Abstract
Background
The number of older patients who undergo emergent major abdominal procedures is expected to increase yet little is known about mortality beyond 30 days after surgery.
Objective
Identify factors associated with mortality among older patients at 30, 180 and 365 days after emergency major abdominal surgery.
Design
A retrospective study of the Health and Retirement Study (HRS) linked to Medicare Claims from 2000-2010.
Setting
N/A
Participants
Medicare beneficiaries > 65.5 years enrolled in the Health and Retirement Study (HRS) from 2000-2010, with at least one urgent/emergent major abdominal surgery and a core interview from the HRS within 3 years prior to surgery.
Main Outcomes and Measures
Survival analysis was used to describe all-cause mortality at 30, 180 and 365 days after surgery. Complementary log-log regression was used to identify patient characteristics and postoperative events associated with worse survival.
Results
400 patients had one of the urgent/emergent surgeries of interest. Of these 24% were > 85 years; 50% had coronary artery disease, 48% had cancer, and 33% had congestive heart failure; and 37% experienced a postoperative complication. Postoperative mortality was 20%, 31% and 34% at 30, 180 days and 365 days. Among those > 85 years, 50% were dead one year after surgery. After multivariate adjustment including postoperative complications, dementia (Hazard ratio (HR) 2.02, 95%CI 1.24-3.31), hospitalization within 6 months before surgery (HR 1.63, 95% CI 1.12-2.28) and complications (HR 3.45, 95%CI (2.32-5.13) were independently associated with worse one-year survival.
Conclusion
Overall mortality is high up to one year after surgery in many older patients undergoing emergency major abdominal surgery. The occurrence of a complication is the clinical factor most strongly associated with worse survival.
Keywords: Major abdominal surgery, One-year mortality, Surgical complications, Geriatric Surgery, Emergency Surgery
Introduction
In 2011 over half of surgical procedures requiring hospital stays were performed in patients aged 65 or older,1 and the demand for surgery among older adults is expected to rise in coming decades.2 Major abdominal surgeries are among the most common operations in older patients, and are also associated with high rates of postoperative mortality in this group.3 Moreover, older patients are more likely than their younger counterparts to undergo emergent, rather than elective, major abdominal procedures, which are themselves associated with higher rates of morbidity and mortality. Therefore, understanding the short and long-term consequences of emergent major abdominal procedures in older adults is of critical importance in meeting the needs of this growing population.
Compared to younger adults, older patients experience high rates of in-hospital death and complications, have longer lengths of hospital stay, and are more likely to be discharged to locations other than home after emergency major abdominal surgery.4-6 Although a significant portion of deaths after elective surgery occur more than 30 days after surgery,7 relatively little is known about longer-term survival in older patients after emergent abdominal surgery. The most rigorous data on survival after emergency major abdominal surgery focuses on in-hospital or 30-day outcomes.5,8 However, setting expectations for survival and recovery beyond the immediate postoperative period is particularly relevant for older adults who tend to prioritize function and quality of life over longevity,9 and may decline life-saving surgery if the expected outcome is not aligned with their healthcare priorities. A better understanding of the factors associated with survival in the first year after surgery would frame realistic expectations, and help patients and their physicians determine if emergent abdominal surgery is aligned with the patient's preferences and goals.
To address this knowledge gap, we used data from a national sample to characterize older patients who receive emergency major abdominal procedures; to describe survival rates during the first year after surgery; and to identify patient characteristics and hospital course factors associated with mortality during the first postoperative year.
Methods
This study was approved by the Partners Healthcare Human Research Committee and the Icahn School of Medicine at Mount Sinai Institutional Review Board.
Data Source
We used data from the 2000-2010 Health and Retirement Study (HRS) linked to Medicare claims. The HRS is a nationally representative, longitudinal study of older Americans which collects detailed data including demographics, health and function, family and caregiver characteristics, domicile, finances, and end-of-life care. Core interviews are conducted biennially, and exit interviews are conducted with a proxy within 2 years of a subject's death. Death dates were ascertained through 2011 using data obtained from the National Death Index (NDI).
Population
Patients were included if they had an inpatient Medicare claim, between July 1, 2000 and December 31, 2010, indicating an emergent or urgent hospital admission associated with any of the following abdominal surgery procedures: open appendectomy, open cholecystectomy, colectomy, laparotomy, abdominal hernia repair, intestinal resection, adhesiolysis, and pancreatic or gastric procedure (see ICD-9 codes in appendix A). If patients had multiple qualifying admissions during the study period, the primary surgical procedure from the most recent admission was considered the index procedure. The most recent admission was selected a priori to have the most recent data to reflect current practice. Eligible patients were aged > 65.5 years at the time of surgery, had fee-for-service Medicare Parts A and B and no Managed Medicare for a minimum of 6 months prior to surgery, and at least one core HRS interview within the three years prior to surgery.
