Abstract
Despite adenovirus (Ad) vector’s numerous advantages for cancer gene therapy, such as high ability of endosomal escape, efficient nuclear entry mechanism, and high transduction, and therapeutic efficacy, tumor specific targeting and antiviral immune response still remain as a critical challenge in clinical setting. To overcome these obstacles and achieve cancer-specific targeting, we constructed tumor targeting bioreducible polymer, an arginine grafted bio-reducible polymer (ABP)-PEG-HCBP1, by conjugating PEGylated ABP with HCBP1 peptides which has high affinity and selectivity towards hepatoma. The ABP-PEG-HCBP1-conjugated replication incompetent GFP-expressing ad, (Ad/GFP)-ABP-PEG-HCBP1, showed a hepatoma cancer specific uptake and transduction compared to either naked Ad/GFP or Ad/GFP-ABP. Competition assays demonstrated that Ad/GFP-ABP-PEG-HCBP1-mediated transduction was specifically inhibited by HCBP1 peptide rather than coxsackie and adenovirus receptor specific antibody. In addition, ABP-PEG-HCBP1 can protect biological activity of Ad against serum, and considerably reduced both innate and adaptive immune response against Ad. shMet-expressing oncolytic Ad (oAd; RdB/shMet) complexed with ABP-PEG-HCBP1 delivered oAd efficiently into hepatoma cancer cells. The oAd/ABP-PEG-HCBP1 demonstrated enhanced cancer cell killing efficacy in comparison to oAd/ABP complex. Furthermore, Huh7 and HT1080 cancer cells treated with oAd/shMet-ABP-PEG-HCBP1 complex had significantly decreased Met and VEGF expression in hepatoma cancer, but not in non-hepatoma cancer. In sum, these results suggest that HCBP1-conjugated bioreducible polymer could be used to deliver oncolytic Ad safely and efficiently to treat hepatoma.
Keywords: Active targeting, Bioreducible polymer, oncolytic adenovirus, Hepatoma cancer targeting peptide, cancer gene therapy
1. Introduction
The primary goal of gene therapy is development of highly efficient vector or delivery system that can express therapeutic gene at proper target site [1]. To this end, adenovirus (Ad)-based gene delivery system has been extensively evaluated for its therapeutic efficacy and safety in animal models and clinical trials [2, 3]. Oncolytic Ad has numerous advantages for cancer gene therapy such as self-propagation, cancer-specific infection, lysis of infected cancer cells, and secondary infection of neighboring cancer cells within the tumor [4]. Oncolytic Ad as a cancer therapeutic agent has been approved for a clinical phase III trials [5, 6]. However, virotherapy using oncolytic Ad has been strictly limited to local administration [7, 8] as systemic injection can induce acute inflammatory and immune response against Ad, resulting in poor therapeutic efficacy and adverse side effects [9].
Generation of hybrid vector system combining viral and non-viral carriers has been extensively studied as a potential solution to immunogenic nature and nonspecific sequestration of systemically administered naked Ad. Non-viral vector has low immunogenicity and therapeutic genes of large size can be delivered efficiently. Various polymeric carriers have been complexed with plasmid DNA to shield and deliver plasmid DNA to targeted cells [10]. However, these plasmid DNA-based hybrid vectors often exhibit polymer-induced cytotoxicity and low transfection efficiency due to inefficient endosomal escape mechanism and nonbiodegradable attributes of the polymer [11]. Thus, further modification of polymeric carriers is required for clinically efficacious gene delivery.
Previously, our group developed a bio-reducible poly(cystamine bisacrylamide-diaminohexane, CBA-DAH), which showed significantly higher transfection efficiency and lower cytotoxicity than 25 kDa polyethylenimine (PEI) [12]. Additionally, we synthesized arginine-grafted bio-reducible poly(CBA-DAH) polymer (ABP) using backbone of poly (CBA-DAH) [13]. Arginine has known cell penetrating functions and thus arginine-grafted non-viral carriers should elicit high gene delivery efficiency. In this regard, many studies reported that surface modification of Ad with varying nanomaterials, such as ABP [14], methoxy poly(ethyleneglycol)-b-poly{N-[N-(2-aminoethyl)-2-aminoethyl]-l-glutamate [15, 16], mPEG-PEI-g-Arg-S-S-Arg-g-PEI-mPEG [17], bile acid-conjugated PEI [18], and multi-degradable bioreducible PEI [19], through electrostatic interaction resulted in enhanced transfection and lowered immunogenicity of Ad. Cationic polymer coated Ad can easily dissociate in the endosomes, effectively releasing the Ads through the proton-sponge effect [20] which enhances transduction efficacy of Ad. However, excess cationic surface charge of Ad and cationic polymer complex shows poor tumor selectivity due to their nonspecific uptake into the normal cells, restricting its clinical application. Moreover, cationic polymer-based delivery vector easily aggregate and nonspecifically interact with serum proteins ultimately reducing delivery efficacy. Thus, development of polymeric vector with cancer-selective targeting function is required. To overcome nonspecific uptake of polymer-coated Ad, cancer-targeting moieties, such as RGD peptide and folate, have been investigated and demonstrated efficient cancer-specific delivery of Ad [21–23]. Furthermore, several biomarkers with high affinity and cancer-selectivity have demonstrated target specific delivery of Ad [9, 24, 25]. Recently, highly cancer-selective hepatoma targeting peptide (HCBP1), which could discriminate between normal and hepatoma cells both in vitro and in vivo, has been identified by phage display method [26]. Moreover, HCBP1 conjugated chitosan-linked PEI/DNA complex showed high gene transfer efficiency and antitumor efficacy in hepatoma [27].
In this study, we report the development of a novel hepatoma cancer-targeting hybrid delivery system using HCBP1-conjugated polymer and oncolytic Ad. A bioreducible polymer, HCBP1 conjugated PEGylated ABP, was electrostatically complexed with oncolytic Ad generating Ad-ABP-PEG-HCBP1 nanocomplex. The hepatoma-specific cell killing effect and suppression of both c-Met and VEGF expression was investigated using Met-inhibiting oncolytic Ad (RdB/shMet) coated with the ABP-PEG-HCBP1. Furthermore, we demonstrated that the Ad-ABP-PEG-HCBP1 nanocomplex protected Ad against the serum and reduced immunogenicity. In sum, our results show for the first time that Ad-ABP-PEG-HCBP1 hybrid system can efficiently target hepatoma with higher safety profile than naked Ad.
2. Materials and Methods
2.1. Cell lines and preparation of Ad vectors
The human hepato carcinoma (Huh7, and HepG2), Human fibrosarcoma (HT1080), human embryonic kidney cell line expressing the Ad E1 region (HEK 293) cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL) and penicillin/streptomycin (GIBCO-BRL) at 37°C in a humidified atmosphere containing 5% CO2. The adenoviral transduction efficiency was examined with a green fluorescence protein (GFP)-expressing replication-incompetent Ad (Ad/GFP). The Ad/GFP was constructed and characterized in our previous study [9, 28]. The construction and generation of oncolytic Ad backbone was previously described (RdB) [19, 29]. The oncolytic Ad (RdB/shMet, or oAd) was generated and inserted expressing short hairpin RNA (shRNA) against c-Met RNA into the RdB by homologous recombination. The Ad/GFP and RdB/shMet were propagated in HEK293 cells and purified by the CsCl gradient method. Purified viruses were stored at −80°C until use.
