Abstract
H2O2 mediates autocrine and paracrine signaling in the vasculature and can propagate endothelial dysfunction. However, it is not clear how endothelial cells withstand H2O2 exposure and promote H2O2-induced vascular remodeling. To understand the innate ability of endothelial cells for sustaining excess H2O2 exposure, we investigated the genotypic and functional regulation of redox systems in primary HUVECs following an H2O2 treatment.
Primary HUVECs were exposed to two types of H2O2 treatments of both transient and consistent H2O2 exposure. Following H2O2 treatments for 24, 48 and 72 hr, we measured O 2− production, mitochondrial membrane polarization (MMP), and gene expressions of pro-oxidative enzymes, peroxidase enzymes, and cytoprotective intermediates.
Our results showed that the 24 hr H2O2 exposure significantly increased O2− levels, hyperpolarized MMP, and downregulated CAT, GPX1, TXNRD1, NFE2L2, ASK1, and ATF2 gene expression in HUVECs. At 72 hr, HUVECs in both treatment conditions were shown to adapt to reduce O2− levels and normalize MMP. An upregulation of GPX1, TXNRD1, and HMOX1 gene expression and a recovery of NFE2L2 and PRDX1 gene expression to control levels were observed in both consistent and transient treatments at 48 and 72 hr.
The response of endothelial cells to excess levels of H2O2 involves a complex interaction amongst O2− levels, mitochondrial membrane polarization and anti- and pro-oxidant gene regulation. As a part of this response, HUVECs induce cytoprotective mechanisms including the expression of peroxidase and antioxidant enzymes along with the downregulation of pro-apoptotic genes. This adaptation assists HUVECs to withstand subsequent exposures to H2O2
Keywords: Endothelial dysfunction, hydrogen peroxide, systems biology, redox homeostasis, HUVEC, HMOX1, GPX1, TXNRD1, ASK1, UCP1, cytoprotection
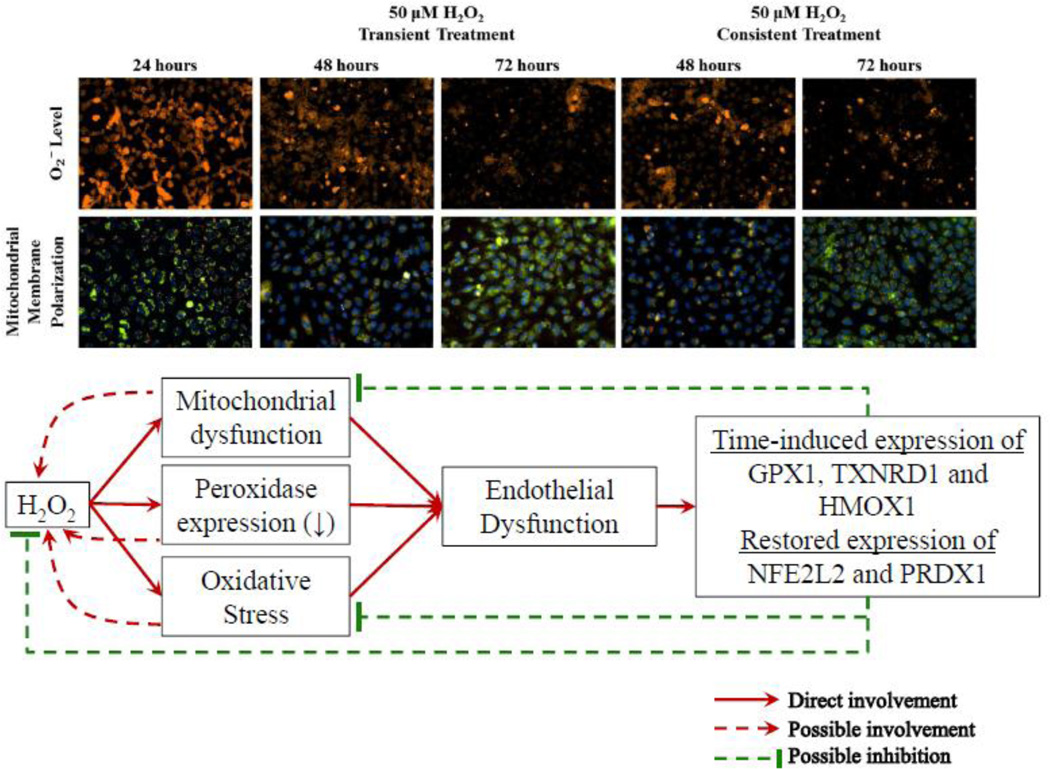
Graphical abstract
Introduction
Cardiovascular diseases (CVD) are one of the leading causes of morbidity and mortality (Kones and Rumana, 2014). Vascular dysfunction and remodeling, which is a result of dysfunctional endothelium, plays a significant role in CVD pathologies (Polovina and Potpara, 2014). The endothelium level of reactive oxygen species (ROS), specifically superoxide (O2− ) and hydrogen peroxide (H2O2), is increased in these diseases (Dhalla et al., 2000). ROS plays an integral role in the functioning of the vasculature (D’Autreaux and Toledano, 2007; Kavdia, 2011). Elevated ROS levels have been shown to vary with the degree of oxidative stress and vascular diseases. H2O2, which is more stable than other ROS, has been postulated to mediate both autocrine and paracrine signaling in the vasculature and to propagate endothelial dysfunction (Ardanaz and Pagano, 2006).
H2O2 has been reported to regulate endothelium mediated vascular tone, oxidative stress, angiogenesis, apoptosis, and permeability. In physiologic conditions, human plasma contains ~ 1–8 µM H2O2. During acute vascular pathology such as ischemia/reperfusion, the H2O2 levels can increase as high as 100–160 µM (Burgoyne et al., 2013). High levels of vascular H2O2 are also observed in the transient and progressive vascular pathologies of wounds and hyperglycemia, respectively (Patel et al., 2013; Roy et al., 2006). In addition, H2O2 is an integral part of the cellular redox system. H2O2 may constitutively regulate the ROS homeostasis (D’Autreaux and Toledano, 2007).
Recent studies of H2O2 exposure have focused on the reduction of pathological effects and protection of endothelial cells. In endothelial cells, protein thiols have been reported to sense H2O2 and to transduce biological responses to H2O2(Burgoyne et al., 2013). Peroxidases that contain an active thiol group include glutathione peroxidases, peroxiredoxins, thioredoxins, and glutaredoxins. Thus, these peroxidases may play a major role in neutralizing exogenous H2O2 (Marinho et al., 2014; Presnell et al., 2013). Conversely, high H2O2 levels oxidize and inactivate peroxidases while causing damage to endothelial cells (Breton-Romero and Lamas, 2014). Similarly, H2O2 disrupts the Nrf-2- antioxidant response element (ARE) pathway; thus, H2O2 affects Nrf-2 mediated transcription. Nrf-2 promotes de novo synthesis of antioxidant enzymes and protects endothelial cells from oxidative stress (Cortese et al., 2008; Ning et al., 2010). In addition, the exposure to H2O2 for less than 24 hr downregulates the gene expression of SODs and CAT, and promotes the gene expression of both antiproliferative and cell repairing genes (Desaint et al., 2004; Wen et al., 2013). These studies have shown that H2O2 exposure not only overwhelms peroxidase defenses but also disrupts antioxidant defense mechanisms, and renders endothelial cells dysfunctional (Kar et al., 2012). Several chemical and medicinal compounds are reported to protect endothelial cells during exposure to H2O2 at the pathologic levels (Cortese et al., 2008; Liang et al., 2011; Liu et al., 2009; Wen et al., 2013). The cytoprotective abilities of these compounds may result from reducing oxidative stress and activating antioxidant mechanisms.
In the vascular system, dysfunctional endothelial cells are a major source of ROS production during vascular dysfunction (Esper et al., 2006). Using a systems biology approach, we showed that hyperglycemia induces the production of H2O2 in human umbilical vein endothelial cells (HUVEC) (Patel et al., 2013). Additionally, the presence of high ROS levels are shown to promote a pro-oxidative redox response in endothelial cells (Coyle et al., 2006; Kar and Kavdia, 2013). Thus, the interplay between a high H2O2 level and a dysfunctional endothelium can lead to a system wide progression of endothelial dysfunction in a feed-forward manner (Kar et al., 2012). The feed-forward progression in endothelial dysfunction is observed with an acute increase in vascular H2O2 levels in the first 48 hr after post-ischemic reperfusion (Horwitz et al., 1994). The endothelial dysfunction is shown to be sporadic and reversible over a period of 2 days to 9 weeks following post-ischemic reperfusion (Horwitz et al., 1994). Thus, the feed forward progression of endothelial cell dysfunction is reversible. The burst of H2O2 in the first 48 hrs may induce the mechanism to protect endothelial cells against progressive damage.
The reversal of endothelial dysfunction after excess H2O2 exposure may be a result of the inherent protection mechanisms. These inherent mechanisms can be important for maintaining the integrity of the endothelium, regulating vascular pathogenesis and vasoregulation following the H2O2 exposure. An understanding of these interactions will explain how vasculature and endothelial cells withstand an increase in oxidative stress levels.
Because of the importance of H2O2 in the function and dysfunction of endothelial cells, we investigated the gene expression and functional level response of HUVEC to transient and consistent H2O2 exposure using a systems approach. The transient and consistent exposure may represent acute and chronic conditions, respectively (Burgoyne et al., 2013). The transient H2O2 exposure condition may be observed in post-ischemic reperfusion, whereas the consistent H2O2 exposure may be observed in diabetes and hypertension, (Lacy et al., 2000; Msolly et al., 2013). In particular, we studied the functional change of O2− levels and mitochondrial membrane polarization, and the gene expression change of pro-oxidative enzymes, peroxidase enzymes, and cytoprotective intermediates in HUVECs following both transient and consistent H2O2 exposure for 24, 48 and 72 hr.
Materials and Methods
Cell Culture and H2O2 exposure
Primary HUVEC (Lonza, MD) were grown in EGM-2 supplemented with bullet kit (Lonza, MD). To maintain consistency, all experiments were carried out using the fourth passage of HUVECs. HUVECs were grown in 6-well culture plate (2×105 cells/well, Becton Dickinson, NJ) for gene expression measurements and 96-well flat bottom black plate (1.5×104 cells/well, Becton Dickinson, NJ) for live-cell fluorescence assays. Cells were treated with freshly prepared 10, 25, and 50 µM H2O2(EMD Millipore, MA) in EGM-2 media at confluency using a transient and consistent H2O2 exposure protocol, which is described next.
