post-training effects of irradiation on contextual fear memory.
In this study, we assessed whether post-training gamma irradiation exposure will affect subsequent contextual fear memory. In addition, immunohistochemistry was used to determine the brain regions involved in the effects of post-training irradiation and whether altered activation of GABA-ergic cells might be involved in these effects.
2. Material and Methods
2.1 Animals
Five-week-old male C57Bl6/J wild-type mice (n = 52) purchased from the Jackson Laboratory (Bar Harbor, ME) were used for this study described below in detail. The mice were housed under a constant 12 hr light: 12 hr dark cycle. Food (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO) and water were provided ad libitum. All procedures were approved by Institutional Animal Care and Use Committee at the Oregon Health & Science University (OHSU, Portland, Oregon).
2.2 Contextual fear conditioning
Sixteen mice were cognitively trained in a contextual fear conditioning paradigm, involving a five-shock paradigm, consisting of 2-second 0.35 mA shocks, separated by 2-minute inter-shock-intervals (ISI), with the first shock at 2 minutes from the beginning of the trial. Sixteen mice were cognitively trained in an object recognition test (see below). The total length of the training session was 10 minutes. Two hours after training, all mice were brought to a room within the animal facility containing an X-ray irradiator (Rad Source RS2000 Biological Research Irradiator, Suwanee, GA). Half of the mice (n = 8 mice) were placed in a new mouse cage fitting in the irradiator and received whole body irradiation at a dose of 1 Gy (dose rate: 1.25 Gy/min). This dose and exposure time could be relevant to nuclear accidents, dirty bomb scenarios, and military missions. This is a relative low dose and not expected to cause significant cell death but has been shown to induce DNA damage that is repaired within 24 hours [43]. The other half of the mice (n = 8 mice) were placed in a new mouse cage and received a sham-irradiation procedure by being placed into the new cage for the same duration of time. Mice were randomly assigned to experimental group (irradiated or sham-irradiated). After fear-conditioning training, and prior to irradiation, mice were randomly sorted until all initial values (bodyweight, baseline-freezing, freezing levels after acquisition, etc.) were not significantly different between groups. The next day, or 24 hours after training, the mice were tested for recall of conditioned fear during a six min trial. All freezing data were analyzed using Med Associates software (Georgia and St. Albans, Vermont), as previously described [44]. The software analyzes freezing based on a proprietary algorithm scoring with freezing defined as no movement except respiration.
2.3 Object exploration test
To compare to the training and testing received for contextual fear conditioning, sixteen mice were cognitively trained and tested for novel object recognition test performed as described [45] but without habituation of the mice three days prior to the training day. In an independent experiment, twenty mice were cognitively trained and tested for novel object recognition following habituation of the mice three days prior to the training day. Mice were placed in an open field (16 × 16 inches, Kinder Scientific, Poway, CA) containing two identical objects and they were allowed to freely explore for 15 minutes. The next day, mice were placed again in the open field, but one familiar object was replaced with a novel object. Mice were allowed to explore for 15 minutes. Movement and time spent exploring each object was recorded and analyzed using Ethovision XT video tracking system (Noldus Information Technology, Sterling, VA). The open field arena and objects were cleaned with 5% acetic acid between mice and trials.
2.4 C-Fos Immunohistochemistry
Two hours following testing for contextual fear conditioning or novel object recognition, thirty-two mice (sixteen mice per cognitive test) were intracardially perfused with 20 ml phosphate-buffered saline (PBS) followed by 40 ml 4% paraformaldehyde. Brains were removed, stored overnight in 4% paraformaldehyde, and then transferred to 30% sucrose. The 2-hour time point was chosen based on previous studies demonstrating extensive induction of c-Fos in the mouse brain following an environmental exposure at that time point [46, 47]. Fixed brains were sectioned coronally into three-series of free-floating sections at 40 μm using a cryostat (Microm HM505E, MICROM international GmbH, Walldorf, Germany). One series of sections, containing a 1/3 representation of the brain with sections 120 μm apart was processed for immunohistochemical detection of c-Fos. A second series of sections was processed for c-Fos and parvalbumin double labeling (see below). For c-Fos alone immunohistochemistry, sections were rinsed in phosphate buffered saline (PBS), incubated in 1% hydrogen peroxide and 0.3% Triton-X (TX) in PBS (PBS-TX, Sigma T-9284)) for 10 minutes, again rinsed in PBS, then incubated in 10% normal goat serum (NGS) in PBS-TX for 1 hour. After rinsing in PBS, sections were incubated in primary antisera (c-Fos rabbit polyclonal: 1:5,000, Santa Cruz Biotechnology, sc52, Billerica, MA, USA) in 4% Normal Goat Serum (NGS) and PBS-TX overnight at room temperature. Sections were rinsed in PBS and incubated for 1 hour in biotinylated goat-anti rabbit antibody in PBS-TX (1:500, Vector Laboratories, Burlingame, CA) followed by rinses in PBS and a 1 hour incubation in avidin-biotin peroxidase complex (ABC Elite kit PK-6100 standard, Vector Laboratories). Following rinses in Tris buffered saline (TBS), sections were developed for visualization of c-Fos positive cells in a hydrogen peroxide/diaminobenzidine/TBS solution for 10 minutes, after which sections were rinsed in PBS and immediately mounted on slides. The following day, sections were dehydrated in ethanol, defatted in xylene, and coverslipped with Permount (Sigma Chemical Co., St. Louis MO, USA).
2.5 Colocalization- Dual Label Immunohistochemistry
To determine the co-localization of c-Fos and parvalbumin immunoreactivity in the mouse brain, we performed dual label immunohistochemistry. For the hippocampus, we only analyzed the dorsal hippocampus. For c-Fos/parvalbumin double-labeling, free-floating sections were rinsed with PBS 3 times, then blocked with 4% donkey serum in PBS-TX for 90 min. Sections were incubated in anti-c-Fos antibodies (1:250, rabbit, Santa Cruz Biotechnology, sc52, Billerica, MA) overnight. Sections were subsequently incubated in 1:200 donkey anti-goat Dylight 594 antibodies (Abcam, Cambridge, MA, USA) for 3 hours at room temperature. Sections were then rinsed in PBS 4 times (20 minutes each rinse) after which the same protocol was repeated using anti-parvalbumin (1:1500, mouse, Sigma, P3088) as primary antibody and 1:200 donkey anti-mouse Alexa 488 (Life Technologies) as the secondary antibody. Sections were slide mounted and coverslipped with antifade reagent to preserve fluorescent signal (Vectashield with 4′6-diamidoino-2-phenylindole (DAPI), Vector), light protected, and stored at 4°C.