2.2. Synthesis of hepatoma targeting peptide conjugated ABP (ABP-PEG-HCBP1)
The poly (cystaminebisacrylamide-diaminohexane) (CBA-DAH) and arginine-grafted poly (cystaminebisacrylamide-diaminohexane) (ABP) were synthethesized as described previously [12, 13]. Then, ABP was activated with NHS-PEG2K-Mal (M.W. = 2 kDa, JenKem Technology, Plano, TX). Briefly, ABP was dissolved in 0.1 M phosphate buffered saline (PBS, pH 7.2, 0.15 M NaCl, 2.0 mM EDTA). 2.4 equivalents of NHS-PEG-Mal were added to the ABP solution and stirred for 1 h at room temperature and then the mixture was dialyzed (MWCO = 3500, Spectrum Laboratories, Inc., Rancho Dominguez, CA) and lyophilized. The molecular weight of ABP-PEG was estimated to be 8.85 × 103 Da/mole by gel-permeation chromatography (GPC). For the thiolation of HCBP1 (FQHPSFI, University of Utah HSC Cores Research Facility, Salt Lake City, UT), Peptide was dissolved in 0.1 M PBS (pH 8.0, 0.15M NaCl, 2.0 mM EDTA). The 2 equivalents of Traut’s reagent (Thermo scientific Inc, Rockford, IL) were added to the peptide solution, and the mixture was further reacted for 2 h at room temperature. Then, the mixture was dialyzed and lyophilized. For the conjugation of target peptides with polymer, PEGylated ABP (ABP-PEG2kDa-Mal) was dissolved in 50 mM PBS (pH 7.2 0.15 M NaCl, 10 mM EDTA). The 1.7 equivalents of thiolated peptide per maleimide groups of ABP-PEG2kDa-Mal were added to the solution. The mixture was further reacted for 4 h and then the mixture was dialyzed and lyophilized. The molecular weight of ABP-PEG-HCBP1 as determined by GPC was 10.6 × 103 Da/mole its PDI value was 1.41. All reaction was monitored by Thin-Layer Chromatography with ninhydrin staining, UV spectroscopy and 1H NMR (400 MHz, D2O).
2.3. Cytotoxicity of ABP, ABP-PEG-HCBP1
The ABP or ABP-PEG-HCBP1 polymers were analyzed for cytotoxicity, including 25 kDa branched PEI (Mw 25,000 Da, Sigma-Aldrich, St. Louis, MO). The cell viability determination was performed by measuring conversion of MTT to formazan as a function of time. Huh7, HepG2, and HT1080 cells were grown to 50% confluence in 96 well plates and were then treated with varying polymer concentrations, up to 20 μg/mL. 48 h following polymer treatment, 100 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, 2 mg/mL in PBS; Sigma) in PBS was added to each well and incubated for 4 h at 37°C. The supernatant was discarded, and the precipitate was dissolved in 200 μL dimethyl sulfoxide (DMSO, Sigma). Plates were read on a microplate reader (Tecan Infinite M200; TecanDeutschland GmbH, Crailsheim, Germany) at 540 nm. The number of living cells in a PBS-treated cell group was analyzed similarly as a negative control.
2.4. Complexation and Physical characterization of polymer coated Ad
Complexes between cationic polymers and Ad were formed by ABP or ABP-PEG-HCBP1 cationic components and the Ad particles (1 × 1010 VP) in an E-tube using PBS (pH 7.4). The molar ratios of cationic molecules to Ad particle (1 × 105 ~ 1 × 106). The diluted cationic polymers were added drop-wise to the solution of diluted Ad particles, mixed by inversion or tapping in a tube diluted to total volume of 100 μl with PBS solution. The hydrodynamic diameters of naked Ad, ABP or ABP-PEG-HCBP1 coated Ad nanocomplex were measured with a dynamic light scattering (DLS). DLS was measured with an argon ion laser set at 488 nm and a fixed 90-degree scattering angle (Malvern Instruments, Inc, Worcestershire, UK). Surface charge was measured using a zeta potentiometer (Zetasizer 3000HS, 10mW HeNe laser, 633 nm; Malvern Instruments) at 25°C. Ad particles (1 × 1010) were gently added to each polymer (1 × 105 ~ 1 × 106 polymer molecules/Ad particle) diluted in PBS for 30 min. The naked Ad, or after formation of Ad-ABP, Ad-ABP-PEG-HCBP1 complexes, HEPES (H0887; Sigma, St. Louis, MO) was added to a final volume at 1 mL before analysis. The sizes and potential values were presented as the average values from five measurements.
2.5. Cellular uptake of Ad/polymers
The synthesis of fluorescein isothiocyanate (FITC, Sigma)-labeled Ad was based on the reaction between the isothiocyanate group of FITC and the primary amine groups of Ad. The Ad (2 × 1011 VPs) was conjugated with FITC (FITC/Ad molar ratio = 1 × 105 in 1mL PBS in the dark at room temperature for 4 h then Ad-FITC was dialyzed at 4°C (10K cut off, Slide-A-Lyzer™ Dialysis Cassettes, Life Technologies, Grand Island, NY) to remove unconjugated FITC and Ad until no fluorescence was detected in the dialysis buffer (PBS). Ad-FITC was sequentially complexed with either ABP or ABP-PEG-HCBP1 for subsequent cellular uptake assay. Huh7 or HT1080 cells were plated onto 24-well plates at 70 – 80% confluence. After 24 h, cells were treated with Ad-FITC, Ad-FITC-ABP, or Ad-FITC-ABP-PEG-HCBP1 at multiplicity of infection (MOI, refers to the number of virion that are added per cell during infection.) of 100 (Huh7) or 500 (HT1080) in 5% FBS containing DMEM for 2 h, and then the cells were washed with ice cold-PBS three times. Cellular uptake activity was quantified by measuring the fluorescence intensity with Tecan Infinite M200 (fluorescence reader).
2.6. Assay for the hepatoma cancer specific transduction efficiency of Ad/GFP-ABP-PEG-HCBP1
Huh7, HepG2, and HT1080 cancer cells were seeded onto 24-well plate at 50 ~ 60% of confluence 24 h in 5% FBS containing DMEM before transduction assay, the cells were treated with naked Ad (Ad/GFP) at a multiplicity of infection (MOI) of 20 (Huh7 or HepG2) and 50 (HT1080) or Ad coated with each polymer (ABP or ABP-PEG-HCBP1) at variable ratio (1 × 105, 3 × 105, 6 × 105, and 1 × 106 molecules/VP). After 48 h of transduction at 37°C, the cells were observed with a fluorescence microscope (Olympus IX81; Olympus Optical, Tokyo, Japan). For quantifying transgene expression, each GFP expression level was quantified by measuring the absorbance at 485 nm for excitation and 535 nm for emission in a plate reader (Tecan Infinite M200) and indicated as values of mean fluorescence intensity (MFI).