To simulate the transient H2O2 exposure condition, HUVECs were treated with H2O2 for the first 24 hr of the experiment. For 48 and 72 hr of experiment duration, the cells were grown in fresh EGM-2 media without H2O2 in the transient H2O2 exposure. In the consistent exposure, HUVECs were treated with H2O2 every 24 hr of the experiment i.e. at 0, 24, and 48 hr (Patel and Kwon, 2009). In both treatment conditions, O2− levels, mitochondrial membrane polarization, and gene expression were measured at 24, 48, and 72 hr. HUVECs growing in EGM-2 media without H2O2 at 24, 48 and 72 hrs (n=3) were considered as control.
Intracellular O2− measurement
Intracellular O2− was measured using dihydroethidium (DHE) at 24, 48 and 72 hr (Chen et al., 2013; Patel et al., 2013). In brief, cells were incubated with 5 µM of DHE (Life Technologies, CA) in phenol red free treatment medium for 1 hr. Using a fluorescence microplate reader (Synergy 2, Biotek, VT), 2-hydroxyethidium (2-OH-E+) fluorescence (Ex: 508 ± 10 nm and Em: 560 ± 20 nm) was measured. Following microplate reader based O2− measurement, cells fluorescence images were captured using Nikon Eclipse Ti microscope and CoolSNAP HQ2 CCD camera (Photometrics, AZ). For uniform image acquisition, camera exposure duration and intensity histogram range settings were kept constant using Nikon-Elements AR software Ver 4. All quantitative measurement of 2-OH-E+ were normalized with the respective time point control.
Following DHE based O2− measurement, HUVECs were incubated with 1 µM Hoescht 33342 (Life technologies, CA) for 10 minutes and fluorescence intensities were measured using fluorescence microscope and microplate reader (340 ± 15 nm (Ex)/460 ± 20 nm (Em)). We used Hoescht 33342 fluorescence intensities to account for variation in cell number and viability.
Mitochondrial membrane polarization measurement
HUVECs mitochondrial membrane polarization levels were measured using JC-10 (Enzo Life Sciences, NY) as previously described (Patel et al., 2013). At 24, 48 and 72 hr following initial H2O2 exposure, cells were incubated with 1 µM JC-10 prepared in phenol-red free EGM media for 15 minutes. Following JC-10 incubation, cell nuclei were stained with 1 µM Hoescht 33342 for 10 minutes. Fluorescence intensities were measured using fluorescence microscope and microplate reader. Level of green fluorescence, specific to J-monomer in cytosol, and red fluorescence, specific to J-aggregates in mitochondria, were measured at 485 ± 10 nm (Ex)/516 ± 10 nm (Em) and 528 ± 10 nm (Ex)/590 nm ± 10 (Em), respectively. Fluorescence intensity of Hoescht 33342 (340 ± 15 nm (Ex)/460 ± 20 nm (Em)) was used to normalize JC-10 fluorescence measurements.
Gene expression measurement and analysis
Gene expression measurements were performed for 50 µM H2O2 treatment for both transient and the consistent treatments. At 24, 48 and 72 hr, total RNA was extracted using RNeasy mini kit (Qiagen, CA) with DNase I digest treatment. After each set of extractions, total RNA was checked for integrity and quantity through agarose based gel electrophoresis and microplate reader. Using high capacity cDNA reverse transcription kit (Life Technologies, CA) 1.5 µg of extracted RNA from each treatment and controls were converted to cDNA in a 20 µl reverse transcription reaction. We measured expression of pro-oxidative enzyme (NADPH oxidase enzyme 1 (NOX1)), superoxide dismutase 1 and 2 (SOD1 and 2), peroxidase enzymes (catalase (CAT), peroxyredoxin1 (PRDX1), glutathione peroxidase1 (GPX1), thioredoxin reductase 1 and 2 (TXNRD1 and 2)), and cytoprotective and antioxidant intermediates including inhibitor of kappa light polypeptide gene enhancer in B-cells kinase beta (IKBKB,), nuclear factor erythroid 2-like 2 (NFE2L2), heme oxygenase (decycling) 1 (HMOX1), heat shock 70kDa protein 1A (HSPA1A), mitogen-activated protein kinase kinase kinase 5 (ASK1), activating transcription factor 2 (ATF2), and uncoupling protein1 (UCP1).
Levels of gene expression in cDNA samples were measured using real-time PCR. All real-time PCR reactions were carried out in a 20 µl volume comprising of 50 ng of cDNA, FAST SYBR green expression mix (Life Technologies, CA), and 200 nM of each forward and reverse primers. Using StepOne Plus systems (Life Technologies, CA) real-time PCR reaction was set to one run of 20 seconds at 95°C that was followed by 36 cycles of 3 seconds at 95°C and 30 seconds at 60°C. The cycle threshold (CT) values form all real-time PCR experiments were measured and analyzed using ΔΔCT method. Housekeeping genes, 18s and RPLP0, were used for normalizing gene expression of each target gene. Gene expression profile of HUVECs in EGM-2 media without H2O2 at 24, 48 and 72 hrs was used as respective time control. We calculated ΔΔCT using the normalization with housekeeping genes (i.e. 18s and RPLP0) and time control. The fold changes with respect to control were calculated using 2−ΔΔCT.
Primer pairs specific to each of these gene expression targets were designed using NCBI’s Primer-BLAST feature. Primers pairs sequences used for gene expression assays are in Table 1. For quality control, each PCR assay reaction was analyzed using melt curve analysis and agarose gel based electrophoresis. Furthermore, no reverse transcription, and no template controls were also run for evaluating specificity of PCR amplification.
Table 1.
List of primers for quantitative real-time PCR
| Target Gene |
Forward Primer (5′→3′) |
Reverse Primer (5′→3′) |
|---|---|---|
| NOX1 | ATCCCCCTGAGTCTTGGAAGT | CACTTCCATGCTGAAGCCAC |
| SOD1 | AGCATTAAAGGACTGACTGAAGG | GTCTCCAACATGCCTCTCTTC |
| SOD2 | GTTGGGGTTGGCTTGGTTTC | ATAAGGCCTGTTGTTCCTTGC |
| CAT | AGGGGCCTTTGGCTACTTTG | ACCCGATTCTCCAGCAACAG |
| PRDX1 | TCCTTTGGTATCAGACCCGA | TAAAAAGGCCCCTGAACGAG |
| GPX1 | CCGGGACTACACCCAGATGA | CGTTCTCCTGATGCCCAAAC |
| TXNRD1 | GGAACTAGATGGGGTCTCGG | TCTTGCAGGGCTTGTCCTAA |
| TXNRD2 | GGTGGACTACGTGGAACCTT | TCTGCCATCTTCCTCCAGTCA |
| HMOX1 | CTGCGTTCCTGCTCAACATC | GGCAGAATCTTGCACTTTGTT |
| IKBKB | CTTTGAGTGCATCACGGGCT | TCACTCTTCTGCCGCACTTT |
| NFE2L2 | CAACTACTCCCAGGTTGCCC | AATGTCTGCGCCAAAAGCTG |
| HSPA1A | CTGGAGCAGGTGTGTAACCC | GCAATCTTGGAAAGGCCCCTA |
| ASK1 | GCCGGTTTTTGGCTGAAGAT | AGCATCCCTCCCCTTAGTCT |
| ATF2 | AGTTGGCGAGTCCATTTGAGA | GATAGGTGTTGCAAGAGGGGA |
| UCP1 | GCTCCAGGTCCAAGGTGAAT | ACAGCGGTGATTGTTCCCAG |
| 18s | TCAACTTTCGATGGTAGTCGCCGT | TCCTTGGATGTGGTAGCCGTTTCT |
| RPLP0 | AGACAATGTGGGCTCCAAGCAGAT | GCATCATGGTGTTCTTGCCCATCA |
Data analysis
Each experimental measurement was carried out on HUVECs derived from three independent experiments (n=3). HUVECs grown in EGM-2 media without H2O2 at 24, 48 and 72 hrs (n=3) were used as control.
DHE and JC-10 fluorescence measurement, and gene expression measurement were analyzed by one-way ANOVA coupled with multiple comparisons using either Fisher’s LSD or Tukey’s post-hoc analysis method after performing the normality check on the data. Statistical differences were marked significant for p-value ≤ 0.05. Results are presented as mean ± S.E.M. (standard error of mean).
Results
O2− levels increased following H2O2 exposure at 24 hr and HUVEC adapted for subsequent H2O2 exposure and reduced O2− levels
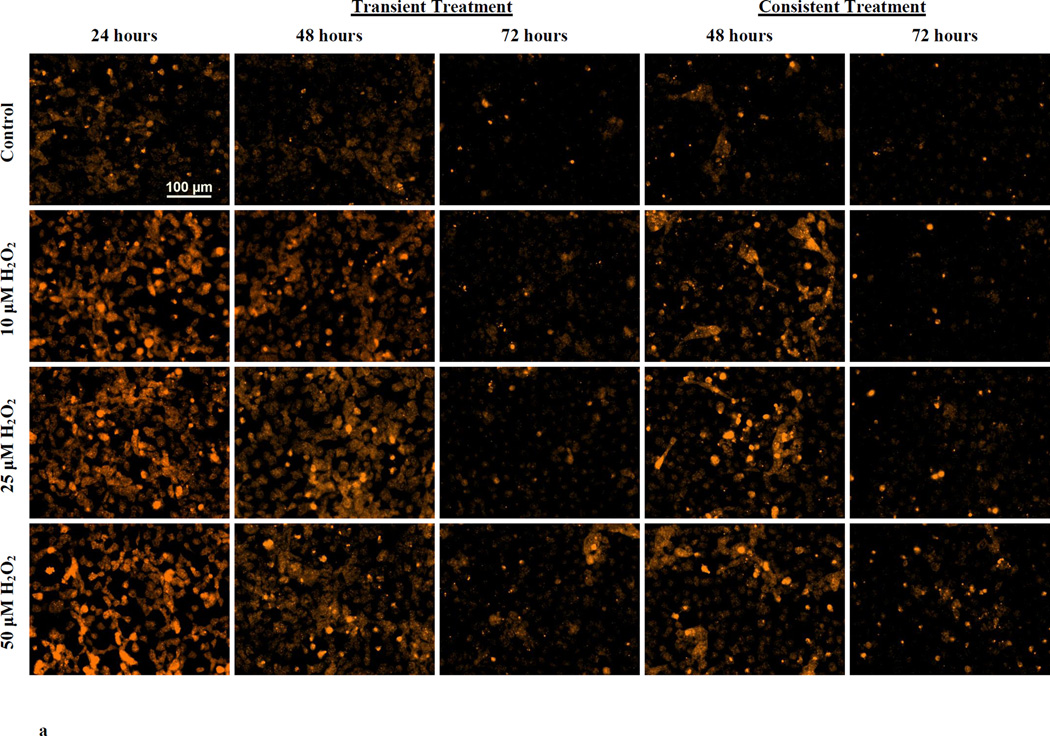
Following H2O2 treatment, O2− levels were measured using DHE with fluorescence microscopy and microplate reader. Figure 1a shows DHE fluorescence in HUVECs at 24 hr and at 48 and 72 hr of the transient and consistent H2O2 treatments. O2− levels were increased in HUVECs treated with 10, 25 and 50 µM H2O2 at 24 hr. The increase in DHE fluorescence persisted at lesser intensity at 48 hr in the transient and consistent treatments (Figure 1a). At 72 hr, DHE fluorescence intensities were similar to control level for all concentration of H2O2 in both treatments (Figure 1a).
