Abstract
Background
Trauma induced coagulopathy (TIC) is associated with a four-fold increased risk of mortality. Hyperfibrinolysis is a component of TIC, but its mechanism is poorly understood. PAI-1 degradation by activated protein C has been proposed as mechanism for deregulation of the plasmin system in hemorrhagic shock, but in other settings of ischemia, tPA has been shown to be elevated. We hypothesized that the hyperfibrinolysis in TIC is not the result of PAI-1 degradation, but is driven by an increase in tPA, with resultant loss of PAI-1 activity through complexation with tPA.
Methods
86 consecutive trauma activation patients had blood collected at the earliest time after injury, and were screened for hyperfibrinolysis using thrombelastography (TEG). Twenty-five hyperfibrinolytic patients were compared to 14 healthy controls using ELISAs for active tPA, active PAI-1 and PAI-1/tPA complex. Blood was also subjected to TEG with exogenous tPA-challenge as a functional assay for PAI-1 reserve.
Results
Total levels of PAI-1 (the sum of the active PAI-1 species and its covalent complex with tPA) are not significantly different between hyperfibrinolytic trauma patients and healthy controls: median 104 pM (IQR 48—201 pM) versus 115 pM (IQR 54—202 pM). The ratio of active to complexed PAI-1, however, was two orders of magnitude lower in hyperfibrinolysis than controls. Conversely, total tPA levels (active plus complex) were significantly higher in hyperfibrinolysis than controls: 139 pM (IQR 68—237 pM) versus 32 pM (IQR 16—37 pM). Hyperfibrinolytic trauma patients displayed increased sensitivity to exogenous challenge with tPA: median LY30 of 66.8% compared to 9.6% for controls.
Conclusions
Depletion of PAI-1 in TIC is driven by an increase in tPA, not PAI-1 degradation. The tPA-challenged TEG, based on this principle, is a functional test for PAI-1 reserves. Exploration of the mechanism of upregulation of tPA is critical to an understanding of hyperfibrinolysis in trauma.
Keywords: hyperfibrinolysis, PAI-1, tPA, aPC, trauma, massive transfusion
Background
Trauma induced coagulopathy (TIC) is present in roughly one-third of severely injured trauma patients at the time of admission and is associated with a four-fold risk of mortality.(1-6) Systemic hyperfibrinolysis is a particularly lethal component of TIC, associated with a greater than 60% mortality rate.(7-9) An understanding of the role of the plasminogen-plasmin system in trauma is in its infancy, but while non-canonical mediators of clot lysis may play a role in the hyperfibrinolysis of TIC, elevated levels of tPA have been observed in coagulopathic trauma patients. (10) Additionally, the reversibility of this pathology with the lysine analog tranexamic acid TXA points to upregulation of the tPA/plasminogen-plasmin system as being chiefly responsible for this phenomenon.(11-19).
Brohi and Cohen et al. proposed a unifying theory of TIC, implicating activated protein C (aPC) in the pathogenesis of both soluble phase coagulopathy and hyperfibrinolysis. In their model, both the degradation of the soluble factors V and VIII as well the indirect upregulation of fibrinolysis due to consumption of plasminogen activation inhibitor (PAI-1) are driven the proteolytic action of aPC. (2, 13, 20-23)
This is plausible, as PAI-1 is the cognate inhibitor of tissue plasminogen activator (tPA), the two enzymes forming a mutually inhibitory covalent complex of 1:1 stoichiometry.(24, 25) Indeed, decreased levels of PAI-1 activity have been demonstrated in trauma patients with hyperfibrinolysis and PAI-1 is known to be inactivated by interaction with a number of proteolytic enzymes including aPC and neutrophil elastase, which may themselves be upregulated in conditions of injury, inflammation and shock. (26-29) It has therefore become widely accepted that degradation of PAI-1 by aPC with resulting unchecked tPA is chiefly responsible for the pathologically high level of plasmin-mediated fibrinolysis in trauma.(21, 22, 30, 31) However, the explicit the molecular mechanisms underlying hyperfibrinolysis have yet to be proven. (21, 22, 30, 32-34) Recent principal component analyses from both our group and Kutcher et al. demonstrate that the upregulation of the plasmin system and depletion of soluble factors are, in fact, independent components of TIC and therefore cannot share a single causal mechanism.(35, 36) Therefore, while aPC upregulation likely plays a key role in soluble factor depletion in TIC, its simultaneous role in hyperfibrinolysis is called into question.