2.7. Competition assay
Huh7 cells were seeded onto 24-well plates at 6 × 104 cells per well in 5% FBS containing DMEM. On the following day, either CAR specific antibody (20 μg/ml) or HCBP1peptide (100 nM) diluted in serum-free DMEM or equivalent amount of PBS was treated for 1 h, before naked Ad/GFP, Ad-ABP, or Ad-ABP-PEG-HCBP1 (20 MOI) transduction. The cells were incubated for 2 days, imaged using fluorescence microscopy (Olympus IX81), and analyzed by the fluorescence plate reader (Tecan Infinite M200).
2.8. Cancer cell killing effect of oncolytic Ad complexed with ABP-PEG-HCBP1
To evaluate the hepatoma cancer specific killing effects of oAd or oAd-ABP, oAd-ABP-PEG-HCBP1, Huh7 and HT1080 cells were grown to 50% confluence in 24-well plates in 5% FBS containing DMEM, treated with PBS, naked oAd, oAd-ABP, or oAd-ABP-PEG-HCBP1 (10 MOI for Huh7 cells, 50 MOI for HT1080 cells), and incubated at 37 °C. Two days post-infection, 200 μL of MTT (Sigma) was added to each well. The samples were incubated at 37 °C for 4 h, the supernatants were discarded, and the precipitates were dissolved in 1.0 mL DMSO (Sigma). The plates were read on a microplate reader at 540 nm. The number of living cells in a PBS-treated cell group was analyzed similarly as a negative control.
2.9. Suppression of Met and VEGF
Huh7 and HT1080 cells were seeded on 12-well plate with a number of 2 × 105 cells per well in 5% FBS containing DMEM. After 24 h, the plated cells were treated with RdB/shMet, RdB/shMet coated with ABP, or ABP-PEG-HCBP1 at an MOI of 2 (Huh7), 20 (HT1080), respectively. 72 h later, each conditioned medium was harvested and the knockdown level of Met or VEGF was measured by human c-Met (Invitrogen, life technology. Grand Island, NY 14072) or VEGF (R&D Systems, Inc. Minneapolis, MN 55413) ELISA assay kit.
2.10. Sability test of Ad/polymer complex
After coating Ad/GFP with polymers (ABP or ABP-PEG-HCBP1, 25 kDa PEI, 3 × 105 molecules/VP) for 30 min at room temperature, respectively, naked Ad and each Ad/polymer were incubated with 30% FBS containing PBS at 37°C for 1, 4 h. Then, naked Ad was applied to Huh7 cells at 20 MOI, after 2 days, GFP expression was analyzed and quantified by fluorescence microscope and fluorescent analyzer.
2.11. Assay for Innate and adaptive immune response
For evaluating innate immune response, murine RAW264.7 macrophage cells were seeded on a 6-well plate at a cell density of 5 × 105/well in 5% FBS containing DMEM. After 24 h, cells were infected with PBS, oAd (1 × 1010 VPs) or oAd complexed with ABP (3 × 105 molecules/VP, 1 × 1010 VPs), or ABP-PEG-HCBP1 (3 × 105 molecules/VP, 1 × 1010 VPs). The supernatants from the infected cells were then collected and analyzed for the presence of Interleukin (IL)-6 by the mouse ELISA kit (R&D Quantikine; M6000B). For evaluating adoptive immune response, we used serum from Ad immunized Balb/c mice, as previously describe [15]. The naked Ad/GFP, Ad/GFP-ABP, or Ad/GFP-ABP-PEG-HCBP1 (50 MOI) were exposed to serum with or without Ad-specific neutralizing Ab, for 1h at 37°C and then treated to Huh7. After 2 days of incubation, each GFP expression was analyzed and quantified by fluorescence microscope and fluorescent analyzer.
2.12. Statistical analysis
The data was expressed as the mean ± standard deviation (SD). The Mann–Whitney test was used for statistical comparisons (SPSS 18.0 software; SPSS, Chicago, IL). Groups with P values less than 0.05 were considered statistically significant.
3. Results and discussion
3.1 Synthesis and characterization of HCBP1-conjugated bioreducible polymers
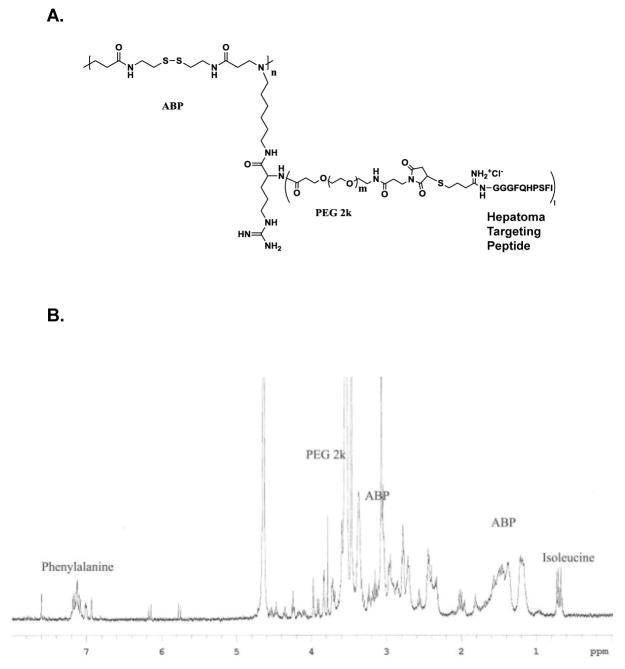
The bioreducible polymer, ABP, showed the greatly enhanced transfection efficiency with significantly less cytotoxicity compared to the PEI due to its bioreducible property [13]. To further impart the hepatoma cancer specific targeting ability, ABP was conjugated with HCBP1 peptide. For enhancing the further in vivo blood circulation and stability, PEG was introduced between ABP and HCBP1 peptide (Figure 1A). The synthesis of ABP-PEG-HCBP1 was confirmed by 1H NMR. The poly (CBA-DAH) was synthesized by using N-Boc-DAH and CBA as repeated monomers. Based on the results of the 1H NMR spectra, poly (CBA-DAH) was composed of eight repeating units and 100% of primary amine of poly (CBA-DAH) was conjugated with arginine. The conjugation ratio of peptide was calculated with unique peaks for ABP (1.2 ppm), PEG (3.6 ppm), isoleucine (0.7 ppm) and phenylalanine of peptide (7.2 ppm) (Figure 1B). This results showed 25% PEG-HCBP1 had been successfully conjugated to the ABP.
Figure 1.