Figure 1. O2− production during transient and consistent H2O2 exposure.
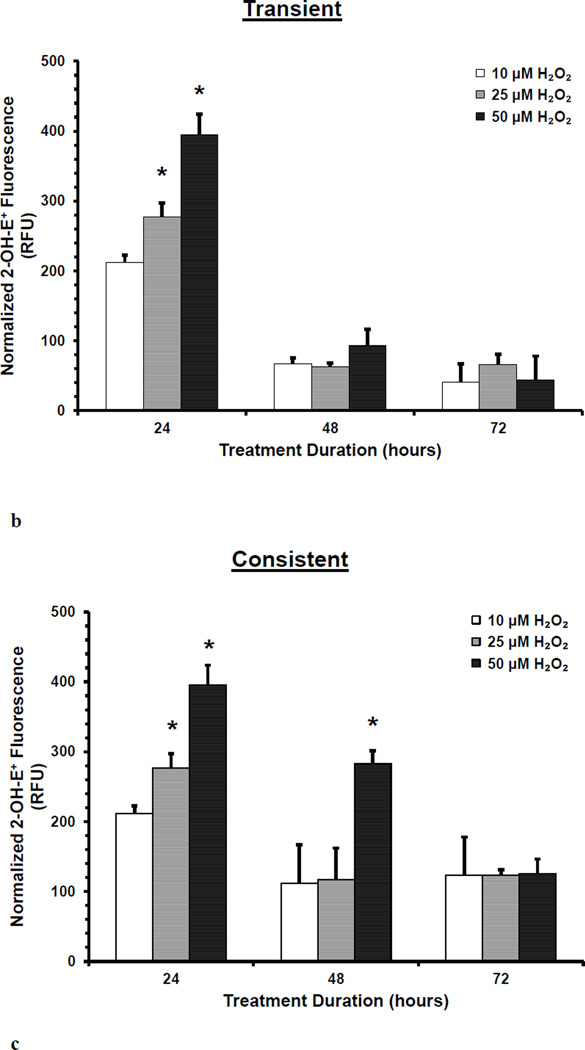
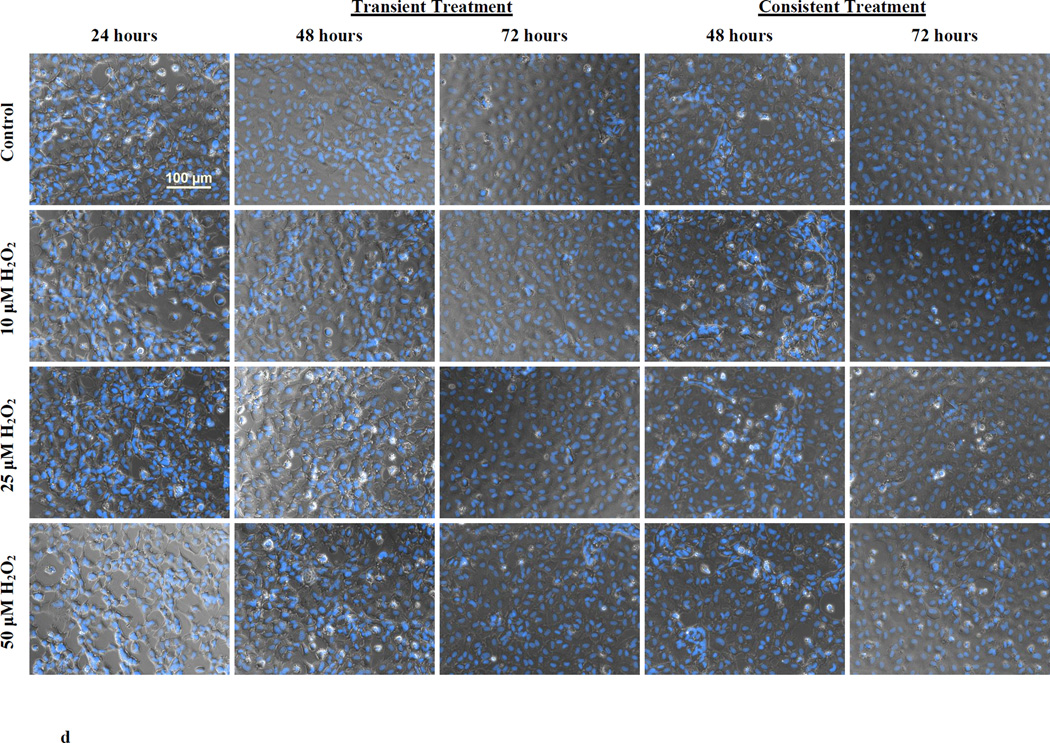
O2− levels in HUVEC were measured during transient and consistent treatment of 10, 25, and 50 µM H2O2 at 24, 48, and 72 hrs. Using DHE, levels of O2− were both qualitatively and quantitatively assessed using fluorescence microscopy (a) and microplate reader (b and c), respectively. Furthermore, the cell viability was observed using Hoescht 33342 stained fluorescence images (d). HUVECs grown in EGM-2 media without H2O2 at 24, 48 and 72 hrs were used as control (n=3). (a) Representative fluorescence microscopy images to measure DHE fluorescence were taken at 24, 48 and 72 hours (n=3). All fluorescence images were captured using the same settings (i.e. 20x objective, 600ms exposure duration, and 2×2 binning). Intensity histograms of all images were kept at a fix setting for uniform visualization. Scale bar represents 100 µm. (b and c) 2-OH-E+, a specific product of DHE and O2− interaction, was measured using fluorescence microplate reader for transient (b) and consistent (c) H2O2 exposures. Results are presented as mean ± S.E.M. Oneway ANOVA followed by multiple comparisons using Fisher’s LSD method was used to analyze fluorescence intensities. Statistically significant differences compared to controls at respective time point are shown with an asterisk (* p≤ 0.05, n=3). (d) Representative fluorescence microscopy images of Hoescht 33342 based nuclei staining were taken at 24, 48 and 72 hours (n=3). All fluorescence images were captured using the same settings (i.e. 20x objective, 61ms exposure duration, and 2×2 binning). Scale bar represents 100 µm. Furthermore, all Hoescht 33342 stained fluorescence images were overlaid with the respective phase image for comprehensive presentation.
The levels of 2-OH-E+, which is a specific product from the interaction between DHE and O2−, is shown in Figure 1b and 1c for the transient and consistent H2O2 treatment, respectively. 2-OH-E+ levels were significantly higher (*p<0.05) and dependent on H2O2 concentration at 24 hr. 2-OH-E+ levels were high compared to respective controls for all H2O2 concentrations in both treatment conditions at 48 and 72 hr. The level of 2-OH-E+ was significantly higher for only 50 µM H2O2(*p<0.05) in the consistent treatment at 48 hr (Figure 1c).
These results showed that the O2− levels initially increased following H2O2 exposure, however, HUVEC s adapted to reduce O2− levels as observed in subsequent H2O2 exposure over longer duration (consistent treatment at 72 hr). Furthermor, H2O2 treatment did not affect HUVEC viability. Nuclei staining with Hoescht 33342 indicated consistent cell number throughout the treatment duration of 72 hr (Figure 1d).