Variables
The primary outcomes of interest were postoperative mortality rates at 30, 180 and 365 days calculated from the date of surgery until the date of death.
To describe the cohort and examine factors associated with survival, we ascertained baseline patient characteristics from the HRS dataset and Medicare claims, including: date and type of surgery, age (categorized as 65-74, 75-84, ≥85 years), gender, race and ethnicity (African-American, Hispanic, White or Other), comorbidities, nursing home residence, hospitalization in the 6-months prior to surgery, functional status, cognitive status, and self-reported health-related quality of life (excellent/very good/good vs. fair/poor).10 Functional status was measured as one's ability to perform 6 activities of daily living (ADLs) independently (vs. receiving help with any ADL). Cognitive status was measured using the telephone interview for cognitive status (TICS) score (normal >8, mild cognitive impairment [MCI] / demented 0-8, or missing/not measured).11 TICS scores were not measured if the core interview was conducted before the patient was 65 years old, or if the interview was conducted with a proxy. Previous efforts to determine the prevalence of frailty among the HRS cohort using three models of frailty showed that although 30.2% of all respondents were frail according to at least one model, only 3.1% were frail according to all three models.12 Therefore due to the imprecise nature of measuring frailty in the HRS, it was not included in this analysis. We identified comorbidities in Medicare claims up to 12 months before surgery using ICD-9 diagnosis codes. 13 We included cancer, diabetes, coronary artery disease, congestive heart failure (CHF), renal failure, and dementia. 14,15
We collected data from Medicare claims to describe the index hospitalization, during which the surgery occurred, including: length of stay (LOS), admission to the intensive care unit (ICU) and ICU LOS, postoperative complications and discharge destination (home, skilled nursing facility (SNF), other or unknown, or expired). The following complications5 (ICD-9 codes in Appendix B) were included: pulmonary, urinary tract infection, renal, infectious, cardiac, delirium, wound, and procedure-related.
Data Analysis
The unit of analysis was the most recent qualifying admission. We derived the date of surgery from Medicare Claims, and, for those with more than 1 qualifying surgical admission, calculated the elapsed time between admissions for each patient. We derived the date of death from the HRS-linked NDI, and verified this date with Medicare Claims when available. We identified patients as alive at one year if either the date of death was more than one year after surgery, or they had no date of death and either a follow up interview in HRS or additional Medicare Claims to verify that they were alive at one year.
We performed bivariate analyses using Fisher's exact test to compare characteristics between patients who were dead or alive at 30, 180 and 365 days, and reported results as means with standard deviations, percentages and p-values, as appropriate. One-year survival curves, stratified by age, were estimated using the Kaplan-Meier method.
All patients were accounted for at one year. Thus, because there was no censoring for mortality within one year, we estimated unadjusted mortality rates at 30, 180 and 365 days using the unadjusted proportions of patients who died within each time point. To identify patient characteristics associated with mortality, complementary log-log binary regression models were fit to survival dichotomized at each time point. The regression parameters of a complementary log-log regression model can be directly interpreted as log hazard ratios; fitting separate complementary log-log regression models at the three time points is semi-parametric in that it allows the hazard ratios to change over time (a typical Cox regression model assumes constant hazard ratios over time). In the primary analysis, we only included baseline patient factors available to the clinician before surgery. In secondary analysis, we included both patient factors and postoperative complications in our model as these would be available to the clinician during postoperative hospitalization. Data on discharge location were not included in the multivariable analysis. Both analyses included independent variables associated with mortality at a p<0.1 level in unadjusted bivariate analysis. To account for differences among patients in the time from the core interview to the date of surgery, we included days from interview to surgery as a variable in our analysis. To account for potential survivor bias, we also conducted sensitivity analysis, repeating the survival and multivariate analyses in a cohort which excluded patients with multiple procedures within the 10 year study period. We used STATA 11.1 (StataCorp LP, College Station, TX). A p-value of <0.05 was considered statistically significant.
Results
Among the 11,629 Medicare beneficiaries in the HRS cohort between 2000 and 2010, 400 (3.4%) had at least one of the emergent/urgent major abdominal surgeries of interest and at least one core HRS interview within the 3 years prior to surgery. These 400 patients comprised our study cohort. The mean time from the core interview to surgery was 401 days (SD +/- 246 days, median 388 days). Of 400 patients in our cohort, 49 had more than one qualifying admission during the study period (median days 333.0, IQR 113-1,084 days); 11 had two admissions within 90 days. Among the latter, we judged 4 procedures to be clinically related to the prior qualifying procedure: two were incisional hernia repairs (2 months and 1 month apart), one was reopening laparotomy (11 days), and one was endoscopy for bleeding after a bowel resection (7 days).