Brain sections incubated without primary antibody were used as negative controls to test for c-Fos and parvalbumin immunoreactivity and showed no labeling.
2.6 Microscopy
Quantification of c-Fos positive cells was performed using an Olympus IX81 microscope (Olympus, Center Valley, PA, USA) equipped with Slidebook software (Intelligent Imaging Innovations, Inc., Denver, CO, USA). Brain regions were identified using the mouse brain atlas of Franklin and Paxinos (2007). Bilateral images of the dorsal dentate gyrus (dDG, Bregma −0.82 to −0.94), dorsal CA1 (dCA1, Bregma −0.82 to −0.94), dorsal CA3 (dCA3, Bregma −0.82 to −0.94), ventral dentate gyrus (vDG, Bregma −2.70 to −2.80), ventral CA1 (vCA1, Bregma −2.70 to −2.80), ventral CA3 hippocampal (vCA3, Bregma −2.70 to −2.80) regions, prelimbic area (PL, Bregma 1.94 to 1.78), infralimbic region (IL, Bregma 1.94 to 1.78), basolateral amygdala (BLA, Bregma −1.34 to −1.46), central nucleus of the amygdala (CEA, Bregma −1.34 to −1.46), suprachiasmatic nucleus (SCN, Bregma −0.82 to −0.94) and paraventricular nucleus (PVN, Bregma −0.82 to −0.94) were captured within 2 sections (120 μm apart) using a 10X objective. c-Fos immunoreactive cells (identified by black nuclear label) were quantified bilaterally within fixed area frames; PVN (box, 275 × 450 μm), paraventricular thalamus (PVT) (box, 790 × 410 μm each), bed nucleus of the stria terminalis (BNST) (box, 335 × 620 μm), central nucleus of the amygdala (CEA) (circle, 575 μm diameter), cingulate cortex (box, 425 × 400 μm), dentate gyrus (box, 850 × 420 μm), CA1 (box, 850 × 420 μm) hippocampal region, and CA3 (box, 850 × 420 μm) hippocampal region. Counting was performed using a double blinded non-stereological method as previously reported in our laboratory [28].
For colocalization of c-Fos/parvalbumin cells, confocal images were captured bilaterally within 3 sections using an Olympus IX81 confocal microscope with a 20X water objective. C-Fos/parvalbumin cells were quantified in the dDG, dCA1, dCA3, PL, IL, and BLA. Cells were considered colocalized if they expressed both c-Fos and parvalbumin immunoreactivity. Within brain regions, c-Fos and parvalbumin immunopositive cells were counted, in addition to cells expressing both c-Fos and parvalbumin immunoreactivity. These counts were used to determine both the total number and percentage of c-Fos positive cells that expressed parvalbumin. For all cell quantifications, cells were counted in both hemispheres of a given region in each section and summed. Cell counts for the three sections were averaged and are presented as cells per section.
2.7 Statistical Analysis
All statistical analyses were carried out using GraphPad (San Diego, CA) and SPSS (Chicago, IL, USA) software. For c-Fos quantification, data is presented as the average number of cells expressing c-Fos in each brain region. For dual-labeling quantification, data is presented as the percentage of parvalbumin cells expressing c-Fos. A student’s t-test was utilized for comparison of positive cells in sham versus irradiation conditions for each brain region individually. To analyze the percentage of c-Fos/parvalbumin positive cells, a repeated measures analysis was used first, with brain region as the repeated measure. If Mauchly’s Test of Sphericity was violated, a Greenhouse-Geisser correction was used.
3. Results
3.1 Cognitive Testing
Mice received post-training as illustrated in Fig. 1A. Mice that received post-training irradiation showed a dramatically increased contextual fear memory, as compared to sham-irradiated mice (t = 4.911, p < 0.0001, Fig. 1B). In the object recognition test of mice that were not habituated to the environment without the objects prior to the training day, there was a trend towards an increased exploration of the objects over the 15-min test on the testing day in irradiated compared to sham-irradiated mice but that did not reach significance (t = 1.734, p = 0.093, Fig. 1C). During the 15-min test, neither group showed preferential exploring of the novel object and there was no difference in exploring of the novel object between the two groups (data not shown). In the object recognition test of mice that were habituated to the environment without the object prior to the training day, there was a robust preference of preferential exploring of the novel object on the testing day (Fig. 1D). However, post-training irradiation did not affect novel object recognition.
Fig. 1.
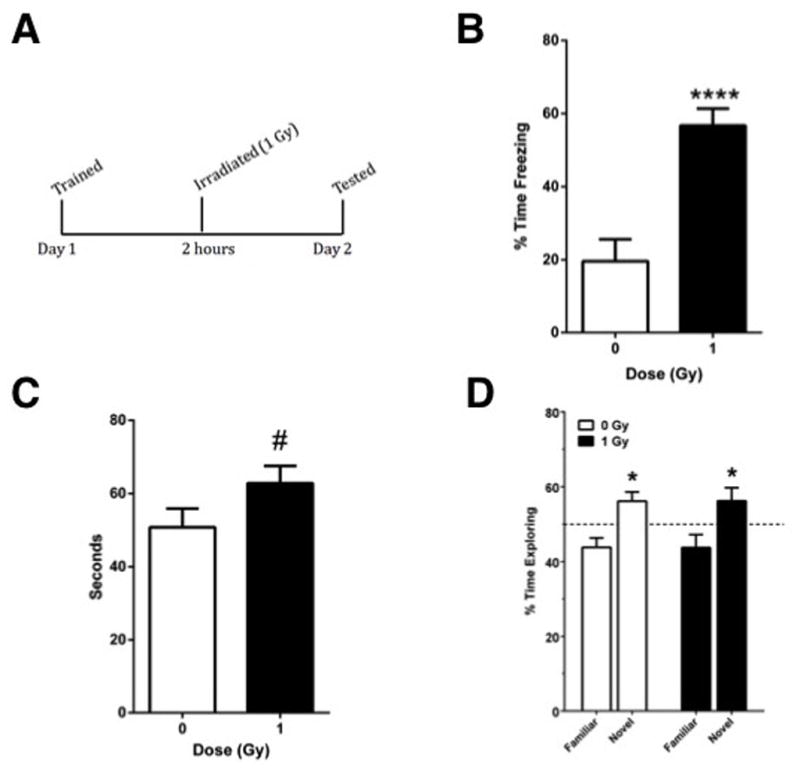
1A. Mice received post-training irradiation and were tested for contextual fear memory or object recognition the following day. 1B. Mice that received post-training irradiation showed increased contextual fear memory. 1C. In the object recognition test of mice not habituated to the environment without the objects prior to the training day, there was a trend towards an increased exploration of the objects over the 15-min test in irradiated compared to sham-irradiated mice (p = 0.093). ****p < 0.0001; n = 8 mice/test/radiation condition. 1D. Mice habituated to the environment without the objects prior to the testing day showed robust preferential exploring of the novel object but there was no effect of post-training irradiation on novel object recognition. *p < 0.05 versus the familiar object. n = 10 mice/radiation condition.
