Abstract
Objectives
To quantify the prognostic importance of pre-frailty and frailty among a population-based sample of cancer survivors.
Design
The Third National Health and Nutrition Survey mortality-linked prospective cohort study.
Setting
Eighty-nine survey locations across the United States.
Participants
Population-based sample of older adults (average age 72.2 years) with a self-reported diagnosis of non-skin-related cancer.
Measurements
The primary outcome was all-cause mortality. Frailty components included: 1) low weight-for-height; 2) slow walking; 3) weakness; 4) exhaustion; and 5) low physical activity. Participants with 1–2 or ≥3 of the 5 above-described criteria were classified as pre-frail or frail, respectively. Participants who did not meet any of the above-described criteria were considered non-frail.
Results
Among 416 cancer survivors, the prevalence of pre-frailty and frailty was 37.3% and 9.1%, respectively. During a median follow-up of 11.2-years, 319 (76.7%) participants died. The median survival of participants classified as non-frail, pre-frail, and frail were 13.9-, 9.5-, and 2.5-years, respectively. Compared to non-frail cancer survivors, those classified as pre-frail [Hazard Ratio (HR): 1.84 (95% CI: 1.28–2.65); P=0.001] or frail [HR: 2.79 (95% CI: 1.34–5.81); P=0.006] had a higher risk of premature mortality.
Conclusion
Pre-frailty and frailty are prevalent clinical syndromes that may confer an increased risk of premature mortality among older adult cancer survivors. Identifying frail cancer survivors and targeting interventions for them may be a strategy to improve survivorship after cancer.
Keywords: disability, fatigue, exhaustion, weakness, mortality
INTRODUCTION
Cancer survivors are living longer after a diagnosis of cancer as a result of earlier detection and efficacious therapies.1 However, many cancer survivors are left with poor global health after receiving the treatments that cured their primary disease, with possible consequences for their long-term prognosis. Frailty is a syndrome of poor global health that includes unintentional weight loss, impaired physical function, weakness, exhaustion, and low levels of physical activity.2 Frailty is strongly associated with premature mortality in older adults,2 but the association between frailty and mortality among cancer survivors is unknown. Characterizing the relationship between frailty and mortality may provide insight to the physiologic and functional capacity of cancer survivors.3
Prior studies demonstrate that cancer survivors frequently report symptoms synonymous with frailty,4–8 which may persist for years after completion of treatment for cancer.9, 10 Treatment for cancer is hypothesized to accelerate the aging process.11, 12 Perhaps as a result, cancer survivors are 46% more likely to be frail compared to adults of similar age without a history of cancer.3 However, given that cancer treatment is a transient exposure, frailty among cancer survivors may represent a unique entity with etiologies and trajectories distinct from those without a history of cancer. As such, the long-term consequences of pre-frailty and frailty among cancer survivors are largely unknown. We tested the hypothesis that pre-frailty and frailty confer an increased risk of premature mortality among a nationally-representative sample of older community-dwelling cancer survivors.
METHODS
Study Design
The Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III) was a stratified multistage study designed to provide health information on a nationally representative sample of U.S. civilians. All participants provided written informed consent.
Study Participants
Adults aged ≥60 years who self-reported a diagnosis of non-skin-related cancer.
Frailty Definition
We implemented a definition of frailty that has been operationalized previously in the NHANES III database.13 The five criteria for frailty included:
Low weight-for-height, defined using a body mass index (BMI) ≤18.5 kg/m2;
Slow walking speed, defined using the slowest quintile adjusted for sex, in a timed 2.4-meter walk;
Weakness, defined as having any level of difficulty or inability to lift or carry something as heavy as 4.5-kilograms;
Exhaustion, defined as having any level of difficulty or inability to walk from one room to another on the same floor;
Low levels of physical activity, defined as a self-report of being less active compared to men or women of a similar age.
Participants who met 1–2 or ≥3 of the 5 above-described criteria were classified as pre-frail or frail, respectively.13 Participants who did not meet any of the above-described criteria were considered non-frail.