A total of 24% of patients were ≥ 85 years, 60% were female and 78% were non-Hispanic white. The proportion of patients with comorbidities at baseline were: coronary artery disease (CAD), 50%; cancer, 48%; congestive heart failure (CHF), 33%; diabetes, 34%; renal failure, 19%; and dementia 13%. Forty-nine percent of patients experienced at least one postoperative complication. Most patients (53%) were discharged home, however, 19% were discharged to a SNF and 16% died in the hospital.(Table 1) The mean hospital length of stay was 13 days (SD+/-11 days); 32% were admitted to the ICU and the mean ICU LOS was 5.79 days (SD + 7.93).
Table 1. Patient Characteristics and Mortality over One Year (univariate analysis).
| Mortality | |||||||
|---|---|---|---|---|---|---|---|
| 0-30 Day | 0-180 Day | 0-365 Day | |||||
| % | % | p-value | % | p-value | % | p-value | |
| Overall sample (N=400) | 19.8 | 31.0 | 34.3 | ||||
| Age at surgery | |||||||
| 65-74 | 36.0 | 12.5 | 20.8 | 22.9 | |||
| 75-84 | 40.0 | 19.4 | 0.002 | 29.4 | <0.001 | 35.0 | <0.001 |
| 85+ | 24.0 | 31.3 | 49.0 | 50.0 | |||
| Sex | |||||||
| Female | 60.0 | 20.8 | 0.53 | 31.7 | 0.74 | 35.4 | 0.59 |
| Male | 40.0 | 18.1 | 30.0 | 32.5 | |||
| Race & Ethnicity | |||||||
| African American | 14.5 | 22.4 | 36.2 | 37.9 | |||
| Hispanic | 5.8 | NR | 0.69 | 34.8 | 0.61 | 43.5 | 0.48 |
| White | 78.0 | 19.9 | 30.1 | 33.3 | |||
| Other | 1.8 | NR | NR | NR | |||
| Comorbidities | |||||||
| Coronary artery disease | 49.8 | 23.1 | 0.10 | 37.2 | 0.009 | 40.2 | 0.02 |
| Cancer | 47.5 | 17.9 | 0.38 | 30.5 | 0.91 | 34.2 | >0.999 |
| CHF | 32.8 | 32.1 | <0.001 | 46.6 | <0.001 | 50.4 | <0.001 |
| Diabetes | 34.0 | 25.0 | 0.06 | 35.3 | 0.21 | 38.2 | 0.27 |
| Renal failure | 19.3 | 33.8 | 0.001 | 53.2 | <0.001 | 53.2 | <0.001 |
| Dementia | 12.5 | 32.0 | 0.04 | 54.0 | <0.001 | 60.0 | <0.001 |
| Hospital admission within 6 months of surgery | |||||||
| Yes | 37.5 | 24.0 | 0.12 | 42.7 | <0.001 | 48.0 | <0.001 |
| No | 62.5 | 17.2 | 24.0 | 26.0 | |||
| Nursing home resident | |||||||
| Yes | 3.0 | 41.7 | 0.07 | 50.0 | 0.20 | 50.0 | 0.35 |
| No | 97.0 | 19.1 | 30.4 | 33.8 | |||
| Functional Status | |||||||
| ADL - Independent | 82.5 | 17.6 | 0.02 | 27.9 | 0.004 | 31.5 | 0.02 |
| ADL - Any Dependence | 17.5 | 30.0 | 45.7 | 47.1 | |||
| TICS categorical | |||||||
| TICS >8 Normal | 89.3 | 20.4 | 31.4 | 34.5 | |||
| 0-8 MCI or Demented | 2.8 | NR | 0.57 | 45.5 | 0.34 | 54.5 | 0.21 |
| TICS Missing / Unknown | 8.0 | NR | 21.9 | 25.0 | |||
| Self reported health | |||||||
| Excellent / Very Good / Good | 51.5 | 13.6 | 0.002 | 23.3 | 0.001 | 25.7 | <0.001 |
| Fair/Poor | 48.5 | 26.3 | 39.2 | 43.3 | |||
| Any complication | |||||||
| Yes | 37.5 | 37.3 | <0.001 | 52.0 | <0.001 | 56.7 | <0.001 |
| No | 62.5 | 9.2 | 18.4 | 20.8 | |||
| Discharge location | |||||||
| Home | 53.8 | 2.3 | 7.9 | 11.2 | |||
| SNF | 18.8 | NR | 0.002 | 30.7 | <0.001 | 36.0 | <0.001 |
| Other or Unknown | 11.5 | 15.2 | 43.5 | 47.8 | |||
| Expired | 16.0 | 98.4 | <0.001 | 100 | <0.001 | 100 | <0.001 |
*Complications include urinary tract infections, pulmonary embolus, pneumonia, surgical site infection, respiratory failure, acute kidney failure, and acute myocardial infarction
NR = not reported (HRS guidelines do not permit reporting categories with < 5 respondents)