3.2 Quantification of c-Fos positive cells
See also Table 1 for a total overview the number of c-Fos positive cells in sham-irradiated and irradiated cells mice tested for contextual fear conditioning and object recognition. Fig. 2 shows representative immunohistochemical images of c-Fos positive cells in sham-irradiated and irradiated mice tested for contextual fear conditioning. Figs. 3–5 show the quantification. There was a significant interaction effect between radiation and test condition in the dDG (two-way ANOVA (F(1,27) = 4.290, p = 0.0480). There were significant effects of test in the dCA3 (F(1,28) = 10.08, p = 0.0036), dCA1 (F(1,27) = 6.617, p = 0.0159), and the vDG (F(1,27) = 4.378, p = 0.0459) as determined by two-way ANOVAs. In the IL of mice tested for contextual fear memory, there were less c-Fos positive cells in irradiated than sham-irradiated (t = 2.415, p = 0.0300, Fig. 3) mice. There was no significant difference in the number of c-Fos positive cells between sham and irradiated mice in any other brain region in mice tested for conditioned fear (Figs. 3–5). There were no significant differences in the number of c-Fos positive cells between sham and irradiated mice in any brain region in mice tested for object recognition (Fig. 3–5).
Table 1.
Number of c-Fos immunoreactive cells in mice of mice tested for object recognition and contextual fear memory.
| Object Recognition | Contextual Fear Memory | |||
|---|---|---|---|---|
| 0 Gy Mean ± SEM | 1 Gy Mean ± SEM | 0 Gy Mean ± SEM | 1 Gy Mean ± SEM | |
| dDG | 93.58 ± 5.010 | 103.6 ± 5.605 | 68.06 ± 3.807 | 58.47 ± 4.796 |
| dCA1 | 46.92 ± 3.750 | 43.96 ± 7.672 | 32.97 ± 3.584 | 33.38 ± 3.696 |
| dCA3 | 64.78 ± 7.218 | 64.57 ± 7.460 | 37.56 ± 3.165 | 50.59 ± 7.110 |
| vDG | 61.63 ± 2.422 | 68.50 ± 4.131 | 62.09 ± 4.350 | 51.94 ± 4.909 |
| vCA1 | 57.09 ± 5.197 | 57.75 ± 4.803 | 54.22 ± 3.955 | 48.27 ± 4.373 |
| vCA3 | 38.20 ± 3.000 | 44.77 ± 5.991 | 35.84 ± 3.044 | 41.03 ± 4.759 |
| IL | 196.3 ± 12.64 | 181.8 ± 20.59 | 178.7 ± 11.11 | 146.2 ± 7.580 |
| PL | 258.9 ± 25.12 | 269.3 ± 43.81 | 252.6 ± 27.96 | 219.2 ± 19.69 |
| BLA | 50.57 ± 4.508 | 55.46 ± 5.815 | 47.96 ± 3.910 | 43.58 ± 4.021 |
| CEA | 47.77 ± 5.479 | 46.91 ± 2.618 | 55.64 ± 3.607 | 50.75 ± 2.808 |
| SCN | 428.9 ± 29.89 | 411.9 ± 21.55 | 445.4 ± 24.42 | 409.4 ± 38.67 |
| PVN | 255.3 ± 18.24 | 265.1 ± 14.54 | 309.3 ± 23.16 | 271.4 ± 22.57 |
Fig. 2.
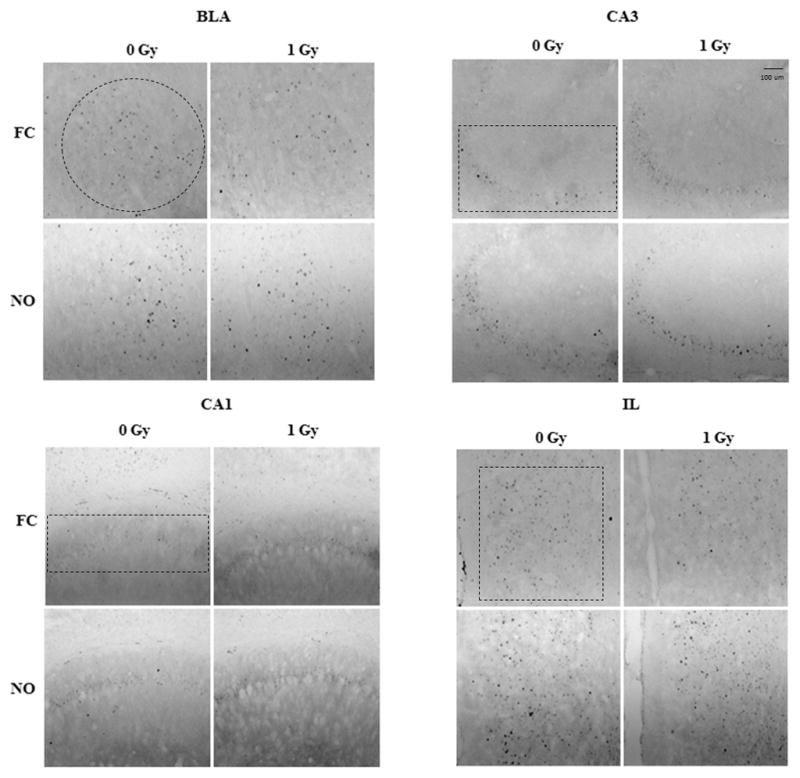
Representative images of c-Fos immunoreactive cells in the BLA, CA3, CA1, and IL of sham-irradiated and irradiated mice tested for contextual fear memory and object recognition.
Fig. 3.