Mortality Outcome
The primary outcome of this study was all-cause mortality. Vital status was identified using the National Death Index (NDI) database through December 31, 2006. Participants were linked to the National Death Index (NDI) database using a probabilistic matching algorithm that included 12 identifiers including Social Security Number, sex, date of birth, race, state of residence and birth, and marital status.14 The National Center for Health Statistics removed select subject characteristics in the file to prevent the re-identification of study participants. The publically released survival data are nearly identical to the restricted-use NHANES III linked mortality file.15
Covariates
Demographic information including date of birth and sex were self-reported using a standardized questionnaire. Clinical information including type of cancer, date of cancer diagnosis, smoking history, alcohol consumption, hospitalizations in the prior year, self-reported health status, and frequency of physical activity were assessed using a standardized questionnaire. Cognitive function was assessed using the short portable version of the mini mental status exam.16 The presence of comorbid health conditions was determined by asking participants if a doctor had ever told them that they had any of the following: hypertension, diabetes, hyperlipidemia, asthma, arthritis, myocardial infarction, stroke, and congestive heart failure.
Height in meters and body mass in kilograms were measured by study technicians. Body mass index was calculated as weight divided by the square of height (kg/m2). Hemoglobin was quantified using a Coulter S-Plus Jr electronic counter (Coulter Electronics, Hialeah, FL). Albumin was quantified using a Hitachi 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). C-reactive protein was determined using latex-enhanced nephelometry immunoassay (Behring Diagnostics, Somerville, NJ). Plasma fibrinogen was measured using enzyme assay methods with Coag-A-Mate XC plus (Scarborough, Ontario, Canada).
Statistical Analysis
We used Cox proportional hazards regression models to estimate the hazard ratio (HR), and 95% Confidence Interval (95% CI). We examined log-log plots to confirm the assumption of proportional hazards. Sample weights were incorporated into the statistical analyses to account for nonresponse bias, and we employed multistage sampling probabilities to provide estimates for the U.S. population. Stata/SE v.13.1 statistical software was used for all analyses. Additional exploratory analysis methods are presented in the eMethods.
RESULTS
We identified 498 adults aged ≥60 years who self-reported a prior diagnosis of non-skin-related cancer. Sufficient information was available on 416 (84%) participants to define frailty. Participants with insufficient information necessary to define frailty were more likely to have arthritis (63.3% versus 45.2%; P=0.022), lower albumin (3.9 g/dL versus 4.0 g/dL; P=0.015), and poorer self-reported health (3.5 versus 3.0; P=0.004), compared to those with sufficient information necessary to define frailty, respectively. No other differences were observed between participants with versus without sufficient information necessary to define frailty.
Cohort Characteristics
The mean age of study participants was 72.2 years and 61.0% were female (Table 1). Participants were diagnosed with cancer at a variety of sites including breast (26.3%), gastrointestinal (18.4%), genitourinary (21.3%), gynecologic (14.2%), lung (14.2%), and hematologic (2.7%). The mean time since cancer diagnosis was 11.2 years and 34.2%, 21.5%, and 44.3% were <5, 5–10, and ≥10 years since cancer diagnosis, respectively.
Table 1.