**Not 100% due to in-hospital death beyond 30 days.
Mortality
Overall mortality rates were 19.8%, 31.0%, and 34.3% at 30, 180, and 365 days respectively. (Figure 1) Among 137 patients who died in the first year after surgery, 46.7% (n=64) died in hospital, 57.7% (n=79) died within 30 days after surgery and 90.5% (n=124) died within 180 days after surgery. In unadjusted analysis, mortality rates were highest among patients ≥ 85 years at 30 days (31.3% vs. 12.5% (among those 65-74 years), p=0.001), 180 days (49.0% vs. 20.8%, p<0.001), and 365 days (50.0% vs. 22.9%, p<0.001). Other patient characteristics associated with mortality at 30 days, 180 days and 365 days included CHF, renal failure, dementia, dependence in any ADL and fair/poor self reported health. CAD and hospital admission in the 6 months prior to surgery were associated with mortality at 180 and 365 days. Complications and discharge to a location other than home were also associated with mortality at 30 days, 180 days and 365 days. (Table 1)
Figure 1. One Year Survival for Older Adults After Major Emergency Abdominal Surgery Stratified by Age.
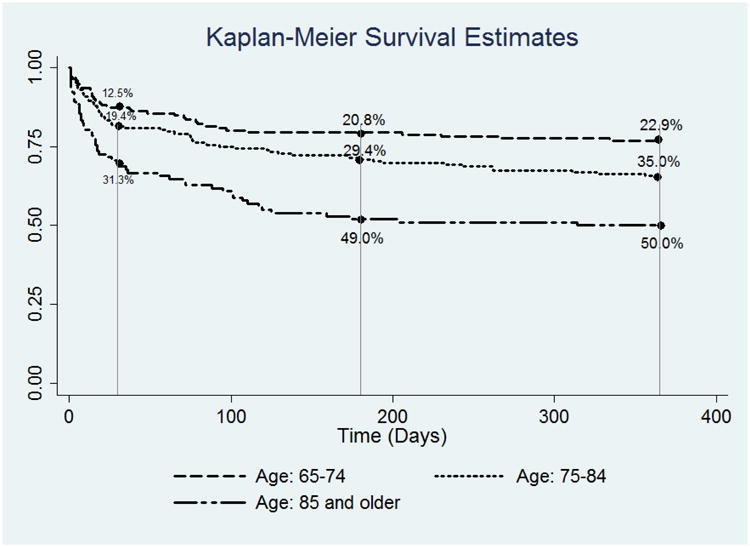
In the primary adjusted analysis (including only factors known prior to surgery), two factors were associated with increased mortality at all time points over the first year after surgery: age > 85 (30 days: Hazard Ratio, 2.18; 95% Confidence Interval (1.16 - 4.08); 180 days: 2.07 (1.25-3.43); 365 days: 1.93 (1.18 - 3.15)) and renal failure (30 days: 1.67 (1.00 - 2.77); 180 days: 1.96 (1.30-2.98); 365 days: 1.64 (1.09 - 2.47)). CHF was associated with increased mortality at 30 days. Dementia and hospitalization in the 6 months prior to surgery were associated with death at 180 and 365 days. CHF and fair/poor self-reported health increased the hazard of death at 365 days. (Table 2)
Table 2. Baseline Patient Factors Associated with Mortality Up to One Year After Emergent Major Abdominal Surgery.
| Characteristic | 0-30-Day Hazard Ratio (95% Confidence Interval) | 0-180-Day Hazard Ratio (95% Confidence Interval) | 0-365-Day Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|
| Age | |||
| Age: 75-84 | 1.35 (0.74 - 2.45) | 1.23 (0.76 - 1.98) | 1.41 (0.90 - 2.20) |
| Age: 85+ | 2.18 (1.16 - 4.08) | 2.07 (1.25-3.43) | 1.93 (1.18 - 3.15) |
| Comorbidities | |||
| Coronary artery disease (CAD) | 0.90 (0.54 - 1.49) | 1.08 (0.72 - 1.63) | 0.99 (0.67 - 1.47) |
| Cancer | 0.89 (0.56 - 1.40) | 0.98 (0.68 - 1.42) | 0.99 (0.70 - 1.41) |
| Congestive heart failure (CHF) | 1.86 (1.08 - 3.19) | 1.48 (0.95 - 2.28) | 1.52 (1.00 - 2.29) |
| Diabetes | 1.20 (0.74 - 1.96) | 0.94 (0.63 - 1.42) | 0.95 (0.64 - 1.39) |
| Renal failure | 1.67 (1.00 - 2.77) | 1.96 (1.30 - 2.98) | 1.64 (1.09 - 2.47) |
| Dementia | 1.38 (0.76 - 2.51) | 1.63 (1.01 - 2.65) | 1.82 (1.15 - 2.90) |
| Hospital admit within 6 months prior to surgery | 1.03 (0.63 - 1.68) | 1.58 (1.07 - 2.33) | 1.75 (1.21 - 2.54) |
| Assistance received for any Activities of Daily Living (ADL) | 1.01 (0.56 - 1.81) | 1.00 (0.61 - 1.62) | 0.87 (0.54 - 1.39) |
| Self-Reported Health: Fair/Poor | 1.62 (0.98 - 2.70) | 1.36 (0.90 – 2.05) | 1.52 (1.03 - 2.24) |
| Days between core interview and surgery | 1.00 (1.00 – 1.00) | 1.00 (1.00 – 1.00) | 1.00 (1.00 – 1.00) |
Base categories are Age: 65-74, Self-reported health: Excellent/very good/good, ADL: Independent.