Mitochondrial membrane polarization remained similar at 72 hr irrespective of H2O2 transient or consistent treatment
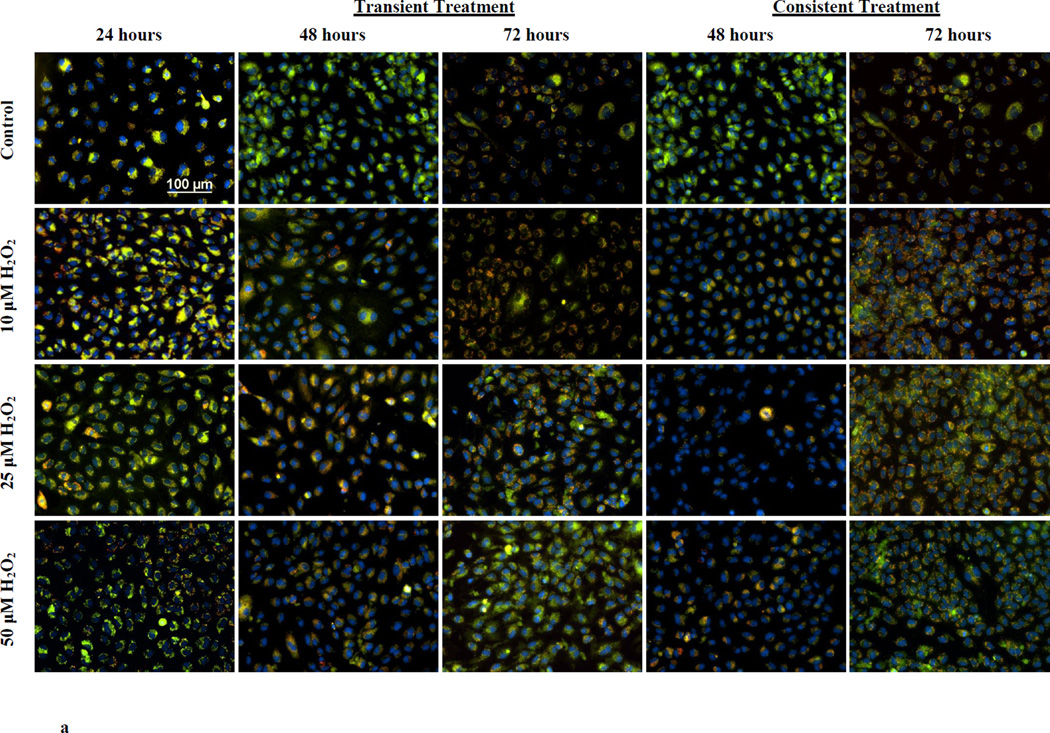
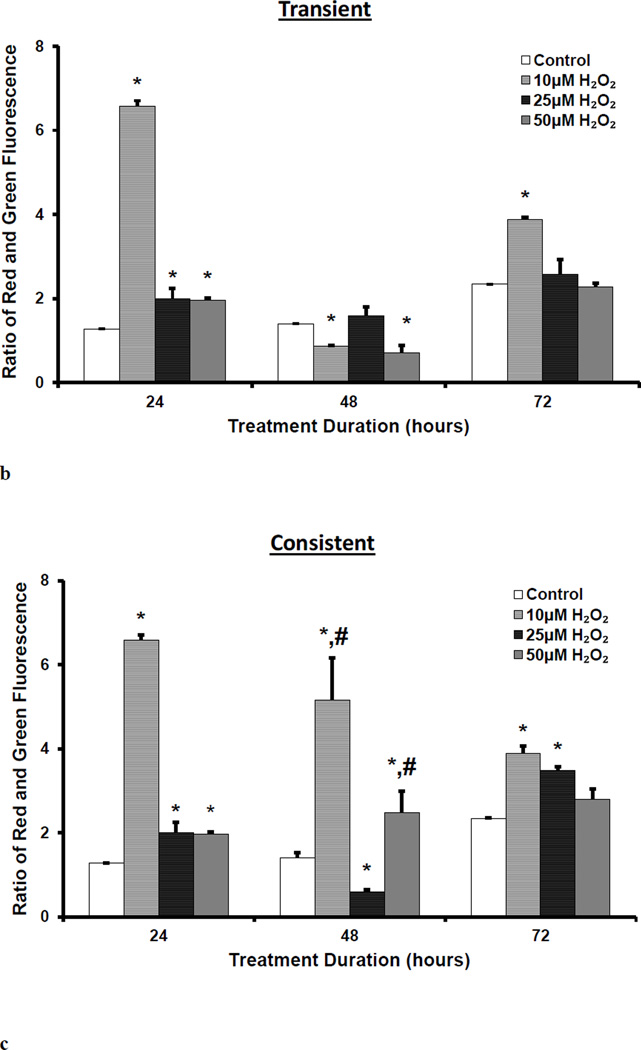
Mitochondrial membrane polarization was measured using JC-10. Red and green fluorescence intensities were measured in HUVECs following JC-10 incubation. Red and green fluorescence are specific to J-aggregate in mitochondria and J-monomer in cytosol, respectively. Fluorescence images were taken (Figure 2a) and fluorescence was quantified using plate reader (Figure 2b and 2c). Following 24 hr of treatment to 10, 25, and 50 µM H2O2, mitochondrial membranes were hyperpolarized in HUVECs (Figure 2a). The ratio of J-aggregates (red) to J-monomers (green) fluorescence intensity significantly increased (*p<0.05) for all H2O2 concentrations. The membrane hyperpolarization was highest for 10 µM H2O2(Figure 2b). At 48 hr, there was a significant difference in the membrane polarization between the transient and consistent treatments for 10 and 50 µM H2O2 concentrations (# p <0.05, Figure 2c). The membrane remained hyperpolarized at 72 hr for 10 µM H2O2 concentration for both transient and consistent treatment as compared to control (Figure 2c). For 50 µM H2O2, mitochondrial membrane polarization reduced back to the control level at 72 hr in both treatments.
Figure 2. Mitochondrial membrane polarization for transient and consistent H2O2 exposure.
Mitochondrial membrane polarization was measured in HUVECs using JC-10 for the transient and consistent treatments of 10, 25, and 50 µM H2O2 at 24, 48, and 72 hr. Fluorescence intensity specific to J-aggregates (red) and J-monomers (green) was measured using fluorescence microscopy (a) and microplate reader (b and c). HUVECs grown in EGM-2 media without H2O2 at 24, 48 and 72 hr was used as respective time control (n=3). (a) Representative fluorescence images are shown for J-aggregates and J-monomers measurement. Hoescht 33342 fluorescence was measured for stained nucleus. All fluorescence images were captured using settings of 20x objective, and 200ms (Hoescht 33342), 700ms (J-monomer) and 500ms (J-aggregates) exposure duration. Intensity histograms of all images were kept at the same settings. Overlay image of all captured fluorescence intensities are shown. Scale bar represents 100 µm. (b and c) J-aggregates (red) and J-monomers (green) fluorescence was measured using microplate reader for transient (b) and consistent (c) H2O2 exposures. The ratio of red to green fluorescence is shown. A higher ratio of red to green fluorescence intensity as compared to that of control shows hyperpolarization of mitochondrial membrane, whereas a lower ratio of red to green fluorescence intensity ratio as compared to that of control shows depolarization. Results are presented as mean ± S.E.M. One-way ANOVA followed by multiple comparisons using Fisher’s LSD method was used to analyze the ratio of fluorescence intensities. Statistically significant differences compared to controls at respective time point are shown with an asterisk (* p≤ 0.05, n=3).
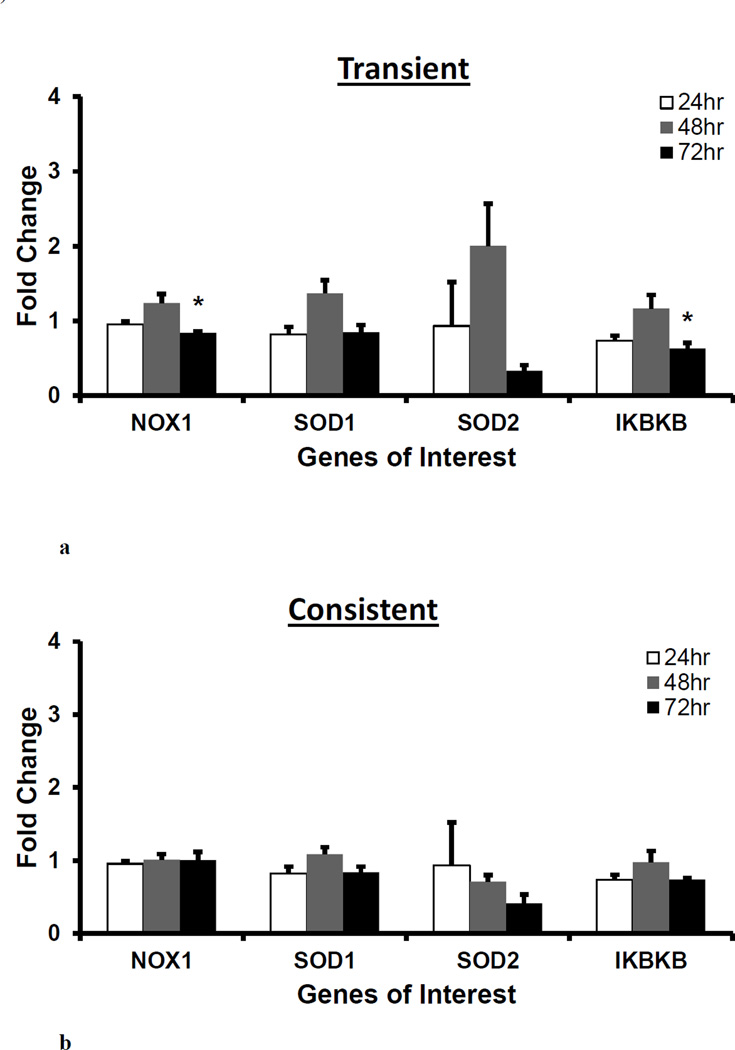
Gene expressions of NOX1, SOD1, SOD2, and IKBKB did not differ between the transient and consistent H2O2 treatment
NOX1, SOD 1 and 2, and IKBKB gene expression was measured for 50 µM of transient and consistent H2O2 treatments at 24, 48 and 72 hrs. As seen in Figure 3, there was no significant difference between the transient and consistent treatment’s gene expressions of NOX1, SOD1 and 2 and IKBKB at all three time points. In the transient treatment, the gene expressions were non-significantly upregulated at 48 hr, whereas NOX1 and IKBKB (*p<0.05, Figure 3a) were significantly downregulated at 72 hr. SOD2 gene expressions was downregulated (not significant) as compared to the respective control in both treatments at 72 hr. The consistent H2O2 treatment reduced SOD2 gene expression over the period of 72 hr (Figure 3b).
Figure 3. Gene expression of pro-oxidant enzyme, superoxide dismutase enzymes and proinflammatory transcription factor following H2O2 treatments.
For 50 µM H2O2 transient (a) and consistent (b) exposure, gene expressions of pro-oxidant enzyme (NOX1), superoxide dismutases (SOD1 and SOD2), and pro-inflammatory transcription factor (IKBKB) were measured at 24, 48, and 72 hr. Gene expression profile of HUVEC in EGM-2 media without H2O2 at 24, 48 and 72 hrs (n=3) was used as respective control for data normalization and statistical analysis. Fold changes in gene expression were normalized against housekeeping genes (i.e. 18s and RPLP0) and control using ΔΔC(t) method. Results are presented as mean ± S.E.M. Gene expression resulting from three individual experiments (n=3) were statistically compared with control at respective time points using one-way ANOVA followed by multiple comparisons using Tukey’s post-hoc analysis. Statistical difference with p<0.05 is marked with an asterisks (*). Gene expression results presented in subsequent figures were also analyzed and presented in same way.