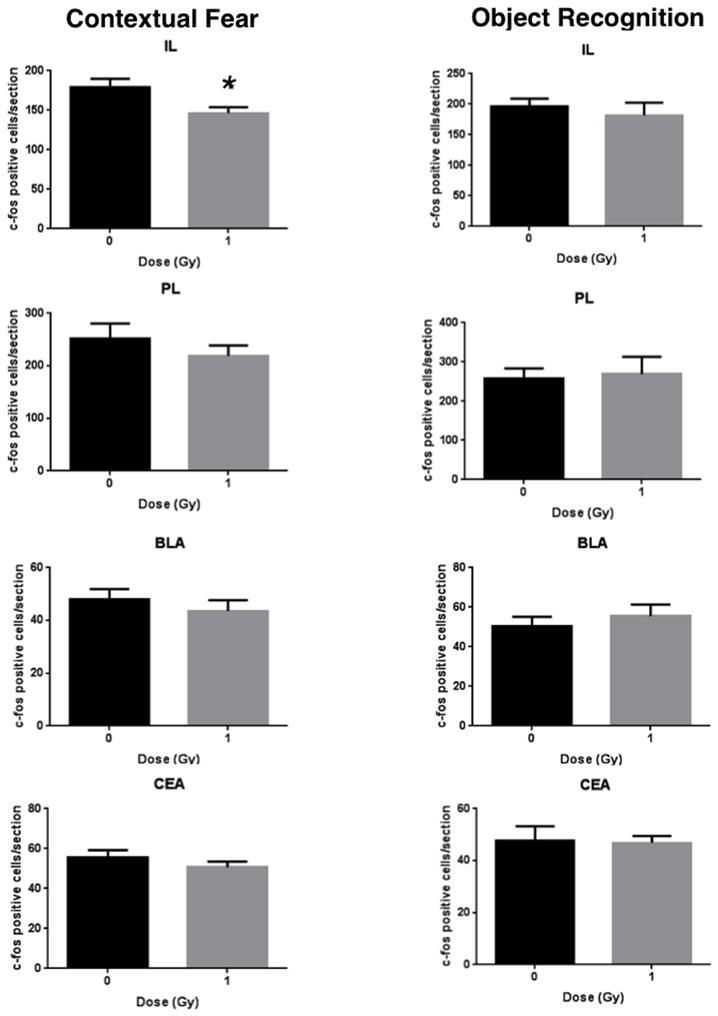
Number of c-Fos immunoreactive cells in sham-irradiated and irradiated mice tested for contextual fear memory and object recognition in the IL, PL, BLA, and CEA. *p < 0.05; n = 8 mice/test/radiation condition.
Fig. 5.
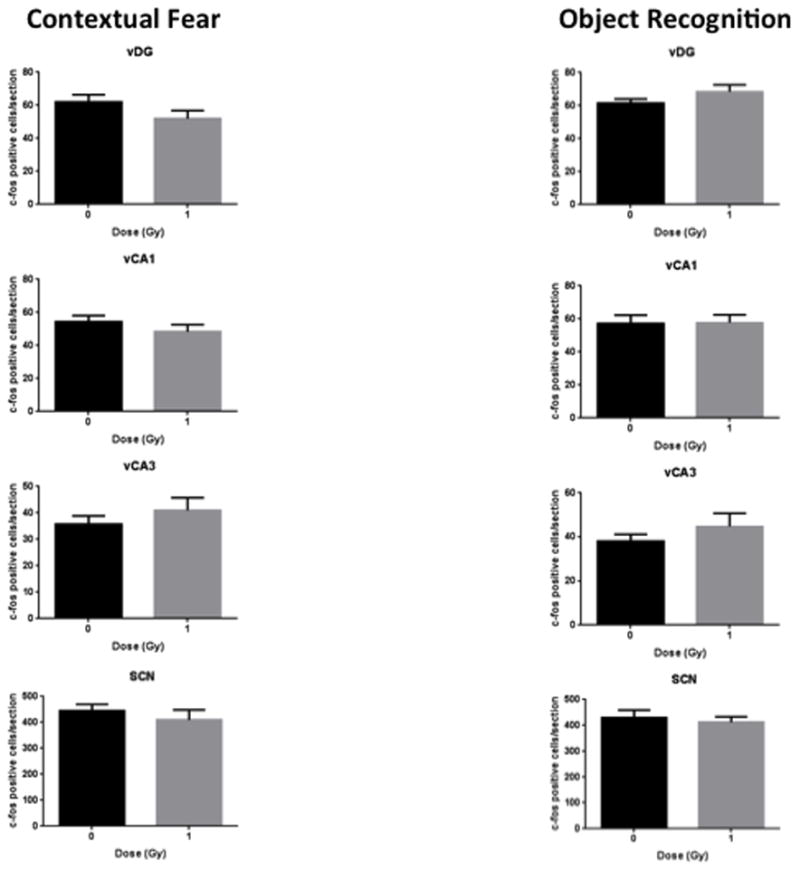
Number of c-Fos immunoreactive cells in sham-irradiated and irradiated mice tested for contextual fear memory and object recognition in the ventral hippocampus and SCN. n = 8 mice/test/radiation condition.
3.3 Quantification of dual labeled c-Fos and parvalbumin positive cells
Representative image of c-Fos parvalbumin co-labeled cells in sham-irradiated and irradiated mice tested for contextual fear conditioning and object recognition are shown in Fig. 6. Figs. 7 and 8 show the quantification. A repeated measures analysis, with brain region as the repeated measure, was first performed with both test groups. Mauchly’s Test of Sphericity indicated that the assumption of sphericity had been violated (χ2(2) = 33.006, p = 0.003), and therefore a Greenhouse-Geisser correction was used. There was a significant interaction between radiation, test condition, and brain region for the percentage of c-Fos/parvalbumin positive cells (F(1,27) = 3.061, p = 0.024). In mice tested for contextual fear memory, there was no significant difference in the percentage of parvalbumin/c-Fos-positive cells/section in any brain region but there was a trend towards a lower percentage of parvalbumin/c-Fos co-labeled cells in irradiated than sham-irradiated mice (Fig. 7). In contrast, in mice tested for object recognition, the percentage of parvalbumin/c-Fos co-labeled cells in the CA1 region of the hippocampus was lower in irradiated than sham-irradiated mice (t = 2.919, p = 0.009, Fig. 8).
Fig. 6.