Demographic and Clinical Characteristics, Stratified by Survival Status
| Characteristic | Overall (n=416) [mean (SE) or (%)] |
Died During Follow-Up | P-Value | |
|---|---|---|---|---|
| Yes (n=319) | No (n=97) | |||
| Age — yr. | 72.2 (0.46) | 74.4 (0.53) | 66.9 (0.60) | <.001 |
| Sex — (%) | ||||
| Male | 39.0 | 47.5 | 18.1 | <.001 |
| Female | 61.0 | 52.5 | 81.9 | |
| Race — (%) | ||||
| White | 94.3 | 94.3 | 94.3 | .695 |
| Black | 5.4 | 5.3 | 5.7 | |
| Other | 0.3 | 0.4 | 0.0 | |
| Education — Yr. | ||||
| 0–8 | 20.1 | 21.9 | 15.8 | .745 |
| 9–11 | 19.2 | 19.1 | 19.7 | |
| 12 | 33.5 | 33.2 | 34.0 | |
| ≥13 | 27.2 | 25.8 | 30.5 | |
| Type of Cancer — (%) | ||||
| Breast | 26.3 | 23.9 | 32.1 | .003 |
| Gastrointestinal | 18.4 | 19.7 | 15.4 | |
| Genitourinary | 21.3 | 25.0 | 12.4 | |
| Gynecologic | 14.2 | 10.1 | 24.2 | |
| Lung | 2.7 | 3.8 | 0.0 | |
| Hematologic | 2.5 | 1.4 | 5.0 | |
| Other, missing, or can’t remember | 14.6 | 16.1 | 10.9 | |
| Time Since Cancer Diagnosis — Yr. | ||||
| Mean (Continuous) | 11.2 (0.65) | 10.7 (0.72) | 12.3 (1.4) | .340 |
| <5 | 34.2 | 35.4 | 31.2 | .567 |
| 5–10 | 21.5 | 22.5 | 19.1 | |
| ≥10 | 44.3 | 42.1 | 49.7 | |
| Body Mass Index — kg/m2 | ||||
| Mean (Continuous) | 25.8 (0.31) | 25.7 (0.36) | 26.1 (0.59) | .551 |
| <18.5 | 3.7 | 4.7 | 1.3 | .626 |
| 18.5–24.9 | 42.5 | 41.6 | 44.7 | |
| 25.0–29.9 | 36.2 | 36.4 | 35.7 | |
| ≥30.0 | 17.6 | 17.3 | 18.4 | |
| Smoking Status — (%) | ||||
| Never | 42.2 | 41.8 | 43.2 | .915 |
| Former | 45.4 | 46.2 | 43.4 | |
| Current | 12.4 | 12.0 | 13.4 | |
| No. of Alcoholic Drinks Consumed in Past Wk. | 1.1 (0.15) | 0.9 (0.16) | 1.5 (0.31) | .101 |
| Cognitive Function | 12.8 (0.22) | 12.4 (0.27) | 13.9 (0.35) | .001 |
| Self-Reported Comorbid Health Conditions — (%) | ||||
| Hypertension | 48.3 | 48.9 | 46.9 | .776 |
| Diabetes | 10.8 | 13.0 | 5.3 | .086 |
| Hyperlipidemia | 32.4 | 27.3 | 45.2 | .010 |
| Asthma | 5.7 | 7.2 | 2.2 | .145 |
| Arthritis | 45.2 | 45.2 | 45.4 | .981 |
| Myocardial Infarction | 15.0 | 20.3 | 1.8 | <.001 |
| Stroke | 7.5 | 9.7 | 2.0 | .012 |
| Congestive Heart Failure | 6.5 | 7.6 | 3.9 | .354 |
| Hospitalization(s) in Past Yr. — (%) | 29.1 | 31.7 | 22.6 | .172 |
| Hemoglobin (g/dL) | 13.5 (0.08) | 13.5 (0.10) | 13.5 (12.8) | .849 |
| Albumin (g/dL) | 4.0 (0.02) | 4.0 (0.03) | 4.1 (0.04) | .179 |
| C-Reactive Protein (mg/dL) | 0.5 (0.05) | 0.6 (0.06) | 0.4 (0.07) | .024 |
| Fibrinogen (mg/dL) | 335.0 (5.77) | 340.5 (6.89) | 322.8 (10.38) | .155 |
| Self-Reported Health (Best–Worst: 1–5) | 3.0 (0.07) | 3.2 (0.08) | 2.5 (0.14) | <.001 |
| Frailty Criteria— (%) | ||||
| Low Weight-For-Height | 3.7 | 4.7 | 1.3 | .185 |
| Slow Walking | 15.8 | 20.0 | 5.5 | .009 |
| Weakness | 29.2 | 32.7 | 20.7 | .065 |
| Exhaustion | 9.5 | 11.1 | 5.8 | .222 |
| Low Physical Activity | 20.2 | 23.1 | 13.1 | .086 |
| Frailty Classification — (%) | ||||
| Non-Frail (0 Frailty Components) | 53.6 | 47.1 | 69.5 | .008 |
| Pre-Frail (1–2 Frailty Components) | 37.3 | 41.9 | 26.2 | |
| Frail (≥3 Frailty Components) | 9.1 | 11.0 | 4.3 | |
Characteristics Associated with Mortality
Among 416 participants, we observed 319 deaths (76.7%) during a median of 11.2-years of follow-up (Table 1). Participants who died during the follow-up period were more likely to be older, male, diagnosed with gastrointestinal, genitourinary, or lung cancer, have poorer cognitive function, report a history of myocardial infarction or stroke, have higher concentrations of C-reactive protein, and self-report poorer health, compared to participants who did not die during the follow-up period. Time since cancer diagnosis, measured as a continuous or categorical variable, was not associated with mortality.