3.2 Generation and characterization of Ad-ABP, Ad-ABP-PEG2k-HCBP1
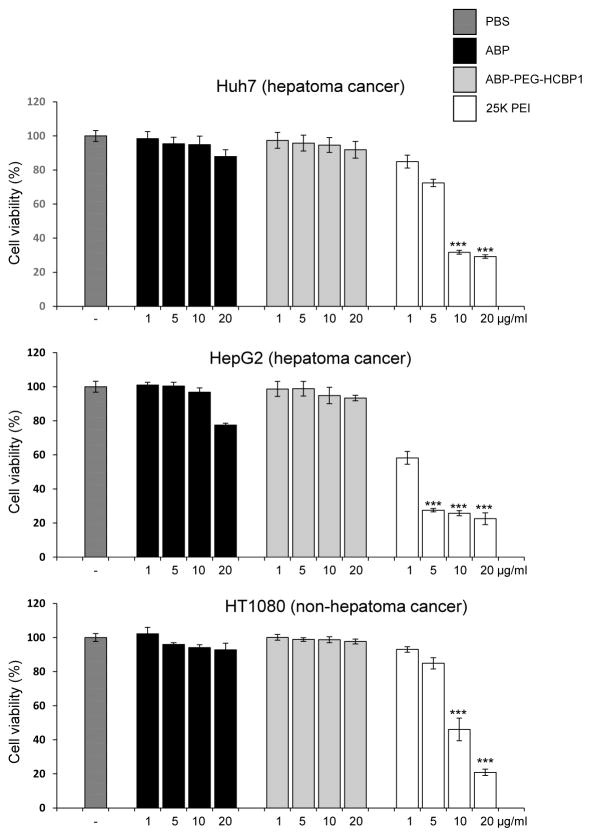
After synthesized polymers, we evaluated the cytotoxicity of ABP-PEG-HCBP1, the viabilities of Huh7, HepG2, and HT1080 cells were measured by MTT assays after treatment with ABP-PEG-HCBP1, 25 kDa PEI, and ABP as controls. As shown in Figure 2, 25 kDa PEI treated cells exhibited significant cytotoxicity. A much lower cytotoxicity was observed for ABP, ABP-PEG-HCBP1 at concentrations below 10 μg/ml (P < 0.001). Meanwhile, ABP exhibited more cytotoxicity compare with ABP-PEG2K-HCBP1 at concentration of 20 μg/ml. The viabilities of the cells treated with the two bioreducible polymers (ABP, ABP-PEG-HCBP1), were above 87.9%, 91.8% for Huh7, 77.5%, 93.4% for HepG2, and 92.8%, 97.7% for HT1080 throughout the up to 20 μg/ml of polymer concentrations, respectively. These findings were correlated with previous report demonstrating that ABP showed a few cytotoxicity. More importantly, ABP-PEG-HCBP1 also showed no cytotoxicity, it may be attributed to the reducible character of ABP as well as conjugation of PEG.
Figure 2.
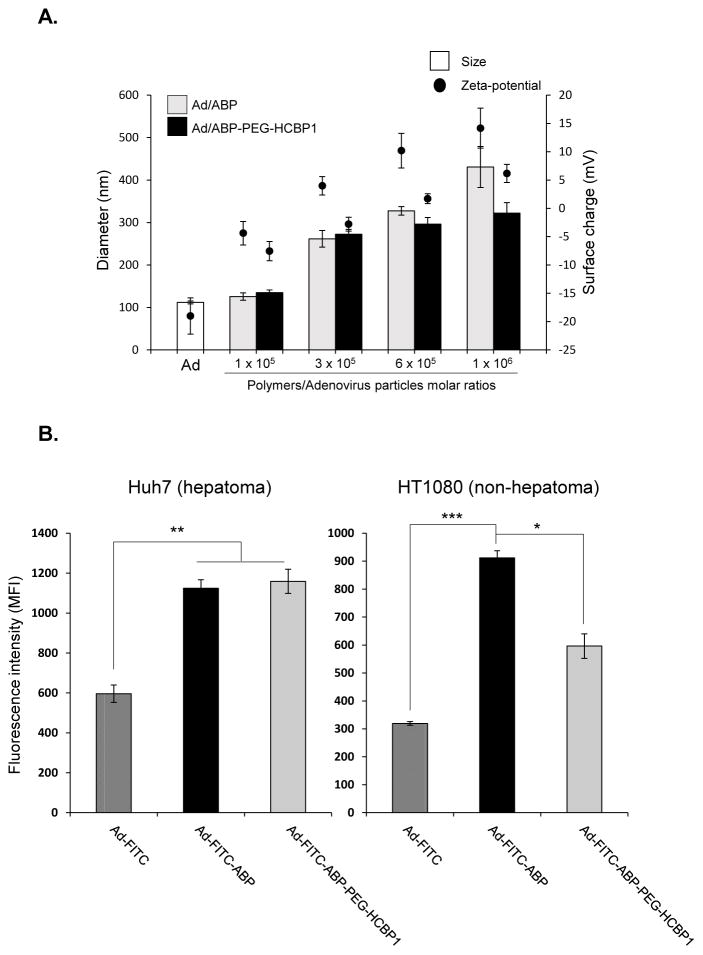
The average size of naked Ad or Ad coated with the polymers (ABP, HCBP1 conjugated PEGylated ABP) were assessed by DLS. As shown in figure 3A, while the average size of naked Ad was about 112 nm, size of polymer coated Ad complexes were shown approximately 125.7, 261.7, 327.3, and 430.6 nm for Ad-ABP, 134.7, 272.3, 296.3, and 322.4 for Ad-ABP-PEG-HCBP1, respectively. This result describes that the positively charged HCBP1-conjugated PEGylated ABP can physically form complex with the negatively charged surface of Ad. In zeta potential results, naked Ad showed negative charge (−19 mV), in contrast the surface charge of ABP coated Ad increased to positive charge (−4.4, 3.9, 10.2, and 14.2 mV) following increasing polymer concentration. Moreover surface charge of Ad-ABP-PEG-HCBP1 was decreased compared to Ad-ABP, due to PEGylation (−7.5, −2.8, 1.7, and 6.2 mV). This suggests that ABP or ABP-PEG-HCBP1 successfully coated the surface of Ad through electrostatic interactions, resulting in a net positive charge (Ad-ABP) or neutral charge (Ad-ABP-PEG-HCBP1). Of note, size of Ad-ABP indicated larger than Ad-ABP-PEG-HCBP1 complex, implying strong positive charge of ABP can induce aggregation of Ad/polymer complex through interaction with negative charge of Ad [14]. One of the advantages with PEGylation method is enable to decrease surface charge of cationic polymer resulting increased stability in the serum. However, reduced surface charge of polymer complex could decrease the transduction efficiency, due to reduce the interaction between cationic polymer and cellular surface. Thus, we introduced HCBP1, hepatoma cancer targeted peptide to the cationic bioreducible polymer for enhancing transduction efficiency.
Figure 3.
To further evaluate hepatoma cancer targeting efficiency of Ad-ABP-PEG-HCBP1, we performed the cellular uptake assay of Ad-ABP-PEG-HCBP1 complex in comparison to naked Ad or ABP-coated Ad by fluorescence labeling with FITC (Figure 3B). Targeting specific binding ligands on the certain tumor site is an efficient way to improve the selectivity of therapeutic vector, and this process is critical to the development of cancer specific therapy. For this purpose, we used previously investigated HCBP1 peptide by phage display technology, which showed high accuracy and selectivity for hepatoma cancer. The cellular uptake was markedly enhanced when Ad-FITC was complexed with either ABP or ABP-PEG-HCBP1 compared to naked Ad-FITC in hepatoma cancer (P < 0.01). However, cellular uptake was significantly reduced when non-hepatoma cancer cells were treated with Ad-FITC-ABP-PEG-HCBP1 in comparison to that with Ad-FITC-ABP (P < 0.05). Ad-FITC-ABP treated cells showed high cellular uptake efficiency both hepatoma and non-hepatoma cancer due to strong net positive surface charge, otherwise, Ad-FITC-ABP-PEG-HCBP1 treated cells showed 1.6-fold increase in hepatoma cancer compare to non-hepatoma cancer (supplementary figure 1, P < 0.01), suggesting that conjugation of HCBP1 has specifically targeting to the hepatoma cancer.