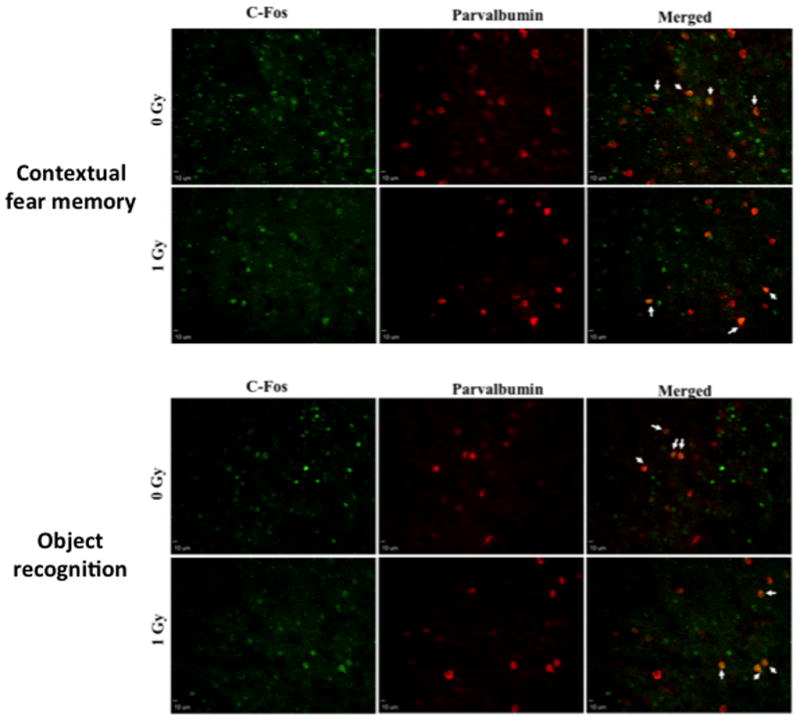
Representative images of c-Fos and parvalbumin immunoreactive cells in the IL of sham-irradiated and irradiated mice tested for contextual fear memory (A) and object recognition (B).
Fig. 7.
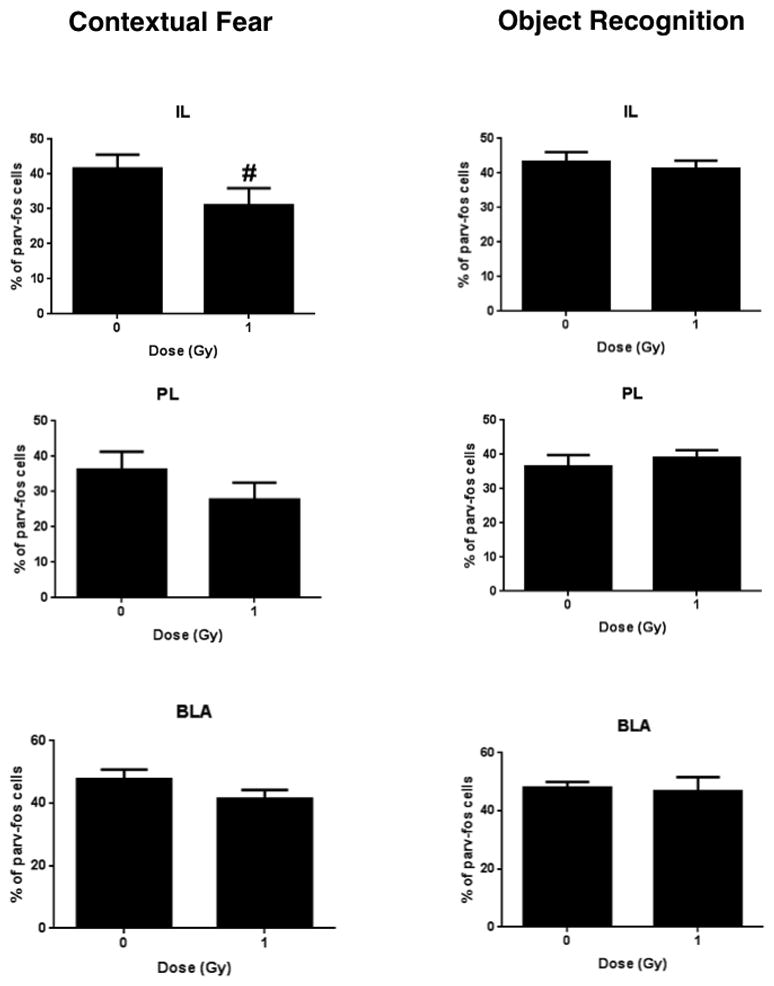
Number of c-Fos and parvalbumin immunoreactive cells in IL, PL, and BLA of mice tested for contextual fear memory and object recognition. There was a trend towards a lower percentage of parvalbumin/c-Fos co-labeled cells in the IL of irradiated than sham-irradiated mice (#p = 0.098). n = 8 mice/test/radiation condition.
Fig. 8.
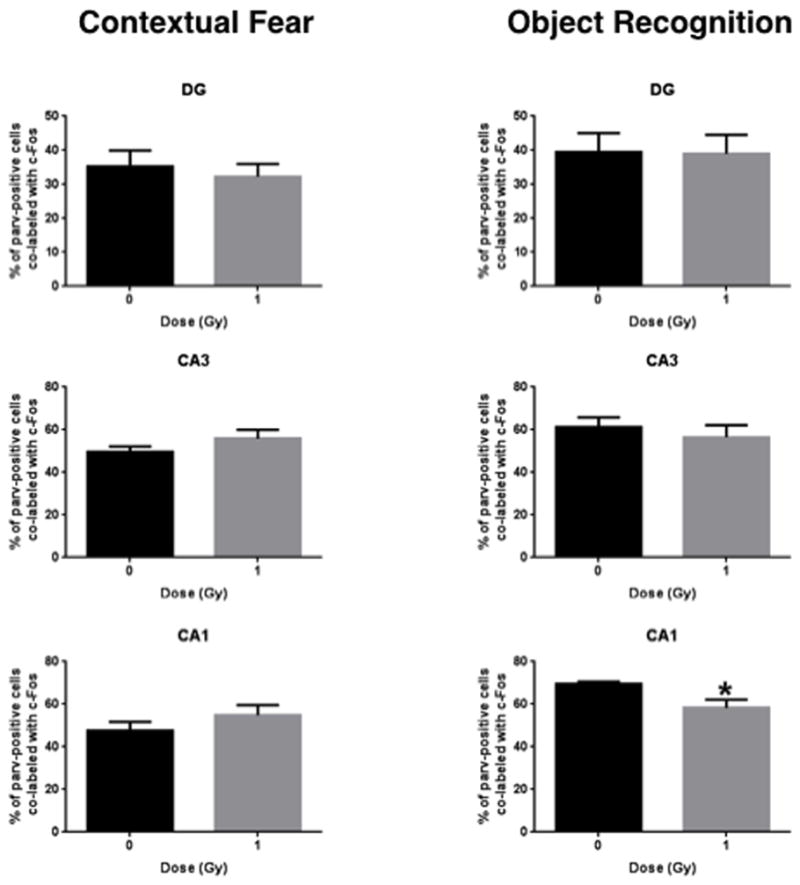
Number of c-Fos and parvalbumin immunoreactive cells in DG, CA3, and CA1 regions of mice tested for contextual fear memory and object recognition. *p = 0.0009; n = 8 mice/test/radiation condition.