Prevalence and Prognostic Importance of Individual Frailty Criteria
The prevalence of the individual criteria for frailty ranged from 3.7% to 29.2% (Table 1). When each of the five frailty criteria were individually entered into a multivariable-adjusted model, low weight-for-height, weakness, and low physical activity were associated with premature mortality (Table 2). When all of the five frailty criteria were simultaneously entered into a multivariable-adjusted model, low weight-for-height and low physical activity were independently associated with premature mortality (Table 2). We observed a relationship between the number of frailty components treated as an ordinal (count) variable (Ptrend<.001), and as a continuous variable [HR: 1.46 (95% CI: 1.22–1.73); P<.001] with the risk of premature mortality (eTable 1).
Table 2.
Association between Frailty and All-Cause Mortality
| Performance Measure | Hazard Ratio (HR) and 95% Confidence Intervals (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model 1a | P-Value | Model 2b | P-Value | Model 3c | P-Value | |
| Individual Components of Frailty | ||||||
| Low Weight-For-Height | 2.51 (0.99–6.38) | .053 | 4.38 (2.14–8.98) | <.001 | 7.30 (3.15–16.90) | <.001 |
| Slow Walking | 2.42 (1.53–3.83) | <.001 | 1.60 (1.11–2.30) | .012 | 1.26 (0.83–1.91) | .274 |
| Weakness | 1.50 (1.08–2.09) | .016 | 1.48 (1.09–2.00) | .012 | 1.70 (1.15–2.51) | .008 |
| Exhaustion | 2.03 (1.05–3.94) | .036 | 1.71 (0.98–2.98) | .057 | 1.35 (0.78–2.34) | .283 |
| Low Physical Activity | 1.81 (1.21–2.72) | .004 | 2.21 (1.51–3.21) | <.001 | 2.71 (1.66–4.42) | <.001 |
| Components of Frailty Adjusted for One-Another | ||||||
| Low Weight-For-Height | 2.50 (1.06–5.93) | .037 | 4.19 (2.21–7.93) | <.001 | 5.31 (2.43–11.59) | <.001 |
| Slow Walking | 2.23 (1.48–3.37) | <.001 | 1.39 (1.02–1.89) | .034 | 1.12 (0.73–1.72) | .596 |
| Weakness | 1.06 (0.74–1.52) | .750 | 1.13 (0.82–1.55) | .466 | 1.41 (0.94–2.11) | .095 |
| Exhaustion | 1.12 (0.55–2.26) | .754 | 1.21 (0.73–2.00) | .461 | 0.96 (0.55–1.66) | .872 |
| Low Physical Activity | 1.60 (1.48–3.37) | <.001 | 1.96 (1.30–2.94) | .001 | 2.15 (1.33–3.49) | .002 |
| Composite Frailty Classification | ||||||
| Non-Frail | 1.00 | 1.00 | 1.00 | |||
| Pre-Frail | 1.72 (1.26–2.35) | .001 | 1.54 (1.15–2.06) | .004 | 1.84 (1.28–2.65) | .001 |
| Frail | 3.17 (1.54–6.52) | .002 | 3.00 (1.57–5.69) | .001 | 2.79 (1.34–5.81) | .006 |
Model 1 is unadjusted.