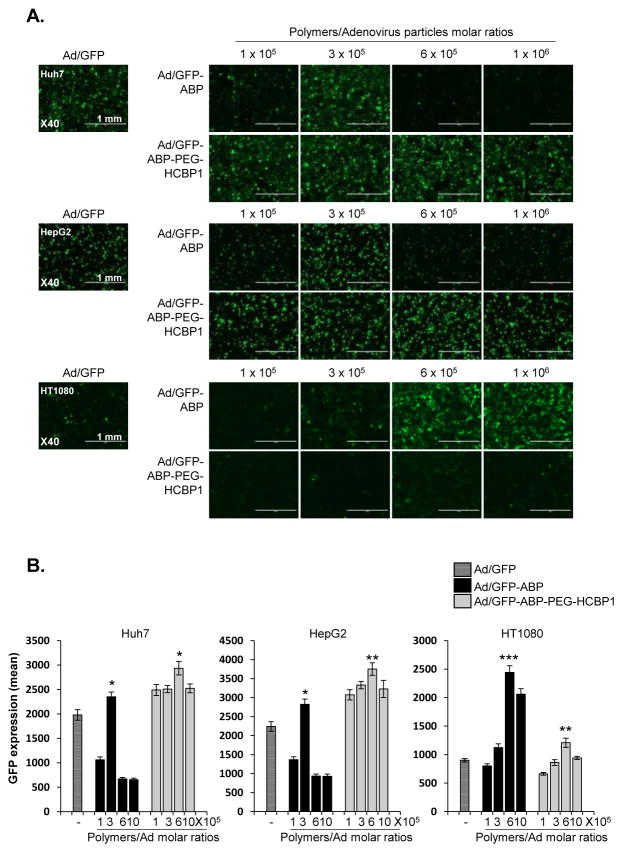
3.3 Hepatoma cancer cell specific transduction efficiency of Ad-ABP-PEG-HCBP1
To test whether the Ad-ABP-PEG-HCBP1 nanocomplexes showed hepatoma specific transduction, GFP expression was measured in both hepatoma cancer and non-hepatoma cancer cell lines. Huh7 and HepG2 hepatoma cancer at a multiplicity of infection (MOI) of 20, and non-hepatoma cancer HT1080 cells (50 MOI) were transduced with Ad/GFP, Ad/GFP-ABP or Ad/GFP-ABP-PEG-HCBP1, and at polymer: Ad molar ratios ranging from 1 × 105 to 1 × 106. After 48 h of incubation, GFP expressing levels were measured by microscopy images and fluorescence intensity. We previously reported that bioreducible ABP polymer coated Ad results in high transduction efficiency and significantly lesser cytotoxicity than 25 kDa PEI due to its bioreducibility [14]. As shown in Figure 4, the transduction efficiency of Ad/GFP-ABP was markedly increased in comparison to naked Ad in Huh7 (polymer:Ad molar ratios of 3 × 105), HepG2 (polymer: Ad molar ratios of 3 × 105), and HT1080 cells (polymer:Ad molar ratio of 6 × 105). This suggests that Ad-ABP can efficiently transduce cancer cells through charge-mediated cell entry mechanism rather than coxsackie and adenovirus receptor (CAR)- and HCBP1 receptor (HCBP1R)-mediated entry. However, HCBP1-conjugated ABP backbone has PEGylated primary amine groups which decrease net cationic surface charge of the polymer resulting in decreased internalization of Ad-ABP-PEG-HCBP1 in comparison to ABP-complexed Ad. In HCBP1R-negative cells, Ad-ABP-PEG-HCBP1 is expected to have lower transduction than Ad-ABP due to issues such as steric hindrance caused by PEG side chains. Of note, Ad-ABP-PEG-HCBP1 exhibits significantly enhanced transduction than Ad-ABP in cancer cells with high HCBP1R expression which confirms efficient targeting by conjugated HCBP1 on the polymer surface. In specific, Ad/GFP-ABP-PEG-HCBP1 induced GFP expression showed higher level compared to Ad/GFP-ABP treated cell in both Huh7 (P < 0.05) and HepG2 (P < 0.01) cell lines, respectively. However, transduction efficiency into HT1080 non-hepatoma cells did not show significant difference with targeting peptide (P < 0.01). Of note, transduction efficiency of Ad/GFP-ABP exhibited significant variations depending on ABP concentration reacted with Ad. Insufficient polymer concentration can lead to inefficient cellular uptake due to low cationic charge of Ad/polymer. However, excess polymer can also decrease polyplex’s transduction efficiency due to increased particle size or poor endosomal escape mechanism. Moreover, Ad/polymer complex has different optimizing conditions for transduction in different cell lines likely due to varying cellular endocytosis or pinocytosis property. Thus optimization of Ad/polymer is required to enhance complex’s cellular uptake. As shown in supplementary figure 2, Ad-ABP-PEG-HCBP1(1: 6 × 105 ratio) treated cells showed 3.3-, 4.0-fold increase transduction efficiency compare to Ad-ABP treated cells in Huh7 and HepG2 hepatoma cancer (P < 0.001) however transduction efficiency decreased 0.5-fold in HT1080 non-hepatoma cancer (P < 0.01). The results demonstrate that ABP-PEG-HCBP1 polymer give Ad to selectivity for the hepatoma cancer and limited delivery into non-hepatoma cells.
Figure 4.
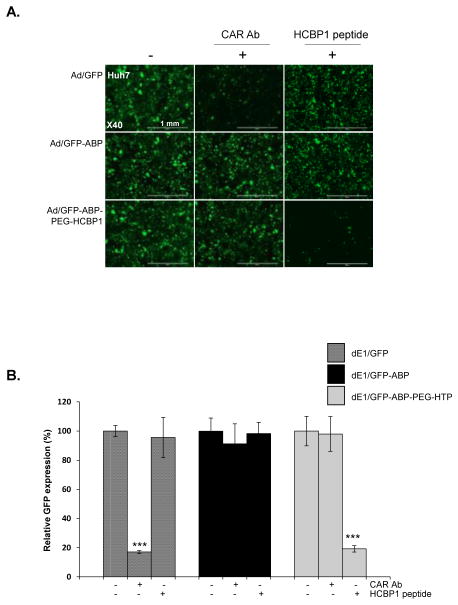
To further demonstrate that Ad-ABP-PEG-HCBP1 transduction was dependent on HCBP1R and independent of the CAR, Huh7 hepatoma cancer, which express both HCBP1R and CAR, were pre-incubated with free HCBP1 peptide or CAR Ab prior to transduction, respectively. (Figure 5). Pretreatment of Huh7 cells with CAR Ab significantly reduced GFP transduction of naked Ad/GFP decreasing 83% (P < 0.001). In contrast, Ad/GFP-ABP or Ad/GFP-ABP-PEG-HCBP1 transduced GFP expression was not reduced by the effects of CAR Ab, indicating that Ad/GFP-ABP or Ad/GFP-ABP-PEG-HCBP1 uptake was not mediated by interaction between CAR and adenoviral fiber. When Huh7 cells were pre-treated with the free HCBP1 peptide, GFP transduction by Ad/GFP-ABP-PEG-HCBP1 was 81% (P < 0.001) lower than in non-treated cells, but HCBP1 had no effect on GFP transduction by both naked Ad/GFP and Ad/GFP-ABP. Thus, these data show that Ad/ABP-PEG-HCBP1 cellular entry is mediated primarily by CAR-independent pathway and HCBP1R-dependent mediated pathway, and it may have hepatoma specific therapeutic value.