4. Discussion
This study shows for the first time a profound increase in contextual fear memory a day following post-training whole body irradiation. The increased fear memory is associated with less c-Fos positive cells in the IL, suggesting an important role for the IL in radiation-enhanced contextual fear memory. The specific role for the IL observed in the current study is consistent with the prominent role of the IL in the regulation of fear learning and memory [48, 49]. Consistent with the post-training radiation enhanced contextual fear memory and fewer c-Fos positive cells in the IL in the current study, activation of the IL reduced conditioned fear and enhanced extinction learning [50]. In contrast to contextual fear memory, post-training whole body irradiation did not affect novel object recognition.
No significant effects of post-training irradiation in mice tested for contextual fear were seen in other hippocampal or cortical brain areas we analyzed. However, the effects of irradiation might be more wide spread in the brain and not limited to contextual fear memory-relevant circuitry under other test and radiation conditions. For example, release rates of GABA from pre-optic mediobasal hypothalamic areas of juvenile female rats were lower following brain only gamma irradiation (Co(60), 5 Gy) [51]. This radiation-enhanced contextual fear memory does not seem limited to gamma irradiation and is not necessarily limited to post-training irradiation either. In mice trained and tested for contextual fear conditioning three months following 28Si irradiation (600 MeV/n, 0.25 Gy), part of the outer-space environment, there was enhanced contextual fear memory associated with enhanced synaptic plasticity in the CA1 region of the hippocampus [52]. The 28Si data show that these radiation effects might be seen for prolonged periods following radiation exposure. Consistent with long-term effects, dis-inhibition involving reduced GABA-ergic neurotransmission and reduced levels of parvalbumin, a calcium binding albumin protein expressed in fast-spiking GABA-ergic inhibitory inter-neurons [37], is seen following in utero irradiation [38–40].
The effect of irradiation on c-Fos positive cells was specific for contextual fear memory and not seen in animals who received post-training irradiation and were subsequently tested for object recognition. Interestingly, 6-hydroxydopamine lesions of either the PL or IL did not affect novel object recognition when the time between the training and testing trial was 10 min, while it did affect recognition of a familiar object placed in a novel location when the time between these two trials was 10 min [53]. Aspiration lesion of the medial frontal cortex using controlled vacuum did not affect novel object recognition either, when the time between the training and testing trial was 5 min [54]. However, in mice tested for object recognition, the percentage of parvalbumin/c-Fos co-labeled cells in the CA1 region of the hippocampus was lower in irradiated than sham-irradiated mice. This in turn might relate to the trend towards increased exploration of the objects in the irradiated mice.
Parvalbumin-positive cells in the CA1 region of the hippocampus might be important for novel object recognition. Under conditions of pharmacological blockade of glutamatergic transmission, activation of muscarinic acetylcholine receptors strongly enhances the excitability of parvalbumin-positive cells in the CA1 region of the hippocampus and genetic elimination of M1 muscarinic receptors from parvalbumin-positive cells impairs novel object recognition [55]. Reduction of the AMPA-medicated current in parvalbumin-positive cells also impaired novel object recognition [56]. It is conceivable that the lower percentage of parvalbumin/c-Fos co-labeled cells in the CA1 region of the hippocampus of irradiated than sham-irradiated mice might relate to the increased time they spent exploring both objects on the test day. The experimental study design limited the behavioral testing for the object recognition and fear conditioning tests to two days only so that performance and brain activation could be more easily compared. Therefore, the mice were not habituated to the arena without the objects. Perhaps as a result of the lack of habituation to the area, there was no bias towards preferential exploration of the novel object of sham-irradiated mice on the test day and potential detrimental effects of post-training irradiation on novel object recognition could not be assessed in this experiment. Consistent with this notion, novel object recognition was seen in an independent experiment in which mice were habituated to the environment without the objects prior to the training day. As post-training irradiation did not affect novel object recognition, the effects of post-training irradiation might be specific for contextual fear memory.
In sham-irradiated and irradiated mice tested for either cognitive test the number of c-Fos positive cells was about twice as high in the dDG than dCA1. In contrast, using a completely different behavioral paradigm with pictures involving six days of training with two sessions per day, each containing two sets of 30 different pictures, the strongest increase in c-Fos was seen in the CA1 and a decrease was seen in the DG [57]. Future efforts are warranted to determine and compare the relative regional brain activation following distinct behavioral stimuli.
In summary, this study shows that post-training irradiation enhances contextual fear memory at a relatively low dose. Within the limitation of the number of brain areas analyzed in this study and the relatively similar number of c-fos or parvalbumin/c-fos positive cells in various brain regions, the IL might play an important role in these radiation effects. Future studies are warranted to determine the dose-response curves for these effects and whether they are dependent on the interval between radiation exposure and memory testing. The radiation-enhanced fear memory might reflect cognitive injury. Enhanced fear memory by itself can be problematic, especially when it is accompanied by impaired extinction of the fear memory.
Fig. 4.