Model 2 is adjusted for age and sex.
Model 3 is adjusted for age, sex, race, education, body mass index (continuous), type of cancer, time since cancer diagnosis (continuous), smoking status, weekly drinking, cognitive function, hypertension, diabetes, asthma, arthritis, myocardial infarction, stroke, congestive heart failure, hospitalization in the prior year, hemoglobin, albumin, C-reactive protein, fibrinogen, and self-reported health status.
Prevalence and Prognostic Importance of Pre-Frailty and Frailty
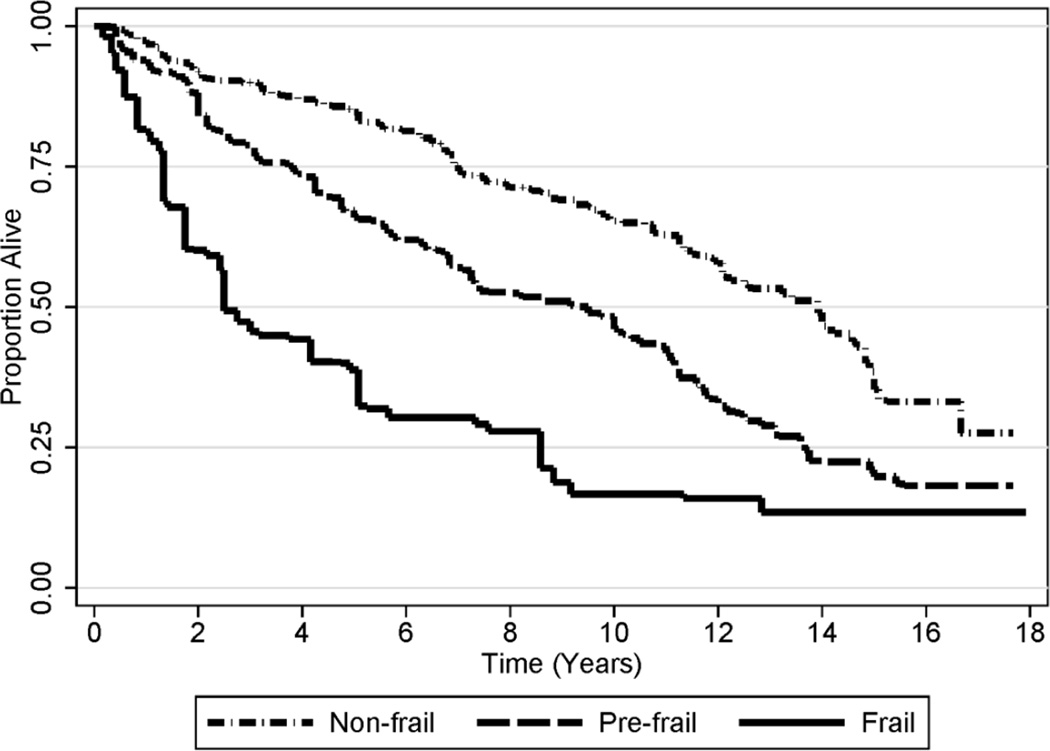
The prevalence of pre-frailty and frailty were 37.3% and 9.1%, respectively (Table 1). Median survival was 9.5- and 2.5-years among participants classified as pre-frail or frail, respectively, as compared to 13.9-years among participants classified as non-frail (Figure 1). Participants classified as pre-frail or frail had a significantly higher risk of premature mortality, as compared to participants who were non-frail (Table 2).
Figure 1. Kaplan-Meier Survival Plot, Stratified by Stage of Frailty.