Bold indicates statistical significance at p<0.05
In the secondary adjusted analysis (including postoperative complications as a covariate), postoperative complications were the factor most strongly associated with higher hazard of death at all time points (30 days: 7.16 (3.73-13.75); 180 days: 3.57 (2.34-5.44); 365 days: 3.45 (2.32-5.13). Hospital admit within 6 months was no longer significant at 180 days and renal failure was no longer significantly associated death at 365 days. (Table 3)
Table 3. Predictors of Postoperative Mortality Up to One Year After Emergent Major Abdominal Surgery (Including Post-Operative Complications).
| Characteristic | 0-30-Day Hazard Ratio (95% CI) | 0-180-Day Hazard Ratio (95% CI) | 0-365-Day Hazard Ratio (95% CI) |
|---|---|---|---|
| Age | |||
| Age: 75-84 | 1.21 (0.66 - 2.23) | 1.17 (0.72 – 1.90) | 1.34 (0.85 - 2.11) |
| Age: 85+ | 1.89 (1.00 – 3.55) | 1.96 (1.18 – 3.26) | 1.77 (1.08 - 2.90) |
| Comorbidities | |||
| Coronary Artery Disease (CAD) | 0.77 (0.47 – 1.27) | 0.98 (0.65 – 1.48) | 0.89 (0.60 – 1.33) |
| Cancer | 1.08 (0.68 – 1.71) | 1.13 (0.78 – 1.65) | 1.12 (0.78 – 1.61) |
| Congestive Heart Failure (CHF) | 1.90 (1.10 – 3.24) | 1.53 (0.98 – 2.38) | 1.60 (1.05 - 2.44) |
| Diabetes | 1.02 (0.62 – 1.68) | 0.82 (0.54 – 1.25) | 0.82 (0.55 – 1.22) |
| Renal Failure | 1.43 (0.85 - 2.39) | 1.83 (1.20 – 2.79) | 1.50 (0.99 - 2.27) |
| Dementia | 1.35 (0.72 - 2.53) | 1.80 (1.08 – 3.01) | 2.02 (1.24 – 3.31) |
| Hospital admit within 6 months prior to surgery | 0.91 (0.58 – 1.50) | 1.46 (0.98 – 2.16) | 1.63 (1.12 - 2.38) |
| Activities of Daily Living (ADL): any dependence | 1.34 (0.72 - 2.47) | 1.26 (0.76 – 2.09) | 1.05 (0.64 – 1.72) |
| Self-Reported Health: fair/poor | 1.46 (0.87 - 2.45) | 1.23 (0.81 – 1.87) | 1.39 (0.94 - 2.07) |
| Any complications | 7.16 (3.73 – 13.75) | 3.57 (2.34 – 5.44) | 3.45 (2.32 – 5.13) |
| Days between core interview and surgery | 1.00 (1.00 – 1.00) | 1.00 (1.00 – 1.00) | 1.00 (1.00 – 1.00) |
Base categories are Age: 65-74, Self-reported health: Excellent or very good, ADL: Independent.
Bold indicates statistical significance at p<0.05
In the sensitivity analyses, overall survival at 30, 180 and 360 days was 21%, 32% and 36% (compared to 20%, 31% and 34% in the study cohort) respectively. In multivariate analyses, the overall pattern of results were unchanged while there were a few small changes in statistical significance, likely due to change in sample size.
Discussion
We used the nationally representative HRS linked to Medicare claims to describe mortality in older patients up to one year after emergency major abdominal surgery. We found that these surgeries were not uncommon and were associated with high one-year mortality. Among patients older than 85, over half died within the first postoperative year. Among patient characteristics that would be available to clinicians before surgery, advanced age, congestive heart failure, renal failure, dementia, and poor self reported health were all associated with increased risk of death during the first year after surgery. However, the occurrence of postoperative complications during the index hospitalization was the factor most strongly associated with worse survival up to one year after the operation.