Figure 5.
3.4 Cancer cell killing effect of ABP-PEG-HCBP1 with oncolytic adenovirus
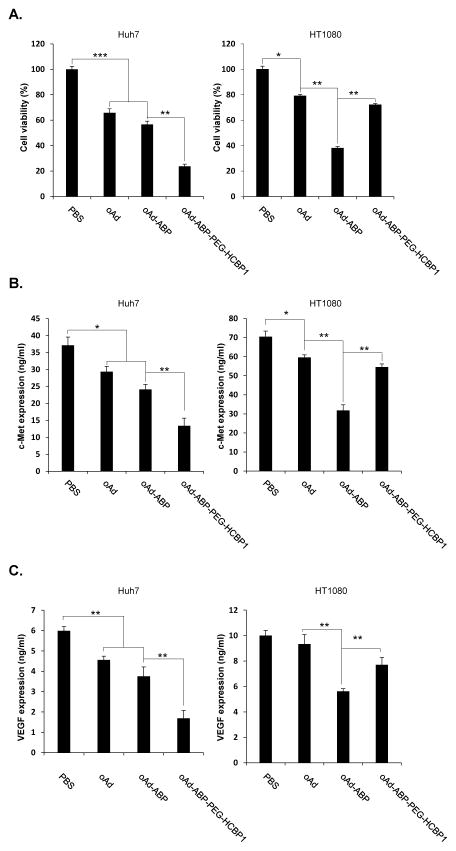
To confirm the hepatoma cell specific killing effect, MTT assay was performed using with oncolytic adenovirus complexed with ABP-PEG-HCBP1 (Figure 6A). The c-Met signaling promotes hepatocyte proliferation and regeneration, suggesting a potential tumor-promoting role in hepatoma [30]. The signaling of c-Met and its ligand is often overexpressed in several cancer cells, and triggered primary tumor growth and metastasis. Thus specific knockdown against c-Met can give rise a promising therapeutic efficacy through abrogate c-Met and HGF signaling in hepatoma cancer. In this study, we investigated a dual tumor-targeting therapy by hepatoma cancer specific targeting peptide HCBP1 conjugated ABP complexed with an oncolytic Ad expressing c-Met specific knockdown by short hairpin Met (RdB/shMet, oAd), which can strongly suppress c-Met signaling for hepatoma. Previously, our group has engineered an Ad expressing c-Met-specific shRNAs which showed potent and effective suppression of endogenous expression of c-Met. [31]. One of the limitation of siRNA system as therapeutic agent is short duration of siRNA expression, resulting in suboptimal therapeutic efficacy in vivo. siRNA-expressing oncolytic Ad system can overcome this limitation as oncolytic Ad selectively-replicates in cancer cells which can amplify and prolong expression of shMet in the tumor microenvironment. In addition, HGF–Met signaling is a potent inducer of endothelial cell growth and promoter of angiogenesis. Therefore, shMet-expressing oncolytic Ad can sequentially downregulate angiogenesis through inhibition of Met. [32]. We hypothesized that the generated oAd-ABP-PEG-HCBP1 would target both the hepatoma-specific tumor and knockdown of overexpressed Met in hepatoma cancer. In the result, Cancer cell killing efficacy was increased when oncolytic Ad was complexed with ABP, showing 43.3% for Huh7 and 62.1% for HT1080 increase of cancer cell killing at 10 (Huh7 and HepG2), 50 (HT1080) MOI, suggesting the facilitated oncolytic Ad delivery mediated by positively charged ABP either hepatoma or non-hepatoma cells (P < 0.001 for Huh7 and P < 0.01 for HT1080). In marked contrast, oAd-ABP-HCBP1 elicited significantly enhanced cancer cell killing efficacy compared to either naked oAd or oAd-ABP in hepatoma cells. In specific, cancer cell killing was observed at 76.3% for Huh7 and 28% for HT1080, showing2.2- and 1.3-fold increase compared to naked oAd, respectively. This results suggest that oAd-ABP-PEG-HCBP1 had potent oncolytic activity, comparable with either naked oAd or oAd-ABP, but it was restricted to non-hepatoma cells. Of interest, oAd-ABP-PEG-HCBP1 treated non-hepatoma cell was much less cancer cell killing than oAd-ABP treatment for non-hepatoma HT1080 cells, suggesting that conjugation with HCBP1 peptide may have provided de-targeting ability against non-target cells oncolytic activity. Together, these data demonstrate that oncolytic Ad coated with ABP-PEG-HCBP1 specifically killed hepatoma cancer, mediated by HCBP1 targeting peptide.
Figure 6.
3.5 Suppression of VEGF and Met by ABP-PEG-HCBP1 with oncolytic adenovirus
Vascular endothelial growth factor (VEGF) has been demonstrated to be one of key regulator of angiogenesis and, it plays a crucial role in tumor growth and progression [33]. Previous studies has reported that c-Met induce the angiogenesis by upregulation of VEGF level in vitro and in vivo [34]. Moreover, cross-talk between the c-Met and VEGF pathways synergistically enhanced proliferation, cytoskeletal remodeling, and migration in endothelial cells [35]. With this background, we hypothesized that shMet-expressing oncolytic Ad-ABP-PEG-HCBP1 can inhibit the both Met and VEGF level, resulted in maximized therapeutic efficacy for hepatoma cancer. To investigate the effects of Met-specific shRNA, the expression level of Met and VEGF were determined in the hepatoma cancer (Huh7) and non-hepatoma cancer cells (HT1080) treated with oAd, oAd–ABP, oAd–ABP-PEG-HCBP1 using ELISA assay, respectively. As shown in Fig. 6B. C, the significant suppression of Met or VEGF expression by Ad-ABP was observed in both cancer cells (Huh7 and HT1080) when compared with the results by naked oAd. However, oAd -ABP-PEG-HCBP1 treated cells showed significantly suppression of Met or VEGF expression in hepatoma cancer compared with oAd-ABP treated group, but no significant suppression of Met or VEGF expression was observed in HT1080 cancer cells. Among all experimental groups, the most noticeable suppression of Met or VEGF expression by oAd-ABP-PEG-HCBP1 complex was investigated with 2.2- for Met or 2.7-fold for VEGF decreased than oAd in Huh7 cells. These results demonstrate that oAd complexed with ABP-PEG-HCBP1 can suppress both Met and VEGF expression in hepatoma cancer, maximized therapeutic value.