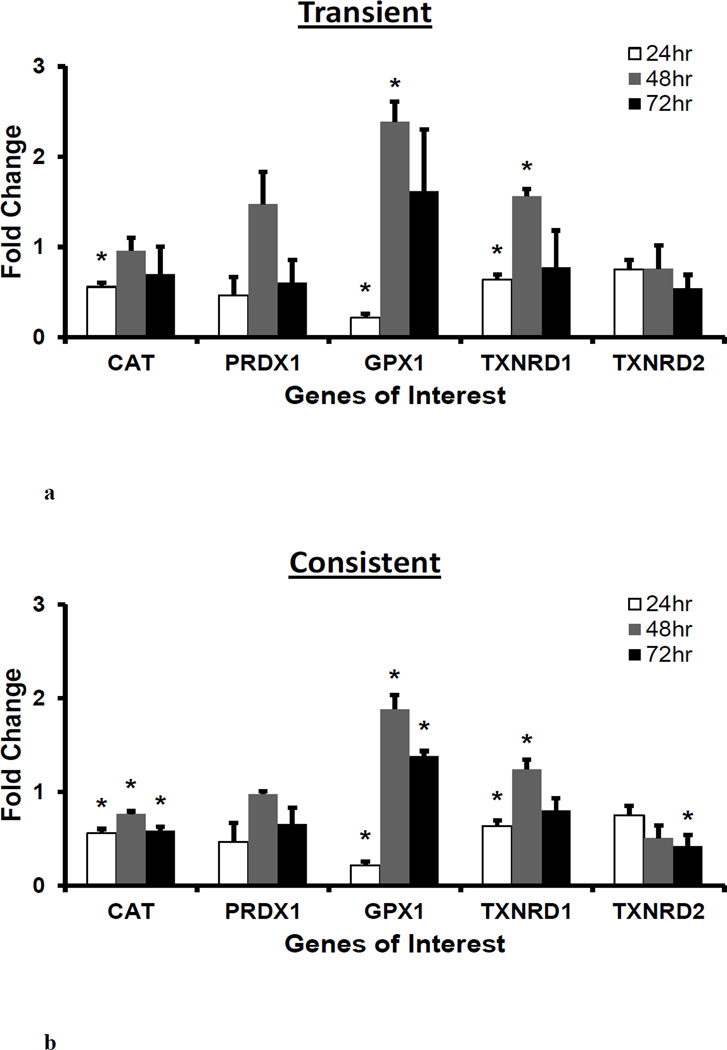
Peroxidase enzymes CAT, GPX1 and TXNRD1 are significantly downregulated at 24 hr and GPX1 is upregulated at 48 and 72 hrs
To understand how the presence of exogenous H2O2 affect the gene expression of peroxidase enzymes, CAT, PRDX1, GPX1, TXNRD1 and TXNRD2 gene expression was measured for 50 µM of transient and consistent H2O2 treatments at 24, 48 and 72 hrs. As seen in Figure 4, the gene expressions of CAT, GPX1, and TXNRD1 were significantly downregulated (*p<0.05) whereas PRDX1, and TXNRD2 were downregulated (non-significant) after 24 hr H2O2 treatment. These peroxidase enzymes, except for TXNRD2, were upregulated at 48 hr in both treatments. Specifically, GPX1 and TXNRD1 were significantly upregulated (*p<0.05) in both H2O2 treatments. There was a downregulation in peroxidase enzymes at 72 hr as compared to the respective gene expression at 48 hr in both treatments. The GPX1 gene expression remained high in both treatments at 72 hr with respect to the control levels.
Figure 4. Gene expression of peroxidase enzymes following H2O2 treatments.
For 50 µM H2O2 transient (a) and consistent (b) exposure, gene expressions of peroxidase enzymes CAT, PRDX1, GPX1, TXNRD1, and TXNRD2 were measured at 24, 48, and 72 hr. Gene expression profile of HUVEC in EGM-2 media without H2O2 at 24, 48 and 72 hr (n=3) was used as respective control for data normalization and statistical analysis. Fold changes in gene expression were normalized against housekeeping genes (i.e. 18s and RPLP0) and control using ΔΔC(t) method. Statistical difference with p<0.05 is marked with an asterisks (*) (n=3).
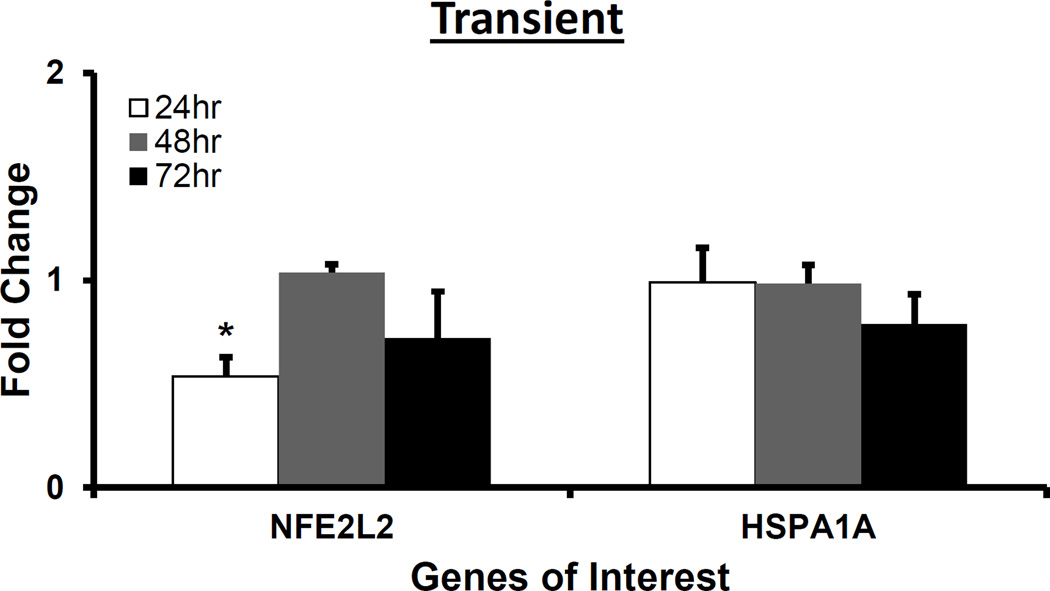
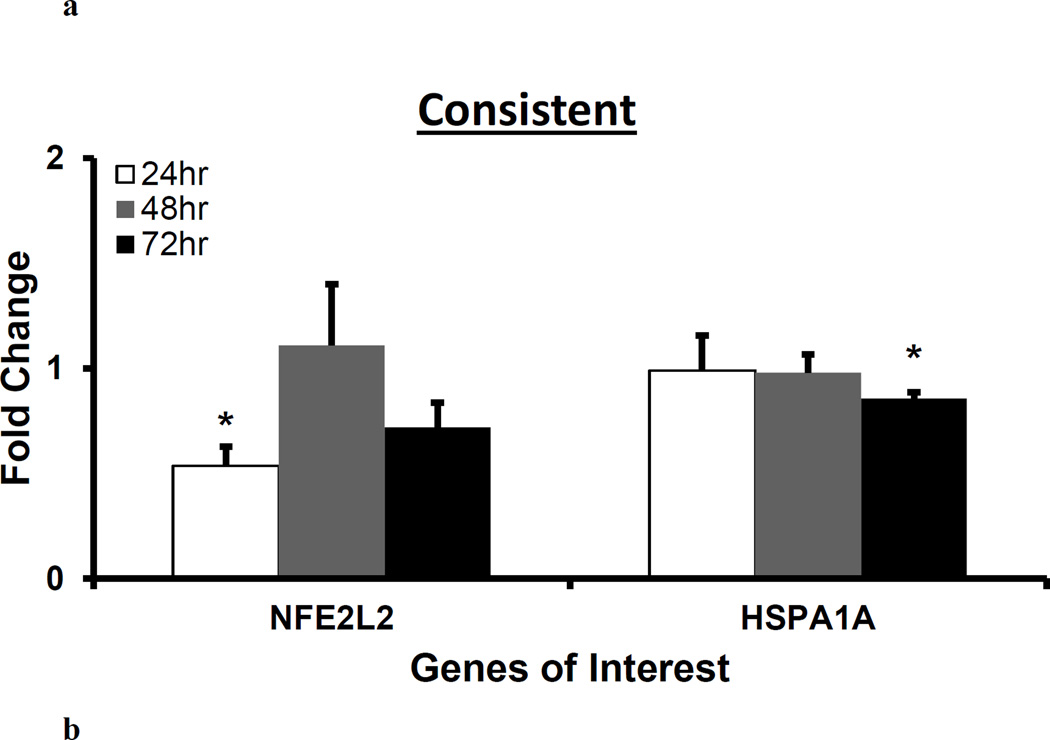
NFE2L2 and HSPA1A was downregulated at 24 and 72 hr, respectively, whereas HMOX1 was upregulated at 48 hr following H2O2 treatment
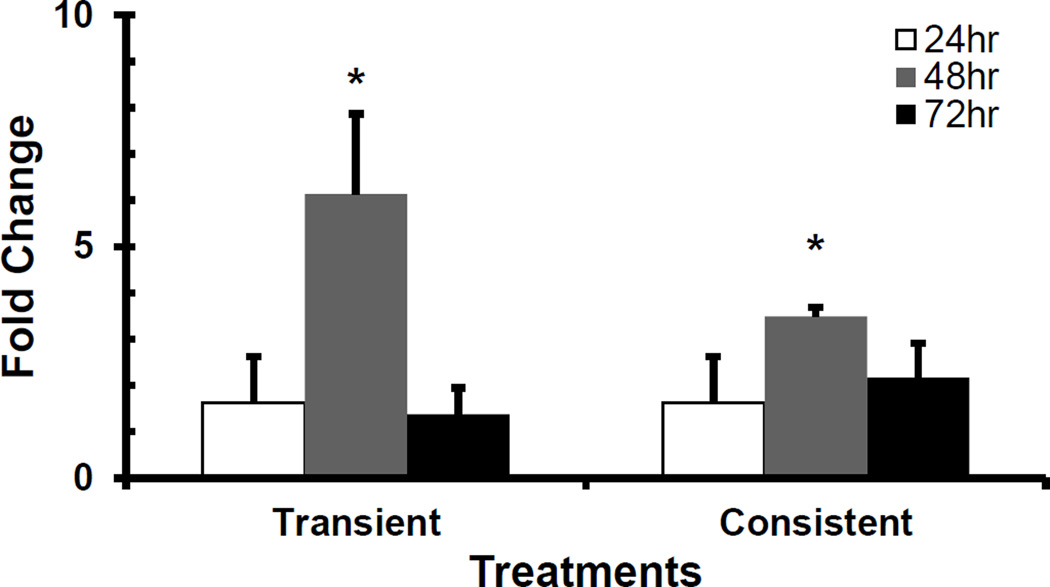
NFE2L2, HSPA1A and HMOX1 are involved in the protection of cells and tissues from oxidative damage (Cortese et al., 2008; Stocker and Perrella, 2006). NFE2L2 regulates the expression of antioxidant proteins. HspA1A aids in DNA repair following stress related damage. HMOX1 is a potent cytoprotective enzyme in diverse conditions including oxidative stress and inflammation (Liang et al., 2011; Stocker and Perrella, 2006). We measured gene expressions of NFE2L2, HSPA1A and HMOX1 following H2O2 treatment. Figure 5 shows NFE2L2 and HSPA1A gene expressions following the transient and consistent H2O2 treatment. The NFE2L2 gene expression (*p<0.05) was significantly downregulated at 24 hr. The HSPA1A gene expression did not change at 24 and 48 hrs. The HSPA1A gene expression was significantly downregulated (*p<0.05) at 72 hr in the consistent H2O2 treatment (Figure 5b). The gene expression of HMOX1 followed similar bell-shaped trend with the maximum upregulation at 48 hr (*p<0.05) in both treatments as compared to control (Figure 6).