Our data corroborate previous studies demonstrating that emergent major abdominal surgeries in older patients are high-risk procedures associated with considerable morbidity and mortality in the short-term, and extends this knowledge by demonstrating that patients remain at high risk of mortality well after the immediate postoperative period. Thirty-day mortality was 20% in this surgical cohort. When compared to 11% 30-day mortality in a medical cohort of hospitalized Medicare Beneficiaries of the same age range and with similarly high prevalence of comorbidities,16 these data suggest that even after surgery, mortality risk is higher. Massarweh et al. used the Washington State database to examine morbidity and mortality up to 90 days after surgery.5 As in our study, these investigators found that morbidity and mortality increased with advancing age and that postoperative complications were strongly associated with death up to 3 months. However, they did not characterize factors most closely associated with mortality over time, nor did they distinguish mortality rates at 30 and 90 days. In a single-center retrospective study, Gazala et al. sent a survey to octogenarians who were within 3 years of having undergone emergency abdominal surgery.l They found that 55% were alive at the time the surveys were sent, and that among survivors, mortality rates were 39%, 45%, and 50% at 1, 2, and 3 years, respectively.17 However, the study design did not allow investigators to report characteristics associated with improved survival, and was limited by survivor bias. Our study shows that most patients, who die in the first year after surgery, die after they leave the hospital and that most of those, die within 6 months. The implications of this are significant in that hospital and 30-day mortality are inadequate to fully capture the increased mortality risk associated with these procedures, and to inform clinical decisions, advance care planning, and frame expectations for recovery. Increased mortality may be due to factors other than surgery. However, mortality measured over a full year after surgery, can inform the evaluation of the individual patient's time until treatment equipoise,18 specifically by weighing the risk of mortality without surgical intervention to that risk after surgery, among a population with limited life expectancy overall. For many patients, risk of other outcomes (e.g., extended hospital admission or loss of functional dependence) may be of greater concern than mortality alone.19
The prevalence of comorbid conditions in this population deserves special consideration. Patients with chronic underlying organ dysfunction have shortened life expectancy, and the association between these conditions and poor surgical outcomes are well described. For example, older patients with end stage renal disease undergoing elective vascular surgery have over four times higher odds of 30-day postoperative mortality,20 and older patients with congestive heart failure have over three times higher odds of in-hospital mortality after elective colon surgery.21 Patients with cardiopulmonary and renal disease have impaired ability to tolerate volume shifts associated with major abdominal surgery, and those with sarcopenia or malignancy may be unable to tolerate the increased metabolic demands of wound healing or infection.22 Frailty, is increasingly recognized as an important predictor of adverse postoperative outcomes including complications, in hospital and 6 month mortality, and institutional discharge among older patients. Unfortunately the HRS lacks physiologic details, and a robust measure of frailty, to fully explain how these conditions contributed to worse postoperative outcomes. Nonetheless, these findings support the need for surgical decision making within the context of the patient's overall health.
Our study also reveals patient factors, specifically dementia, heart failure, and hospitalization within 6 months prior to surgery that were associated with a higher risk of death at one-year. As a consequence, even though hospital discharge is an important milestone in recovery, certain patients continue to have a high mortality risk during the first weeks and months after discharge. Thus, patients at high risk of death in the first six months after surgery could benefit from specialized clinical pathways to reduce postoperative complications, including counseling regarding advance care planning, goal setting, and transitions to more palliative approaches to care when appropriate. This is particularly relevant in this cohort where serious life-limiting illnesses including renal failure, dementia, CHF and cancer were highly prevalent.