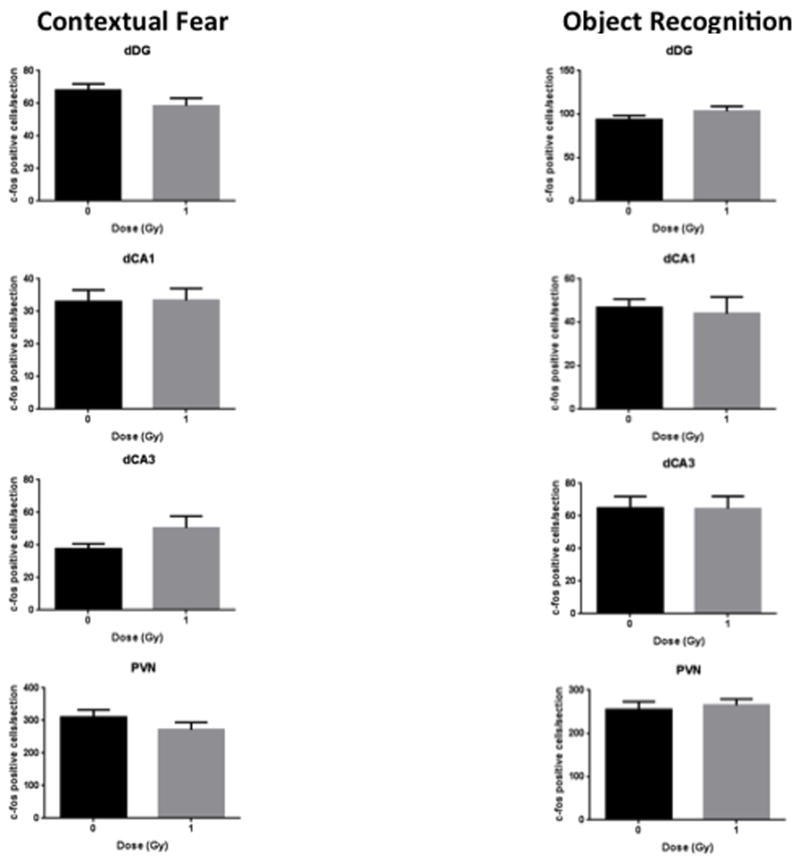
Number of c-Fos immunoreactive cells in sham-irradiated and irradiated mice tested for contextual fear memory and object recognition in the dorsal hippocampus and PVN. n = 8 mice/test/radiation condition.
Highlights.
Post-training irradiation enhances contextual fear memory.
This is associated with reduced neuronal activation in the infralimbic cortex.
This involves reduced GABA-ergic neurotransmission in the infralimbic cortex.
Acknowledgments
This work was supported by NIDA T32 DA007262, NASA NNJ12ZSA001N and NSCOR NNX10AD59G, The Murdock Trust, and the development account of Dr Raber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 2.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–63. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 3.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. International Journal of Radiation Oncology, Biology and Physics. 1995;31:983–98. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 4.Surmaho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56:1285–90. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 5.Raber J, Fan Y, Matsumori Y, Weistein PR, Fike JR, Liu J. Irradation attenuates neurogenesis in the dentate gyrus and exacerbates ischemia-induced functional deficits. Ann Neurol. 2004;55:381–90. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 6.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 7.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg S, Morhardt D, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Villasana L, Acevedo S, Poage C, Raber J. Sex- and ApoE Isoform-dependent effects of radiation on cognitive function. Rad Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H-L, Wu J, Zhu J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Dev Immunol. 2010:186813. doi: 10.1155/2010/186813. online May 31 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villasana L, Pfankuch T, Raber J. Isoform-Dependent Effects of apoE on Doublecortin-Positive Cells and Microtubule-Associated Protein 2 Immunoreactivity following 137Cs Irradiation. Radiat Environ Biophys. 2010;49:421–6. doi: 10.1007/s00411-010-0290-4. [DOI] [PubMed] [Google Scholar]
- 11.Villasana L, Benice T, Raber J. Long-term effects of 56Fe irradiation on spatial memory of mice: role of sex and apolipoprotein E isoform. Int J Radiat Oncol Biol Phys. 2011;80:567–73. doi: 10.1016/j.ijrobp.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Rabin B, Joseph J, Shukitt-Hale B. Heavy particle irradiation, neurochemistry and behavior; thresholds, dose-response curves and recovery of function. Adv Space Res. 2004;33:1330–3. doi: 10.1016/j.asr.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 13.Rabin B, Joseph J, Shukitt-Hale B, McEwen J. Effects of exposure to heavy particles on a behavior mediated by the dopaminergic system. Adv Space Res. 2000;25:2065–74. doi: 10.1016/s0273-1177(99)01014-5. [DOI] [PubMed] [Google Scholar]
- 14.Rabin B, Buhler L, Joseph J, Shukitt-Hale B, Jenkins D. Effects of exposure to 56Fe particles or protons on fixed ratio operant responding in rats. J Radiat Res (Tokyo) 2002;43(Suppl):S225–S8. doi: 10.1269/jrr.43.s225. [DOI] [PubMed] [Google Scholar]
- 15.Rabin B, Shukitt-Hale B, Joseph J, Carrihill-Knoll K, Carey A, Cheng V. Relative effectiveness of different particles and energies in disrupting behavioral performance. Radiat Environ Biophys. 2007;46:173–7. doi: 10.1007/s00411-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 16.Yeiser L, Villasana L, Raber J. ApoE isoform modulates effects of cranial 56Fe irradiation on spatial learning and memory in the water maze. Beh Brain Res. 2013;237:207–14. doi: 10.1016/j.bbr.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus. 2010;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin B, Carrihill-Knoll K, Hinchman M, Shukitt-Hale B, Joseph J, Foster B. Effects of heavy particle irradiation and diet on object recognition memory in rats. Adv Space Res. 2009;43:1193–9. [Google Scholar]
- 19.Manda K, Ueno M, Moritake T, Anzai K. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of alpha-lipoic acid. Beh Brain Res. 2007;177:7–14. doi: 10.1016/j.bbr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Manda K, Ueno M, Anzai K. Memory impairment, oxidative damage and apoptosis induced by space radiation: ameliorative potential of alpha-lipoic acid. Beh Brain Res. 2008;187:387–95. doi: 10.1016/j.bbr.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Haley G, Yeiser L, Olsen R, Davis M, Johnson L, Raber J. Early effects of whole body 5Fe irradiation on hippocampal function in C57BL/6J mice. Radiat Res. 2013;179:590–6. doi: 10.1667/RR2946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haley G, Villasana L, Dayger C, Davis MJ, Raber J. ApoE Genotype-Dependent Paradoxical Short-Term Effects of 56Fe Irradiation on the Brain. Int J Radiat Oncol Biol Phys. 2012;84:793–9. doi: 10.1016/j.ijrobp.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden AM, Baez M, Bergeron M, Schoepp DD. Increased c-Fos expression in the centromedial nucleus of the thalamus in metabotropic glutamate 8 receptor knockout mice following the elevated plus maze test. Neuroscience. 2003;121:167–78. doi: 10.1016/s0306-4522(03)00393-2. [DOI] [PubMed] [Google Scholar]
- 24.Dragunow M, Goulding M, Faull RL, Ralph R, Mee E, Frith R. Induction of c-fos mRNA and protein in neurons and glia after traumatic brain injury: pharmacological characterization. Exp Neurol. 1990;107:236–48. doi: 10.1016/0014-4886(90)90141-e. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. NeuroReport. 1996;7:1871–5. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]
- 26.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–85. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 27.French L, Pavlidis P. Relationships between gene expression and brain wiring in the adult rodent brain. PLoS Computational Biology. 2011;7:e1001049. doi: 10.1371/journal.pcbi.1001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuloaga D, Johnson L, Agam M, Raber J. Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J Neurochem. 2014;129:495–508. doi: 10.1111/jnc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dragunow M, Robertson HA. Brain injury induces c-fos protein(s) in nerve and glial-like cells in adult mammalian brain. Brain Res. 1988;455:295–9. doi: 10.1016/0006-8993(88)90088-1. [DOI] [PubMed] [Google Scholar]