Figure 5. H2O2 exposure-induced gene expression of antioxidant transcription factor and enzyme, and heat shock protein.
For 50 µM H2O2 transient (a) and consistent (b) exposure, gene expressions of antioxidant transcription factor (NFE2L2) and heat shock protein (HSPA1A) were measured at 24, 48, and 72 hrs. Gene expression profile of HUVEC in EGM-2 media without H2O2 at 24, 48 and 72 hr (n=3) was used as respective control for data normalization and statistical analysis. Fold changes in gene expression were normalized against housekeeping genes (i.e. 18s and RPLP0) and control using ΔΔC(t) method. Statistical difference with p<0.05 is marked with an asterisks (*) (n=3).
Figure 6. H2O2 exposure-induced gene expression of HMOX1 gene.
For 50 µM H2O2 transient and consistent exposure, HMOX1 gene expression was measured at 24, 48, and 72 hr. Gene expression profile of HUVEC in EGM-2 media without H2O2 at 24, 48 and 72 hr (n=3) was used as respective control for data normalization and statistical analysis. Fold changes in gene expression were normalized against housekeeping genes (i.e. 18s and RPLP0) and control using ΔΔC(t) method. Statistical difference with p<0.05 is marked with an asterisks (*) (n=3).
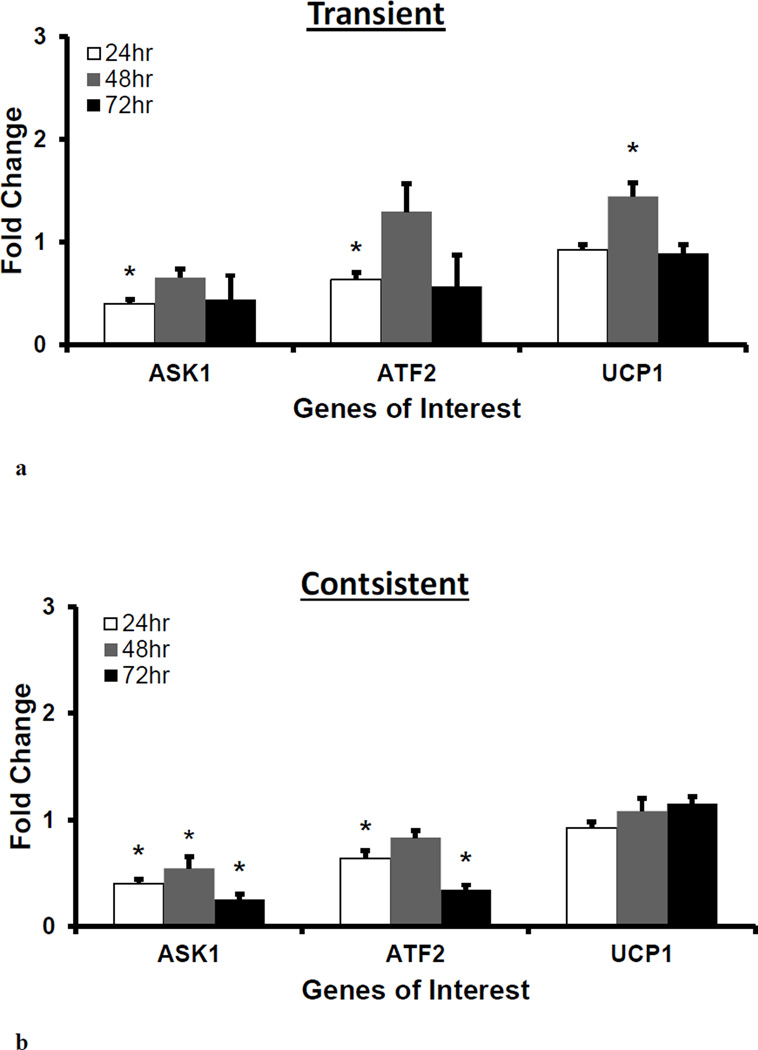
Gene expression of ASK1 and ATF2 were downregulated in consistent H2O2 treatment
Gene expression of ASK1, and ATF2 and UCP1 were measured to evaluate H2O2 treatment induced genetic regulation. In oxidative stress, ASK1 regulates the activation of c-Jun N-terminal kinase and p38 and promotes stress response and dysfunction in endothelial cells (Hattori et al., 2009). At downstream of ASK1 regulated pathway, ATF2 regulates the expression of various genes that are involved in anti-apoptosis, cell growth, and DNA damage response (Bhoumik et al., 2007). ATF2 also interacts with mitochondrial outer membrane and impairs the mitochondrial membrane potential (Lau et al., 2012). UCP1 regulates the activity of mitochondrial electron transport chain to protect mitochondria from oxidative damage (Fink et al., 2005). At 24 hr, the ASK1 and ATF2 gene expressions were significantly downregulated (*p<0.05) as compared to control, whereas the gene expression of UCP1 remained similar to the control level. At 48 and 72 hrs, the ASK1 gene expression remained downregulated for both treatments, and the consistent treatment had significant downregulation (*p<0.05) as compared to control. The ATF2 gene expression significantly decreased (*p<0.05) for the consistent treatment at 72 hr. The UCP1 gene expression was similar to control in both treatments except for significantly higher (*p<0.05) expression at 48 hr for the transient treatment.
Discussion
Because of the critical role of H2O2 in endothelial cell function, we investigated the effect of H2O2 on endothelial cells over the period of 72 hr using a systems approach and correlated gene expression fingerprints and functional changes. Two treatment methods, i.e. transient and consistent, were utilized to study the effect of acute and chronic exposure to H2O2, respectively. Acute exposure to H2O2 may be experienced during post-ischemic reperfusion whereas chronic exposure to H2O2 may be experienced in diabetes and hypertension (Burgoyne et al., 2013; Lacy et al., 2000; Msolly et al., 2013). Furthermore, we extended our study over 72 hr to observe the transition in genotypic and functional changes for supporting HUVEC survival under transient and consistent H2O2 treatments. Results from our study indicated that HUVEC had significant changes in gene expression and O2− and mitochondrial membrane polarization following 24 hr of H2O2 exposure. However, at 72 hr HUVEC regulated gene expression profiles to reach similar levels of O2− and mitochondrial membrane polarization in both the transient and the consistent H2O2 treatment. These results suggest that over a longer term the HUVECs reach a persistent peroxide counterbalancing state after H2O2 exposure over a longer term. In this study, we demonstrated endothelial cells withstand H2O2-induced oxidative stress over the period of 72 hr.
H2O2 treatment related ROS and SOD levels in endothelial cells
H2O2 plays an important role in regulating physiological and pathological conditions in the cardiovascular system. There is a growing body of information about the role of H2O2 in vascular function and homeostasis. Despite the fact that H2O2 has been historically considered an oxidant that causes damage, recent evidence shows that H2O2 is involved in endothelial cell growth and proliferation, survival, endothelium-dependent vasorelaxation, cytoskeletal reorganization, inflammatory responses, and endothelium-regulated vascular remodeling (Breton-Romero and Lamas, 2014; Burgoyne et al., 2013). H2O2 increases the ROS levels of endothelial cells (Wiseman et al., 2007). Various chemical and medicinal compounds have been used to counteract the increase in ROS levels and to improve cell condition during H2O2 exposure (Cortese et al., 2008; Liang et al., 2011; Liu et al., 2009; Potdar and Kavdia, 2009; Wen et al., 2013). Following H2O2 exposure, simultaneous regulation of SOD activity and ROS sources including mitochondrial oxidase, xanthine oxidase, uncoupled-nitric oxide synthase, and NAD(P)H oxidases have been shown to increase ROS levels (Coyle et al., 2006; Szocs, 2004). Moreover, the gene expressions of SOD1 and SOD2 have been reported as both upregulated and downregulated depending on the concentration of H2O2. H2O2 treatment at 600 µM for 4hr was reported to downregulate SOD1 and 2 expressions (Wen et al., 2013), whereas H2O2 treatment at 180 µM for 24 hr was reported to upregulate SOD1 expression (Ugusman et al., 2011). In addition, the causative involvement of expression and activity of NAD(P)H oxidase family enzymes has been reported for inducing the SOD expression in endothelial cells (Frey et al., 2009). In this study, we observed an upregulation of SOD2 in the transient treatment at 48 hr, and a downregulation of SOD2 at 72 hr in both treatments (non-significant). There was no significant change in the NOX1 and SOD1 expression, except for a downregulation in the NOX1 expression at 72 hr under the transient treatment.
In our study, there was a significant increase in O2− levels and hyperpolarization of mitochondrial membrane. However, there was no change in gene expression of NOX1, SOD1 or SOD2 after 24 hr exposure to H2O2. The results suggest a possible involvement of mitochondria and exclude involvement of NOX1, SOD1 and SOD2 for increasing O2− level in H2O2 exposure at 24 hr. However, such a correlation amongst the concentrations of H2O2, and O2− and hyperpolarization of the mitochondrial membrane was not established within 24 hr of our study. This indicates a more complex interaction amongst these parameters as well as the regulation of both the antioxidant mechanism and pro-oxidant mechanism (Cai, 2005; Groleau et al., 2011; Lucchesi et al., 2005).