An advantage of the HRS is the detailed information it provides about functional status, cognitive status and self-reported health over time.23 Our study did not demonstrate increase risk of death with lower TICS score or functional dependence before surgery. This is surprising as functional impairment and frailty are emerging as important predictors of poor outcomes in emergency surgery. In a study of data from the National Surgical Quality Improvement project, Over 80% of patients in our cohort were functionally independent, and fewer than 5% had any cognitive impairment based on the TICS score. Consequently we were underpowered to replicate these findings. Nonetheless, we demonstrate that poor self-reported health predicts longer-term mortality and may be another useful measure for risk stratification in older patients before emergency surgery. As patient reported outcomes become increasingly important in measuring the value of healthcare, tracking self-reported health may be particularly informative.24 This is especially true among older and seriously ill patients where poor self-reported health is associated with worse survival.25,26
Older adults are at higher risk of postoperative complications after emergent abdominal surgery than younger patients 5,27 and complications are highly predictive of death in the perioperative period.28-30 Another study using NSQIP and Veterans Administration data also found that post operative complications after major surgery were more closely linked to death at 30 days, and up to 8 years, than either preoperative risk factors or intraoperative events.7 However, this study included all adult patients and elective surgeries. Surprisingly, in our cohort, where comorbidities were highly prevalent and almost half had cancer, complications were associated with the highest risk of overall mortality even up to one year after surgery. These findings suggest that reducing postoperative complications in older patients may be the best way to improve overall survival for these patients, and that even though complications themselves may not be fatal, patients who have complications die sooner than patients who don't have complications. We hypothesize that complications may be a marker of poorer underlying health beyond what we were able to observe with these data and that complications after surgery may accelerate a decline in health in older patients and increase their overall mortality risk. The concept of failure-to-rescue may be of particular relevance chronically ill and functionally impaired patients where complications are frequently unavoidable. In these cases, it becomes important to identify centers with processes of care and resources associated with better outcomes. For example trauma centers caring for a higher proportion of geriatric patients have lower rates of failure-to-rescue than centers caring for fewer geriatric patients,31 and processes of care specific to older patients (e.g. ACE units and triggered geriatric consultation) may improve failure-to-rescue rates.32,33
There are several limitations to our study. First, this is a retrospective study using administrative claims data. Medicare claims are subject to coding error and lack the detailed clinical data needed for optimal risk adjustment. Second, by drawing our sample from the HRS linked to fee-for-service Medicare, our cohort was smaller than had we solely used Medicare claims. However, the HRS is drawn from a nationally representative sample of older Americans, and survey results from HRS strengthen the data beyond using Medicare Claims data alone. Unfortunately, the low numbers of patients with functional and cognitive impairment in the study cohort render us unable to draw conclusions about the association of functional and cognitive status with mortality during the first year after surgery. Third, core interviews in the HRS are biennial and therefore the time between measurement of HRS variables and the surgical episode are variable across patients. To address this, we controlled for the time from the HRS core interview until surgery in our statistical model.
Conclusion
We found high rates of comorbidity, postoperative complications and mortality up to one year among older patients undergoing emergency major abdominal surgery. A majority of older patients, who die after these procedures, do so within the first 6 months, and complications are more strongly associated with overall mortality during the first year after surgery than baseline patient factors. These findings imply that emergent major abdominal surgery is a major determining event in the health trajectory of older patients, and complications after surgery may hasten death over both the short and long term. Furthermore, findings from this study can facilitate goal-directed care for high-risk patients, and help clinicians identify patients who could also benefit from palliative or supportive care in both the perioperative period and the months following surgery.
Supplementary Material
Acknowledgments
The authors would like to thank Dianali Rivera Morales, MS for her assistance with manuscript preparation.
Funding: Dr. Cooper is supported by a Jahnigen Career Development Award from the American Geriatrics Society and NIA GEMSSTAR 1R03AG042361-01. Dr. Mitchell is supported by a Mid-Career Mentoring Award K24AG033640. Dr. Kelley is supported by a Paul B. Beeson Career Development Award in Aging NIA K23AG040774 and the American Federation for Aging Research.
Sponsor's role: None
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors contributed to study concept and design, data analysis and interpretation, and preparation of manuscript. Dr. Kelley contributed to acquisition of data. Zara Cooper, MD, MSc. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.CMS.gov. Healthcare Expenditures. (online). Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html?redirect=/nationalhealthexpenddata/. Accessed June 1, 2013.
- 2.Etzioni DA, Liu JH, Maggard MA, et al. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deiner S, Silverstein JH. Long-term outcomes in elderly surgical patients. Mt Sinai J Med. 2012;79:95–106. doi: 10.1002/msj.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duron JJ, Duron E, Dugue T, et al. Risk factors for mortality in major digestive surgery in the elderly: a multicenter prospective study. Ann Surg. 2011;254:375–382. doi: 10.1097/SLA.0b013e318226a959. [DOI] [PubMed] [Google Scholar]
- 5.Massarweh NN, Legner VJ, Symons RG, et al. Impact of advancing age on abdominal surgical outcomes. Arch Surg. 2009;144:1108–1114. doi: 10.1001/archsurg.2009.204. [DOI] [PubMed] [Google Scholar]
- 6.Turrentine FE, Wang H, Simpson VB, et al. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamel MB, Henderson WG, Khuri SF, et al. Surgical outcomes for patients aged 80 and older: Morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 9.Fried TR, Bradley EH. What matters to seriously ill older persons making end-of-life treatment decisions? A qualitative study. J PallatMed. 2003;6:237–244. doi: 10.1089/109662103764978489. [DOI] [PubMed] [Google Scholar]
- 10.White K, Borrell LN. Racial/ethnic neighborhood concentration and self-reported health in New York City. Ethn Dis. 2006;16:900–908. [PubMed] [Google Scholar]
- 11.Langa KM, Larson EB, Wallace RB, et al. Out-of-pocket health care expenditures among older Americans with dementia. Alzheimer Dis Assoc Disord. 2004;18:90–98. doi: 10.1097/01.wad.0000126620.73791.3e. [DOI] [PubMed] [Google Scholar]
- 12.Cigolle CT, Ofstedal MB, Tian Z, et al. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. International Classification of Diseases, 9th Revision, Clinical Modification. Centers for Disease Control.(online) [Accessed March 5, 2014]; Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD9-CM/2011/
- 15.PQRS Measures Groups Specifications Manual (version 6.1): Coronary Artery Disease (CAD) Measures Group Overview. American Medical Association. (online) [Accessed March 5, 2014];2013 Available at: http://www.mdinteractive.com/files/uploaded/file/cms2013group/CAD_2013_CMS.pdf.