Frailty components included: low weight-for-height, slow walking, weakness, exhaustion, and low physical activity. Participants classified as non-frail, pre-frail, and frail, had zero, 1–2, and ≥3 frailty components, respectively.
Discriminative Characteristics of Frailty to Predict 5- and 10-Year Mortality
The discriminative characteristics of frailty to predict 5- and 10-year mortality are presented in the eResults (eTables 2 and 3; eFigure 1). The addition of frailty to a model that included age, sex, and type of cancer significantly improved the area under the receiver operating curve to predict 5-year (0.76 vs 0.71; P=.0086) and 10-year (0.80 vs 0.77; P=.0061) mortality, respectively.
DISCUSSION
In this cohort of older adult cancer survivors, pre-frailty and frailty conferred an increased risk of premature mortality. As frailty is likely to be related both to aging and to cancer treatments, providers should be aware of this syndrome and its consequences in older cancer survivors.17 While screening to identify frail older adults has increased in oncologic practice, such screening most often occurs prior to the initiation of cancer treatment to identify patients vulnerable to treatment-related toxicity.18 Less focus has been paid to the impact of frailty after the completion of cancer treatment. The results from this study suggest that screening for the presence of pre-frailty or frailty among older adults who are cancer survivors may result in increased insight on long-term prognosis.
Previous studies have demonstrated that frailty is highly prevalent among older cancer patients, and likely more common than in the general older population. A systematic review of 21 studies of frailty in populations of community-dwelling older adults reported that the mean prevalence of frailty was 9.9% (range: 4–59%).19 In contrast, a systematic review of 20 studies of older cancer patients found that the median prevalence of frailty was 42% (range: 6–86%).20 Although the fact that they have survived cancer and its therapies may label older cancer survivors as uniquely robust older adults, studies have shown that older survivors of cancer routinely report higher rates of decline in functional status and overall health in the years after their cancer diagnosis, compared to age-matched non-cancer controls.21, 22 This accelerated deterioration of functional status and overall health has been hypothesized to be related to cancer treatments,21, 22 and underscores the importance of studying frailty in this unique population.
We observed a strong association between post-cancer frailty and mortality, which may indicate that the presence of frailty after cancer treatment heralds future adverse health events or a lower quality-of-life for older cancer survivors. Given the likely dynamic changes in health that result from cancer and cancer therapy, older cancer patients may benefit from being monitored both before and after treatment for the identification of early frailty symptoms. Experts agree that in the absence of early identification and intervention, pre-frail older adults inevitably progress into frailty,2 and frail older adults will continue a trajectory of worsening health.23 Intervention efforts that prevent or delay the onset of frailty are needed to avoid these outcomes among cancer survivors.11
Exercise or physical activity may be an efficacious lifestyle intervention among older adult cancer survivors with or at-risk for frailty.24 Regular physical activity, such as brisk walking, may reduce frailty among older adults at-risk for disability.25 Exercise may also improve gait speed, balance, and self-reported activities of daily living among frail adults.26 Exercise among cancer survivors may improve patient reported outcomes,27 and observational studies suggest that exercise may reduce the risk of cancer-specific and all-cause mortality.28 Additional research is warranted to better characterize the safety and efficacy of exercise among older adult cancer survivors.
There are limitations to our study. Our modified definition of frailty, based on the definition operationalized by Fried et al.,2 was necessary to accommodate the data available in the nationally-representative NHANES III database. However, this modified definition has been used and validated in prior reports of NHANES.13, 29 There exist other approaches that may be used in clinical practice to identify older adult cancer survivors at-risk for poor outcomes. For example, the comprehensive geriatric assessment (CGA) may provide useful clinical guidance in geriatric oncology.17 The CGA may inform the care of the older adult with cancer by identifying impairments that are amenable to intervention, and predict the risk of adverse outcomes such as treatment-related toxicity, functional or cognitive decline, postoperative complication, and overall survival.30 Another limitation to this analysis is our inability to identify incident cases of frailty. Therefore, it is conceivable that some of the study subjects may have become pre-frail or frail prior to cancer diagnosis or treatment. However, our study focuses on metrics obtained after cancer diagnosis, and our data suggest that pre-frailty and frailty are prevalent issues among cancer survivors and confer and increased risk of premature mortality. We did not have information regarding stage of cancer or specific cancer treatments received. It is likely that these covariates may have attenuated the association between frailty and mortality.