3.6 Stability and safety profiles of Ad/ABP-PEG-HCBP1
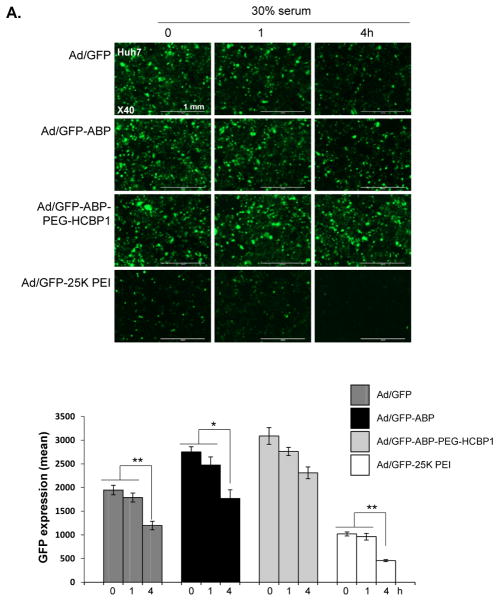
Serum proteins can have a significant impact on the biological activity of Ad or Ad/polymers because of non-specific interaction between Ad or Ad/polymer complexes and serum or other negatively charged components. This interaction may induce complex aggregation or dismantling, which makes the decreasing gene transfection efficiency [36]. Thus, we evaluate the effect of serum on the stability of Ad or Ad/polymers complexes in the presence or absence of 30% serum. To evaluate protection against serum, Ad/GFP, Ad/GFP-ABP, or Ad/GFP-ABP-PEG-HCBP1, or Ad/GFP-PEI 25 kDa PEI were incubated in 30% serum for 1, 4 h, and transgene expression efficiency was estimated. As shown in Figure 7A, the transduction efficiency of Ad/GFP, Ad/GFP-25 kDa PEI were significantly decreased as incubation time increased (P < 0.01), whereas Ad/GFP-ABP were less negatively influenced by serum (P < 0.05). However, Ad/ABP-PEG-HCBP1 was not decrease the adenoviral transduction activity at both 1 h and 4 h serum incubation. These results demonstrate that coating the surface of Ad with ABP-PEG-HCBP1 can protect Ad against serum due to its PEGylation, supporting the potential use of ABP-PEG-HCBP1-coated Ad for clinical application.
Figure 7.
The major hurdle of systemic injection of Ad for maximized therapeutic value is host immune response to inactive foreign pathogens [4]. After systemic injection of Ad, innate immune response factors such as interleukin-6, or tumor necrosis factor-α were elevated resulted in increasing host adverse effect [28, 37]. Moreover, pre-exist anti-Ad neutralizing Ab captured and inactivated the injected Ad could decrease the therapeutic value. Therefore, it is novel strategy for overcoming the limit of therapeutic Ad delivery by masking the surface of the Ad vectors with non-immunogenic viral vectors.
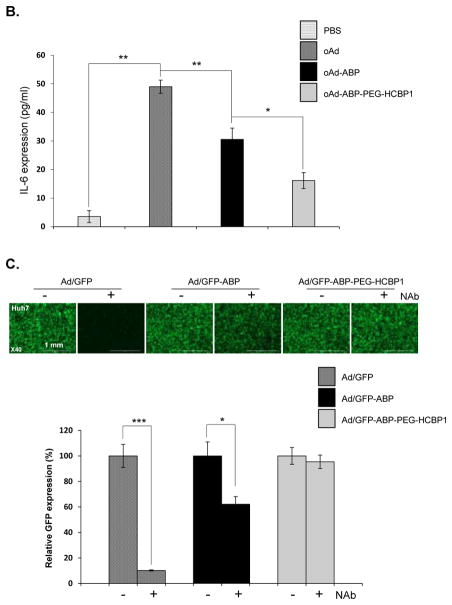
First, we evaluated the attenuation of pro-inflammatory cytokine IL-6 secretion from murine macrophage cell (RAW264.7) by infection with Ad complexed with ABP or ABP-PEG-HCBP1 (Fig. 7B). The secretion of IL-6 in RdB/shMet-infected macrophage cells were 13.8-fold higher than those of PBS-treated macrophage cells, confirming that naked Ad evoked a strong innate immune response (P < 0.01). In contrast, there was no significant secretion of IL-6 from infection with either RdB/shMet-ABP or RdB/shMet-ABP-PEG-HCBP1. Of interestingly, RdB/shMet-ABP-PEG-HCBP1 treated macrophage cells less secretion of IL-6 than RdB/shMet-ABP, due to PEGylation of ABP. This noteworthy results showed that coating the surface of the Ad with non-immunogenic polymer such as ABP or ABP-PEG-HCBP1 can significantly prevent the innate immune response.
To evaluate the adoptive immune evasion of the Ad/polymer complex, naked Ad/GFP, Ad/GFP-ABP, or Ad/GFP-AB-PEG-HCBP1 complexes were incubated for 1 h with serum from Ad-immunized mice or non-immunized serum, respectively. After incubation with non-immunized serum, or serum containing Ad-specific neutralizing Ab (Nab), Huh7 cells were transduced with each experimental group (Figure 7C). The result showed the transduction efficiency of Ad/GFP incubated with Nab treated cells were significantly reduced by 89.9% compared to Ad/GFP incubated with serum without Nab treated cells (P < 0.001), the GFP expression levels of cells treated with Ad/GFP-ABP incubated with Nab serum, less decreasing GFP expression compared to Ad/GFP-ABP treated cells. However, Ad/GFP-ABP-PEG-HCBP1 treated cells have shown no difference of GFP expression levels between the cells whether exposed to the serum with or without the Nab, indicating PEGylation of ABP may more protect Ad against Nab-containing serum than ABP. All together, these data demonstrate that ABP-PEG-HCBP1 masking the surface of Ad particles can substantially reduce the not only innate immune response and but also the adaptive immune response related with Ad-specific NAb.
4. Conclusions
In this study, we synthesized hepatoma-targeting peptide (HCBP1)-conjugated bioreducible polymer to induce hepatoma-specific delivery of potent oncolytic Ad expressing shRNA against c-Met mRNA. Hybrid systems utilizing cationic nonviral carrier and oncolytic Ad for systemic administration can encounter several hurdles such as nonspecific sequestration and instability in the blood due to net cationic surface charge of the polyplex. To this end, conjugation with tumor targeting moiety and PEGylation can increase tumor targeting efficacy and blood circulation time, respectively. ABP-PEG-HCBP1 coated Ad showed highly hepatoma- selective and efficacious transduction and cytolysis. Competition assay with CAR Ab or free HCBP1 peptides confirmed that Ad/ABP-PEG-HCBP1 is internalized by HCBP1R rather than CAR ultimately overcoming CAR-dependency of Ad. Furthermore, Ad/ABP-PEG-HCBP1 induced significantly enhanced Met or VEGF suppression in cancer cells than naked oncolytic Ad. Moreover, Ad-ABP-PEG-HCBP1 complex enabled Ad to evade both innate and adaptive immune responses, implying that Ad/ABP-PEG-HCBP1 nanocomplex is suitable for further in vivo study. To our knowledge, the data presented here is the first to demonstrate hepatoma-specific delivery by hepatoma-targeting peptide conjugated bioreducible polymer coated oncolytic Ad, and its enhanced therapeutic efficacy and safety.