H2O2 triggers a complex regulation in the mitochondrial membrane polarization
Over the short-term (i.e. 1 or 4 hrs), H2O2 exposure of 100 µM to 600 µM has been reported to consistently depolarize the mitochondrial membrane in HUVECs (Liu et al., 2013; Surico et al., 2015; Wen et al., 2013). However, Park el al. (Park, 2013) reported that 24 hr of H2O2 exposure induced a dose dependent response in polarizing the mitochondrial membrane. They showed that 50 and 100 µM H2O2 slightly hyperpolarized whereas 200 and 300 µM H2O2 depolarized the mitochondrial membrane in HUVECs. In this study, we observed that the mitochondrial membrane was hyperpolarized at 10, 25, and 50 µM H2O2 after 24 hr. In addition, mitochondrial membrane polarization levels followed two distinct time-dependent trends in both the transient and consistent H2O2 treatments. For the 10, 25, and 50 µM H2O2 transient and 25 µM H2O2 consistent treatments, the mitochondrial membrane showed an inverted bell shaped response over the period of 72 hr. The corresponding expression of UCP1 in 50µM H2O2 transient treatment showed a complementary bell-shaped increase that peaked and significantly upregulated at 48 hr. Upregulated expression of UCP1 correlates to decreased mitochondrial membrane polarization level in oxidative stress environment (Patel et al., 2013). For the 10 and 50µM H2O2 consistent treatment, a decreasing hyperpolarization was observed over the period of 72 hr, which either remained hyperpolarized or reached to normal polarization at 72 hr. For 50µM H2O2 consistent treatment, corresponding UCP1 expression increased (non-significantly) over 72 hr. These observations indicate that the mitochondria may recover from initial depolarization. The recovery of the mitochondrial membrane polarization is dependent on the concentration and duration of H2O2 exposure. Furthermore, expression of UCP1 might play a critical role in restoring the mitochondrial membrane polarization level following an H2O2 exposure.
In addition to the concentration and duration of H2O2 exposure, H2O2 regulated mitochondrial membrane depolarization is also shown to highly correlate with oxidative stress, depleted nitric oxide (NO) and glutathione (GSH) levels, as well as decreased SOD, GPX and other antioxidant enzyme activity (Liu et al., 2013; Surico et al., 2015; Wen et al., 2013; Xing et al., 2014). In addition, we observed a complex interplay amongst O2− levels, mitochondrial membrane polarization, and various cell redox system related genes, which was dependent on H2O2 exposure duration and concentration. This suggests a complex regulation in mitochondrial functionality that regulates cell survival under exogenous H2O2 exposure.
H2O2 modulates gene expression of peroxidase enzymes
Exposure to H2O2 has been shown to induce cell death, vasorelaxation, vasoconstriction, and neovascularization in vasculature (Ebrahimian et al., 2006; Urao et al., 2013). These effects are dependent on H2O2 concentration (Lucchesi et al., 2005). Peroxidase family enzymes are traditionally associated with sensing and regulating overall H2O2 in endothelial cells (Burgoyne et al., 2013). However, recent studies have reported that the peroxidase enzymes may be responsible for propagating downstream effects of H2O2(Fomenko et al., 2011; Marinho et al., 2014). Furthermore, downregulation in the expression levels of catalase, glutathione peroxidase, and MnSOD are reported to correlate with the cytotoxic effect during exogenous H2O2 treatment (Dernbach et al., 2004). However, it is unclear how H2O2 modulates downstream effects, including peroxidase gene expression.
The results from our study suggest a possible H2O2 regulated mechanism for maintaining sustained H2O2 levels for either cell signaling or cytoprotection, as suggested by Groleau et al. (Groleau et al., 2011). Our study showed that H2O2 treatment induced regulation of peroxidase enzymes expression in both treatments. We observed an overall downregulation in expression of CAT, GPX1, PRDX1, TXNRD1 and TXNRD2 at 24 hr of H2O2 treatment. The downregulation in catalase and glutathione peroxidase was also observed after a 4hr treatment of 600 µM H2O2 in HUVECs (Wen et al., 2013). Interestingly, in our study the regulation in CAT, PRDX1, GPX1, and TXNRD1 expressions was similar in both the transient and the consistent H2O2 treatments. In addition, upregulated GPX1 and TXNRD1 expressions during 48 and 72 hr might have counteracted H2O2 during the consistent treatment. As a result, O2− and mitochondrial membrane polarization levels were similar at 72 hr in both treatments. Overexpression of GPX1 increases resistance against H2O2 toxicity in endothelial cells (Faucher et al., 2003). Hence, the increased expression of GPX1 in our results suggest to the H2O2-counteracting state, which helped HUVECs to endure subsequent H2O2 exposure as administered in consistent treatment.
Unlike GPX1, TXNRD1 plays an indirect role in promoting antioxidant activity by maintaining thioredoxin-1 in a reduced form. In physiological condition, the reduced thioredoxin-1 binds to ASK1 to keep it in an inactive form. However, the treatment with H2O2 promotes interaction with reduced-thioredoxin-1 allowing free ASK1 to promote apoptosis (Zschauer et al., 2011). Thus, the increased expression of TXNRD1 suggests an increased conversion of oxidized-thioredoxin-1 to reduced-thioredoxin-1, which promotes peroxidase as well as anti-apoptotic activities(Dammeyer and Arner, 2011). Thus, the regulation of the GPX1 and TXNRD1 expression suggests the existence of protective mechanisms that may be activated through an initial exposure to H2O2. Unlike other peroxidases, the CAT expression remained downregulated in the consistent H2O2 treatment condition for 72 hr. A consistent downregulation in CAT expression, as observed in the consistent H2O2 treatment, suggested interplay between extracellular H2O2 levels with peroxisome activity.
Current literature characterizes H2O2-exposure with a downregulated expression of peroxidases. However, we were able to show induced expression of peroxidase enzymes with extended experimental duration (i.e., 72 hrs), which followed initial downregulation at 24 hrs. The time-dependent effects, along with concentration dependent responses (Lucchesi et al., 2005), can be used to understand varying effects of H2O2 in vasculature (Ebrahimian et al., 2006; Urao et al., 2013).
We observed increase in HMOX1 in both treatment conditions. HMOX1 has a pleiotropic effect on several pro-oxidant and vasoconstrictor pathway genes (Abraham and Kappas, 2011). These regulations may further protect endothelial cells from subsequent H2O2 exposure. Such regulation may be responsible for protecting endothelial cells from higher H2O2 levels, which usually follow after an initial increase in H2O2 levels in wound (Yoo and Huttenlocher, 2009) and ischemia-reperfusion (Slezak et al., 1995).
H2O2 and apoptotic gene expression
Apoptosis signal-regulating kinase1 (Ask1) is traditionally known to initiate cell apoptosis process through p38 and JNK/SAPK pathways. During the exposure to H2O2, Ask1 has been shown to regulate both angiogenesis and apoptosis in endothelial cells (Izumi et al., 2005; Zhang et al., 2005). Similarly, elevated Ask1 levels are reported in high glucose treated HUVECs, which also have elevated H2O2 levels (Patel et al., 2013). A downregulation of ASK1 protects cells from senescence and inflammation (Pan, 2009; Yokoi et al., 2006). Our results showed H2O2 induced significant downregulation in ASK1 gene expression and a similar trend ATF2 gene expression, which is associated to p38 MAPK and JNK pathway regulation. In addition to these, we also observed an increased expression of TXNRD1, which promotes Ask1 inhibition by improving the level of reduced-thioredoxin-1. Thus, the downregulation of ASK1 and ATF2, along with upregulated TXNRD1, may be responsible for cell protection following H2O2 exposure.
Conclusion
We studied endothelial cell oxidative stress related genotypic and functional changes following exposure to H2O2 in both transient and consistent manners. Our results demonstrated that initial (24 hr) H2O2 exposure alters the redox system at functional levels, i.e., O2− levels and mitochondrial membrane polarization, and gene expression levels in HUVECs. Our results also demonstrated that an initial exposure to H2O2 induced a cytoprotective mechanism in HUVECs that enables the cells to sustain subsequent consistent H2O2 exposure events. Significant gene expression changes were seen for peroxidase enzymes (CAT, GPX1, and TXNRD1), mitochondrial electron transport chain uncoupling protein (UCP1), anti-oxidant enzymes (HMOX1), mitogen-activated protein kinases (ASK1), and transcription factors (ATF2). These gene expression changes may be significant for H2O2-induced cytoprotective mechanism in HUVEC.
Figure 7. H2O2 exposure-induced gene expression of cell signaling intermediates.
For 50 µM H2O2 transient (a) and consistent (b) exposure, gene expressions of cell signaling intermediates ASK1, ATF2, and UCP1 were measured at 24, 48, and 72 hrs. Gene expression profile of HUVEC in EGM-2 media without H2O2 at 24, 48 and 72 hr (n=3) was used as respective control for data normalization and statistical analysis. Fold changes in gene expression were normalized against housekeeping genes (i.e. 18s and RPLP0) and control using ΔΔC(t) method. Statistical difference with p≤0.05 is marked with an asterisks (*) (n=3).
Highlights.
H2O2-induced regulation in redox system were studied in ECs for 72 hrs.
Two treatment conditions: transient and consistent H2O2 exposure.
At 24 hr, H2O2 increased O2− and hyperpolarized mitochondrial membrane.
At 48 hr, upregulation in GPX1, TXNRD1, UCP1 & HMOX1 and a decrease in O2− levels.
At 72 hr, a similar extent of functional & genomic regulation in HUVEC in both H2O2 treatments.
Acknowledgements
We thank Sarah Daoud for her assistance to establish mitochondrial membrane polarization measurement procedure. This study was funded by NIH R01 HL084337.
Abbreviations
- O2−
Superoxide
- H2O2
Hydrogen peroxide
- ROS
Reactive oxygen species
- DHE
Dihydroethidium
- EC
Endothelial cell
- HUVEC
Human umbilical vein endothelial cell
- eNOS
Constitutive nitric oxide synthase
- SOD1
Superoxide dismutase 1
- SOD2
Superoxide dismutase 2
- NOX1
NAD(P)H oxidase 1
- IKBKB
inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta
- CAT
Catalase
- PRDX1
Peroxiredoxin
- GPX1
Glutathione peroxidase 1
- TXNRD1
Thioredoxin reductase 1
- TXNRD2
Thioredoxin reductase 1
- NFE2L2
Oxidative stress activated transcription factor, Nrf-2
- HSPA1A
Heat shock 70kDa protein 1A
- UCP1
Uncoupling protein 1
- HMOX1
heme oxygenase (decycling) 1
- ASK1
Mitogen-activated protein kinase kinase kinase 5
- ATF2
Activating transcription factor 2
- 2-OH-E+
2-hydroxyethidium
- Ex
Excitation
- Em
Emission
- ANOVA
Analysis of variance
- S.E.M.