- 30.Hisanaga K, Sagar SM, Hicks KJ, Swanson RA, Sharp FR. c-fos proto-oncogene expression in astrocytes associated with differentiation or proliferation but not depolarization. Brain Res. 1990;8:69–75. doi: 10.1016/0169-328x(90)90011-2. [DOI] [PubMed] [Google Scholar]
- 31.Onténiente B, Horellou P, Neveu I, Makeh I, Suzuki F, Bourdet C, et al. Cell-type-specific expression and regulation of a c-fos-NGF fusion gene in neurons and astrocytes of transgenic mice. Mol Brain Res. 1994;21:225–34. doi: 10.1016/0169-328x(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 32.Loret C, Sensenbrenner M, Labourdette G, Arenander AT, de Vellis J, Herschman HR. Differential phenotypic expression induced in cultured rat astroblasts by acidic fibroblast growth factor, epidermal g Induction of c-fos and TIS genes in cultured rat astrocytes by neurotransmitters. J Neurosci Res. 1989;24:107–14. [Google Scholar]
- 33.Arenander AT, de Vellis J, Herschman HR. Induction of c-fos and TIS genes in cultured rat astrocytes by neurotransmitters. J Neurosci Res. 1989;24:107–14. doi: 10.1002/jnr.490240115. [DOI] [PubMed] [Google Scholar]
- 34.Bennett M, Schwartz W. Are glia among the cells that express iimmunoreactive c-Fos in the suprachiasmatic nucleus? Neuroreport. 1994;5:1737–40. doi: 10.1097/00001756-199409080-00012. [DOI] [PubMed] [Google Scholar]
- 35.Gahring LC, Cauley K, Rogers SW. Kainic acid induced excitotoxicity and cfos expression in fibroblasts transfected with glutamate receptor subunit, GluR1. J Neurobiol. 1996;31:56–66. doi: 10.1002/(SICI)1097-4695(199609)31:1<56::AID-NEU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–40. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- 37.Hu H, Gan J, Jonas P. Fast-spiking parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science. 2014;345:6196. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 38.Roper S, King M, Abraham L, Boillot M. Disinhibited in vitro neocortical slices containing experimentally induced cortical dysplasia demonstrate hyperexcitability. Epilepsy Res. 1997;26:443–9. doi: 10.1016/s0920-1211(96)01014-5. [DOI] [PubMed] [Google Scholar]
- 39.Roper S, Eisenschenk S, King M. Reduced density of parvalbumin- and calbindin D28-immunoreactive neurons in experimental cortical dysplasia. Epilepsy Res. 1999;37:63–71. doi: 10.1016/s0920-1211(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 40.Deukmedjian A, King M, Cuda C, Roper S. The GABAergic system of the developing neocortex has a reduced capacity to recover from in utero injury in experimental cortical dysplasia. J Neuropathol Exp Neurol. 2004;63:1265–73. doi: 10.1093/jnen/63.12.1265. [DOI] [PubMed] [Google Scholar]
- 41.Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Caroni P. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature. 2011;473:514–8. doi: 10.1038/nature09946. [DOI] [PubMed] [Google Scholar]
- 42.Donato F, Romapani S, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–6. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 43.Hudson D, Kovalchuk I, Koturbash I, Kolb B, Martin O, Kovalchuck O. Induction and persistence of radiation-induced DNA damage is more pronounced in young animals than in old animals. Aging. 2011;3:609–20. doi: 10.18632/aging.100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen R, Marzulla T, Raber J. Impairment in extinction of contextual and cued fear following post-training whole body irradiation. Frontiers. 2014;8:231. doi: 10.3389/fnbeh.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giardino W, Pastor R, Anacker A, Spangler E, Cote D, Li J, et al. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H, Mingler M, McBride M, Murphy A, Valnenzuela D, Yancopoulos GD, et al. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology. 2010;35:1119–32. doi: 10.1016/j.psyneuen.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 49.Sotres-Bayton F, Sierra-Mercado D, Pardilla-Delgrado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–12. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson B, Baratta M, Biedenkapp J, Rudy J, Watkins L, Maier S. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem. 2010;17:591–9. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth C, Schmidberger H, Lakomek M, Witt O, Wuttke W, Jarry H. Reduction of gamma-aminobutyric acid-ergic neurotransmission as a putative mechanism of radiation induced activation of gonadotropin releasing-hormone-pulse generator leading to precocious puberty in female rats. Neurosci Lett. 2001;297:45–8. doi: 10.1016/s0304-3940(00)01663-3. [DOI] [PubMed] [Google Scholar]
- 52.Raber J, Rudobeck E, Allen A, Allen B, Rosi S, Nelson G, et al. 28Silicon radiation-induced enhancement of synaptic plasticity in the hippocampus of naive and cognitively tested mice. Radiat Res. 2014;181:362–8. doi: 10.1667/RR13347.1. [DOI] [PubMed] [Google Scholar]
- 53.Nelson A, Cooper M, Thur K, Marsden C, Cassaday H. The effects of catecholaminergic depletion within the prelimbic and infralimbic medial prefrontal cortex on recognition memory for recency, location, and objects. Behav Neurosci. 2011;125:396–403. doi: 10.1037/a0023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spanswick S, Dyck R. Object/context specific memory deficits following medial frontal cortex damage in mice. PLOSOne. 2012;7:e43698. doi: 10.1371/journal.pone.0043698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi F, Ball J, Stoll K, Satpute V, Mitchell S, Pauli J, et al. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol. 2014;592:3463–94. doi: 10.1113/jphysiol.2014.275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs E, Zivkovic A, Cunningham M, Middleton S, LeBeau F, Bannerman D, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Wan H, Aggleton J, Brown M. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–8. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