Supplementary Material
Acknowledgments
This work was supported by grants from and the National Institutes of Health, USA (CA177932, S-W. Kim), National Research Foundation of Korea (2010-0029220, 2013M3A9D3045879, 2013K1A1A2A02050188, C-O. Yun). The author holds patent rights along with the University of Utah. Although a financial conflict of interest was identified for management based on the overall scope of the project and its potential benefit to the Dr. SW Kim, the research findings included in this publication may not necessarily related to the interests of Dr. SW Kim. The terms of this arrangement have been reviewed and approved by the University of Utah in accordance with its policy on objectivity in research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nature reviews Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 3.Yun CO. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Current opinion in molecular therapeutics. 2008;10:356–361. [PubMed] [Google Scholar]
- 4.Choi JW, Lee JS, Kim SW, Yun CO. Evolution of oncolytic adenovirus for cancer treatment. Advanced drug delivery reviews. 2012;64:720–729. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Human gene therapy. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 6.Raty JK, Pikkarainen JT, Wirth T, Yla-Herttuala S. Gene therapy: the first approved gene-based medicines, molecular mechanisms and clinical indications. Current molecular pharmacology. 2008;1:13–23. doi: 10.2174/1874467210801010013. [DOI] [PubMed] [Google Scholar]
- 7.Alemany R. Cancer selective adenoviruses. Molecular aspects of medicine. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JM. Adenovirus-based cancer gene therapy. Current gene therapy. 2005;5:595–605. doi: 10.2174/156652305774964677. [DOI] [PubMed] [Google Scholar]
- 9.Kim PH, Sohn JH, Choi JW, Jung Y, Kim SW, Haam S, Yun CO. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials. 2011;32:2314–2326. doi: 10.1016/j.biomaterials.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Advanced drug delivery reviews. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Parker AL, Newman C, Briggs S, Seymour L, Sheridan PJ. Nonviral gene delivery: techniques and implications for molecular medicine. Expert reviews in molecular medicine. 2003;5:1–15. doi: 10.1017/S1462399403006562. [DOI] [PubMed] [Google Scholar]
- 12.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjugate chemistry. 2008;19:626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30:658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim PH, Kim TI, Yockman JW, Kim SW, Yun CO. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31:1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Li Y, Kim SW, Lee DS, Yun CO. Therapeutic efficacy of a systemically delivered oncolytic adenovirus - biodegradable polymer complex. Biomaterials. 2013;34:4622–4631. doi: 10.1016/j.biomaterials.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Kim J, Bui QN, Li Y, Yun CO, Lee DS, Kim SW. Tuning surface charge and PEGylation of biocompatible polymers for efficient delivering of nucleic acid or adenoviral vector. Bioconjugate chemistry. 2015 doi: 10.1021/acs.bioconjchem.5b00357. [DOI] [PubMed] [Google Scholar]
- 17.Jung SJ, Kasala D, Choi JW, Lee SH, Hwang JK, Kim SW, Yun CO. Safety Profiles and Antitumor Efficacy of Oncolytic Adenovirus Coated with Bioreducible Polymer in the Treatment of a CAR Negative Tumor Model. Biomacromolecules. 2015;16:87–96. doi: 10.1021/bm501116x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Kasala D, Na Y, Lee MS, Kim SW, Jeong JH, Yun CO. Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression. Biomaterials. 2014;35:5505–5516. doi: 10.1016/j.biomaterials.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 19.Choi JW, Nam JP, Nam K, Lee YS, Yun CO, Kim SW. Oncolytic Adenovirus Coated with Multidegradable Bioreducible Core-Cross-Linked Polyethylenimine for Cancer Gene Therapy. Biomacromolecules. 2015;16:2132–2143. doi: 10.1021/acs.biomac.5b00538. [DOI] [PubMed] [Google Scholar]
- 20.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. Journal of controlled release: official journal of the Controlled Release Society. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kim HA, Nam K, Kim SW. Tumor targeting RGD conjugated bio-reducible polymer for VEGF siRNA expressing plasmid delivery. Biomaterials. 2014;35:7543–7552. doi: 10.1016/j.biomaterials.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benns JM, Mahato RI, Kim SW. Optimization of factors influencing the transfection efficiency of folate-PEG-folate-graft-polyethylenimine. Journal of controlled release: official journal of the Controlled Release Society. 2002;79:255–269. doi: 10.1016/s0168-3659(01)00513-2. [DOI] [PubMed] [Google Scholar]
- 23.Nam HY, Kim J, Kim SW, Bull DA. Cell targeting peptide conjugation to siRNA polyplexes for effective gene silencing in cardiomyocytes. Molecular pharmaceutics. 2012;9:1302–1309. doi: 10.1021/mp200589z. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Nam HY, Kim TI, Kim PH, Ryu J, Yun CO, Kim SW. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials. 2011;32:5158–5166. doi: 10.1016/j.biomaterials.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon OJ, Kang E, Choi JW, Kim SW, Yun CO. Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. Journal of controlled release: official journal of the Controlled Release Society. 2013;169:257–265. doi: 10.1016/j.jconrel.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Zhang Y, Wang J, Zhang Y, Chen J, Pan Y, Ren L, Hu Z, Zhao J, Liao M, Wang S. Screening and identification of a targeting peptide to hepatocarcinoma from a phage display peptide library. Molecular medicine. 2007;13:246–254. doi: 10.2119/2006-00115.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao QQ, Hu YL, Zhou Y, Li N, Han M, Tang GP, Qiu F, Tabata Y, Gao JQ. Gene-carried hepatoma targeting complex induced high gene transfection efficiency with low toxicity and significant antitumor activity. International journal of nanomedicine. 2012;7:3191–3202. doi: 10.2147/IJN.S30909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JW, Jung SJ, Kasala D, Hwang JK, Hu J, Bae YH, Yun CO. pH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesis. Journal of controlled release: official journal of the Controlled Release Society. 2015 doi: 10.1016/j.jconrel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Kim JH, Choi KJ, Kim PH, Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Human gene therapy. 2007;18:773–786. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- 30.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:2310–2318. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS, Oh E, Yoo JY, Choi KS, Yoon MJ, Yun CO. Adenovirus expressing dual c-Met-specific shRNA exhibits potent antitumor effect through autophagic cell death accompanied by senescence-like phenotypes in glioblastoma cells. Oncotarget. 2015;6:4051–4065. doi: 10.18632/oncotarget.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nature reviews Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 33.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulpice E, Ding S, Muscatelli-Groux B, Berge M, Han ZC, Plouet J, Tobelem G, Merkulova-Rainon T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biology of the cell/under the auspices of the European Cell Biology Organization. 2009;101:525–539. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 36.Wu HM, Pan SR, Chen MW, Wu Y, Wang C, Wen YT, Zeng X, Wu CB. A serum-resistant polyamidoamine-based polypeptide dendrimer for gene transfection. Biomaterials. 2011;32:1619–1634. doi: 10.1016/j.biomaterials.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Molecular therapy: the journal of the American Society of Gene Therapy. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.