Standard error of mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Hemang Patel, Email: hemang@wayne.edu.
Juan Chen, Email: ej8568@wayne.edu.
Mahendra Kavdia, Email: kavdia@wayne.edu.
References
- Abraham NG, Kappas A. Mechanism of heme-heme oxygenase system impairment of endothelium contraction in the spontaneously hypertensive rat. Hypertension. 2011;58:772–773. doi: 10.1161/HYPERTENSIONAHA.111.178525. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, et al. ATF2 on the double - activating transcription factor and DNA damage response protein. Pigment Cell Res. 2007;20:498–506. doi: 10.1111/j.1600-0749.2007.00414.x. http://dx.doi.org/10.1111/j.1600-0749.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. http://dx.doi.org/10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, et al. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal. 2013;18:1042–1052. doi: 10.1089/ars.2012.4817. http://dx.doi.org/10.1089/ars.2012.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. http://dx.doi.org/10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann Biomed Eng. 2013;41:327–337. doi: 10.1007/s10439-012-0653-x. http://dx.doi.org/10.1007/s10439-012-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese MM, et al. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. http://dx.doi.org/10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Coyle CH, et al. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic Biol Med. 2006;40:2206–2213. doi: 10.1016/j.freeradbiomed.2006.02.017. http://dx.doi.org/10.1016/j.freeradbiomed.2006.02.017. [DOI] [PubMed] [Google Scholar]
- D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. http://dx.doi.org/10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Dammeyer P, Arner ES. Human Protein Atlas of redox systems - what can be learnt? Biochim Biophys Acta. 2011;1810:111–138. doi: 10.1016/j.bbagen.2010.07.004. http://dx.doi.org/10.1016/j.bbagen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Dernbach E, et al. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. http://dx.doi.org/10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- Desaint S, et al. Mammalian antioxidant defenses are not inducible by H2O2. J Biol Chem. 2004;279:31157–31163. doi: 10.1074/jbc.M401888200. http://dx.doi.org/10.1074/jbc.M401888200. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, et al. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Ebrahimian TG, et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. http://dx.doi.org/10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper RJ, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. http://dx.doi.org/10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher K, et al. Overexpression of cytosolic glutathione peroxidase (GPX1) delays endothelial cell growth and increases resistance to toxic challenges. Biochimie. 2003;85:611–617. doi: 10.1016/s0300-9084(03)00089-0. http://dx.doi.org/10.1016/S0300-9084(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Fink BD, et al. Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E71–E79. doi: 10.1152/ajpendo.00332.2004. http://dx.doi.org/10.1152/ajpendo.00332.2004. [DOI] [PubMed] [Google Scholar]
- Fomenko DE, et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2011;108:2729–2734. doi: 10.1073/pnas.1010721108. http://dx.doi.org/10.1073/pnas.1010721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, et al. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. http://dx.doi.org/10.1089/ARS.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau J, et al. Accelerated vascular aging in CuZnSOD-deficient mice: impact on EPC function and reparative neovascularization. PLoS One. 2011;6:e23308. doi: 10.1371/journal.pone.0023308. http://dx.doi.org/10.1371/journal.pone.0023308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, et al. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. http://dx.doi.org/10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz LD, et al. Time course of coronary endothelial healing after injury due to ischemia and reperfusion. Circulation. 1994;90:2439–2447. doi: 10.1161/01.cir.90.5.2439. http://dx.doi.org/10.1161/01.CIR.90.5.2439. [DOI] [PubMed] [Google Scholar]
- Izumi Y, et al. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005;25:1877–1883. doi: 10.1161/01.ATV.0000174801.76234.bd. http://dx.doi.org/10.1161/01.ATV.0000174801.76234.bd. [DOI] [PubMed] [Google Scholar]
- Kar S, et al. Impact of SOD in eNOS uncoupling: a two-edged sword between hydrogen peroxide and peroxynitrite. Free Radic Res. 2012;46:1496–1513. doi: 10.3109/10715762.2012.731052. http://dx.doi.org/10.3109/10715762.2012.731052. [DOI] [PubMed] [Google Scholar]
- Kar S, Kavdia M. Endothelial NO and O(2).(−) production rates differentially regulate oxidative, nitroxidative, and nitrosative stress in the microcirculation. Free Radic Biol Med. 2013;63:161–174. doi: 10.1016/j.freeradbiomed.2013.04.024. http://dx.doi.org/10.1016/j.freeradbiomed.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavdia M. Mathematical and computational models of oxidative and nitrosative stress. Crit Rev Biomed Eng. 2011;39:461–472. doi: 10.1615/critrevbiomedeng.v39.i5.60. http://dx.doi.org/10.1615/CritRevBiomedEng.v39.i5.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kones R, Rumana U. Prevention of cardiovascular disease: updating the immensity of the challenge and the role of risk factors. Hosp Pract (1995) 2014;42:92–100. doi: 10.3810/hp.2014.02.1096. http://dx.doi.org/10.3810/hp.2014.02.1096. [DOI] [PubMed] [Google Scholar]
- Lacy F, et al. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. http://dx.doi.org/10.1161/01.HYP.36.5.878. [DOI] [PubMed] [Google Scholar]
- Lau E, et al. PKCepsilon promotes oncogenic functions of ATF2 in the nucleus while blocking its apoptotic function at mitochondria. Cell. 2012;148:543–555. doi: 10.1016/j.cell.2012.01.016. http://dx.doi.org/10.1016/j.cell.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, et al. Propofol upregulates heme oxygenase-1 through activation of ERKs in human umbilical vein endothelial cells under oxidative stress conditions. J Neurosurg Anesthesiol. 2011;23:229–235. doi: 10.1097/ANA.0b013e31821c007f. http://dx.doi.org/10.1097/ANA.0b013e31821c007f. [DOI] [PubMed] [Google Scholar]
- Liu HT, et al. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res. 2009;59:167–175. doi: 10.1016/j.phrs.2008.12.001. http://dx.doi.org/10.1016/j.phrs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. Resveratrol attenuates hydrogen peroxide-induced apoptosis in human umbilical vein endothelial cells. Eur Rev Med Pharmacol Sci. 2013;17:88–94. [PubMed] [Google Scholar]
- Lucchesi PA, et al. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- Marinho HS, et al. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. http://dx.doi.org/10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msolly A, et al. Hydrogen Peroxide: an Oxidant Stress Indicator in Type 2 Diabetes Mellitus. JCvD. 2013;1:48–52. [Google Scholar]
- Ning JL, et al. Low- and high-dose hydrogen peroxide regulation of transcription factor NF-E2-related factor 2. Chin Med J (Engl) 2010;123:1063–1069. [PubMed] [Google Scholar]
- Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal. 2009;11:1669–1682. doi: 10.1089/ars.2009.2487. http://dx.doi.org/10.1089/ARS.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WH. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int J Mol Med. 2013;31:471–476. doi: 10.3892/ijmm.2012.1215. http://dx.doi.org/10.3892/ijmm.2012.1215. [DOI] [PubMed] [Google Scholar]
- Patel H, et al. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142. doi: 10.1186/1475-2840-12-142. http://dx.doi.org/10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Kwon S. Interplay Between Cytokine-Induced and Cyclic Equibiaxial Deformation-Induced Nitric Oxide Production and Metalloproteases Expression in Human Alveolar Epithelial Cells. Cell Mol Bioeng. 2009;2:615–624. doi: 10.1007/s12195-009-0092-4. http://dx.doi.org/10.1007/s12195-009-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014;126:38–53. doi: 10.3810/pgm.2014.03.2739. http://dx.doi.org/10.3810/pgm.2014.03.2739. [DOI] [PubMed] [Google Scholar]
- Potdar S, Kavdia M. NO/peroxynitrite dynamics of high glucose-exposed HUVECs: chemiluminescent measurement and computational model. Microvasc Res. 2009;78:191–198. doi: 10.1016/j.mvr.2009.04.001. http://dx.doi.org/10.1016/j.mvr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnell CE, et al. Computational insights into the role of glutathione in oxidative stress. Curr Neurovasc Res. 2013;10:185–194. doi: 10.2174/1567202611310020011. [DOI] [PubMed] [Google Scholar]
- Roy S, et al. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. http://dx.doi.org/10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak J, et al. Hydrogen peroxide changes in ischemic and reperfused heart. Cytochemistry and biochemical and X-ray microanalysis. Am J Pathol. 1995;147:772–781. [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- Surico D, et al. Human Chorionic Gonadotropin Protects Vascular Endothelial Cells from Oxidative Stress by Apoptosis Inhibition, Cell Survival Signalling Activation and Mitochondrial Function Protection. Cell Physiol Biochem. 2015;36:2108–2120. doi: 10.1159/000430178. http://dx.doi.org/10.1159/000430178. [DOI] [PubMed] [Google Scholar]
- Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys. 2004;23:265–295. [PubMed] [Google Scholar]
- Ugusman A, et al. Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. BMC Complement Altern Med. 2011;11:31. doi: 10.1186/1472-6882-11-31. http://dx.doi.org/10.1186/1472-6882-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao N, et al. Critical role of endothelial hydrogen peroxide in post-ischemic neovascularization. PLoS One. 2013;8:e57618. doi: 10.1371/journal.pone.0057618. http://dx.doi.org/10.1371/journal.pone.0057618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, et al. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. http://dx.doi.org/10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman DA, et al. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. http://dx.doi.org/10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- Xing S, et al. Salidroside stimulates mitochondrial biogenesis and protects against H(2)O(2)-induced endothelial dysfunction. Oxid Med Cell Longev. 2014;2014:904834. doi: 10.1155/2014/904834. http://dx.doi.org/10.1155/2014/904834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T, et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55:1660–1665. doi: 10.2337/db05-1607. http://dx.doi.org/10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Huttenlocher A. Innate immunity: wounds burst H2O2 signals to leukocytes. Curr Biol. 2009;19:R553–R555. doi: 10.1016/j.cub.2009.06.025. http://dx.doi.org/10.1016/j.cub.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J Biol Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. http://dx.doi.org/10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
- Zschauer TC, et al. Oxidative stress-induced degradation of thioredoxin-1 and apoptosis is inhibited by thioredoxin-1-actin interaction in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:650–656. doi: 10.1161/ATVBAHA.110.218982. http://dx.doi.org/10.1161/ATVBAHA.110.218982. [DOI] [PubMed] [Google Scholar]