- 16.Joynt KE, Orav E, Jha AK. MOrtality rates for medicare beneficiaries admitted to critical access and non–critical access hospitals, 2002-2010. JAMA. 2013;309:1379–1387. doi: 10.1001/jama.2013.2366. [DOI] [PubMed] [Google Scholar]
- 17.Gazala S, Tul Y, Wagg A, et al. Quality of life and long-term outcomes of octo- and nonagenarians following acute care surgery: A cross sectional study. World J Emerg Surg. 2013;8:23. doi: 10.1186/1749-7922-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noorani A, Hippelainen M, Nashef SM. Time until treatment equipoise: A new concept in surgical decision making. JAMA Surg. 2014;149:109–111. doi: 10.1001/jamasurg.2013.3066. [DOI] [PubMed] [Google Scholar]
- 19.Fried TR, Tinetti ME, Iannone L, et al. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajdos C, Hawn MT, Kile D, et al. The risk of major elective vascular surgical procedures in patients with end-stage renal disease. Ann Surg. 2013;257:766–773. doi: 10.1097/SLA.0b013e3182686b87. [DOI] [PubMed] [Google Scholar]
- 21.Sheer AJ, Heckman JE, Schneider EB, et al. Congestive heart failure and chronic obstructive pulmonary disease predict poor surgical outcomes in older adults undergoing elective diverticulitis surgery. Dis Colon Rectum. 2011;54:1430–1437. doi: 10.1097/DCR.0b013e31822c4e85. [DOI] [PubMed] [Google Scholar]
- 22.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley AS, Langa KM, Smith AK, et al. Leveraging the health and retirement study to advance palliative care research. J Palliat Med. 2014;17:506–511. doi: 10.1089/jpm.2013.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186. doi: 10.1136/bmj.c186. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Costa MF, Cesar CC, Chor D, et al. Self-rated health compared with objectively measured health status as a tool for mortality risk screening in older adults: 10-year follow-up of the Bambui Cohort Study of Aging. Am J Epidemiol. 2012;175:228–235. doi: 10.1093/aje/kwr290. [DOI] [PubMed] [Google Scholar]
- 26.Benjamins MR, Hummer RA, Eberstein IW, et al. Self-reported health and adult mortality risk: an analysis of cause-specific mortality. Soc Sci Med. 2004;59:1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Marusch F, Koch A, Schmidt U, et al. The impact of the risk factor “age” on the early postoperative results of surgery for colorectal carcinoma and its significance for perioperative management. World J Surg. 2005;29:1013–1021. doi: 10.1007/s00268-005-7711-6. discussion 1021-1012. [DOI] [PubMed] [Google Scholar]
- 28.Al-Temimi MH, Griffee M, Enniss TM, et al. When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg. 2012;215:503–511. doi: 10.1016/j.jamcollsurg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Scarborough JE, Pappas TN, Bennett KM, et al. Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Ann Surg. 2012;256:453–461. doi: 10.1097/SLA.0b013e31826578fb. [DOI] [PubMed] [Google Scholar]
- 30.Lidsky ME, Thacker JK, Lagoo-Deenadayalan SA, et al. Advanced age is an independent predictor for increased morbidity and mortality after emergent surgery for diverticulitis. Surgery. 2012;152:465–472. doi: 10.1016/j.surg.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Matsushima K, Schaefer EW, Won EJ, et al. Positive and Negative Volume-Outcome Relationships in the Geriatric Trauma Population. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4834. [DOI] [PubMed] [Google Scholar]
- 32.Baztán JJ, Suárez-García FM, López-Arrieta J, et al. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BM. 2009;338:b50. doi: 10.1136/bmj.b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillou A, Kelley-Quon L, Burruss S, et al. Long-term postinjury functional recovery: Outcomes of geriatric consultation. JAMA Surg. 2014;149:83–89. doi: 10.1001/jamasurg.2013.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