This study possesses several strengths. As a result of the sampling framework of NHANES, our study cohort is a nationally-representative sample of community-dwelling older adult cancer survivors in the United States. Our study had an extensive median length of follow up of 11.2-years, which allowed us to observe a high proportion of deaths. We accounted for a variety of covariates that may confound the association of frailty and mortality including demographic, clinical, and blood chemistry measures.
In conclusion, pre-frailty and frailty are prevalent clinical syndromes that confer an increased risk of premature mortality among older cancer survivors. These findings demonstrate the need for additional research in the area of geriatric oncology. Frailty screening tools should be validated in cohorts of cancer survivors to inform clinical decision-making and characterize the etiologies and trajectories of frailty in this population.3 Efficacious interventions are also needed to prevent or delay the onset of frailty. Exercise or physical activity is a promising intervention with many possible benefits for older cancer survivors. Randomized studies are necessary to determine if exercise can delay or prevent the onset of incident frailty in this population. This knowledge will help to improve the quality and quantity of cancer survivorship.
Supplementary Material
ACKNOWLEDGMENTS
Funding Source: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK096758) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors participated in the study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosaeus I, Daneryd P, Svanberg E, et al. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. International Journal of Cancer. 2001;93:380–383. doi: 10.1002/ijc.1332. [DOI] [PubMed] [Google Scholar]
- 5.Ness KK, Wall MM, Oakes JM, et al. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16:197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Schootman M, Aft R, Jeffe DB. An evaluation of lower-body functional limitations among long-term survivors of 11 different types of cancers. Cancer. 2009;115:5329–5338. doi: 10.1002/cncr.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella D, Davis K, Breitbart W, et al. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard CM, Courneya KS, Stein K, et al. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 9.Baker F, Denniston M, Smith T, et al. Adult cancer survivors: How are they faring? Cancer. 2005;104:2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 10.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: A new challenge for supportive care. Cancer J. 2006;12:432–443. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 12.Monfardini S, Basso U. Oncological causes of frailty in older cancer patients. Eur J Cancer. 2007;43:1230–1231. doi: 10.1016/j.ejca.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm-Leen ER, Hall YN, Tamura MK, et al. Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671. e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–734. doi: 10.1016/0021-9681(86)90155-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilper AP, Woolhandler S, Lasser KE, et al. Health insurance and mortality in US adults. Am J Public Health. 2009;99:2289–2295. doi: 10.2105/AJPH.2008.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obisesan TO, Obisesan OA, Martins S, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–509. doi: 10.1111/j.1532-5415.2007.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 18.Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012;13:e437–e444. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- 19.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 20.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann Oncol. 2015;26:1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 21.Petrick JL, Foraker RE, Kucharska-Newton AM, et al. Trajectory of overall health from self-report and factors contributing to health declines among cancer survivors. Cancer Causes Control. 2014;25:1179–1186. doi: 10.1007/s10552-014-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. Functional status declines among cancer survivors: Trajectory and contributing factors. J Geriatr Oncol. 2014;5:359–367. doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the US older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65:246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: A post hoc analysis of a randomized controlled trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.57.7395. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P Study. J Gerontol A Biol Sci Med Sci. 2015;70:216–222. doi: 10.1093/gerona/glu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou C, Hwang C, Wu Y. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch Phys Med Rehabil. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 28.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit E, Winters-Stone KM, Loprinzi PD, et al. Lower nutritional status and higher food insufficiency in frail older US adults. Br J Nutr. 2012;1:1–7. doi: 10.1017/S000711451200459X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.