Moreover, in other conditions of chronic and acute ischemia such as coronary artery disease, it is known that the total PAI-1 levels (largely in the form of its covalent complex with tPA) are, in fact, elevated.(37, 38) Moreover, it is known that the microvascular endothelium is capable of releasing tPA in response to ischemic stress as well as to signaling by vasopressin and catecholamines, whose levels are both elevated in hemorrhagic shock.(29, 39-43) Recent work from the Houston and London groups adds to the evidence suggesting that total PAI-1 concentration does not change significantly in hyperfibrinolytic TIC, while its activity decreases. (44, 45) If PAI-1 activity decreases to near zero, while total PAI-1 only decreases modestly, then the remaining PAI-1 must not by destroyed by proteolysis, but must simply be rendered inactive; most likely by its cognate inhibitor, tPA. Therefore, we hypothesized that the chief mechanism of decrease in PAI-1 activity in hyperfibrinolytic trauma patients is not enzymatic degradation by aPC, but is the result of complexation with tPA, driven by a massive increase in circulating tPA levels in response to shock.
Methods
Patients and Sample Collection
Consecutive trauma patients (n=86) meeting criteria for the highest level of activation at Denver Health Medical Center had blood collected at the earliest possible time point after injury, either in the field or immediately upon emergency department arrival. The criteria are traumatic injury with any of the following: (a) Blunt trauma with systolic blood pressure SBP <90 mmHg (b) Mechanically unstable pelvic injury (open or obvious by physical exam) (c) Penetrating neck/torso injuries with (SBP) < 90 mmHg (d) gunshot wounds to the neck/torso or stab wounds to the neck/torso that require endotracheal intubation.
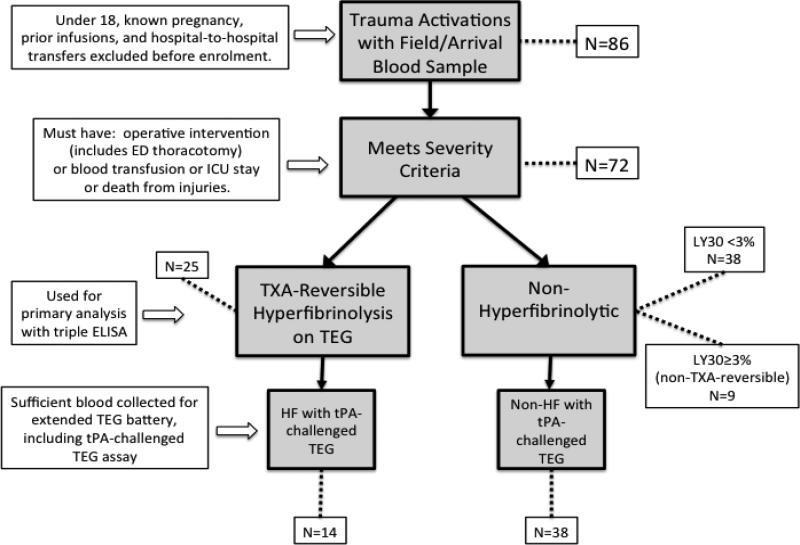
Patients were excluded if less than 18 years old, pregnant, prisoners, the initial blood sample was unable to be collected within one hour of injury, infusion of blood products occurs prior to blood sampling. Patients transferred from external hospitals, or those subsequently found to have documented liver disease or inherited defects of coagulation were also excluded. Patients were also excluded ex post facto if they failed to require at least one of the following: operative intervention, blood transfusion, death from injury or at least one day of ICU stay during their hospital course, yielding an n=72 for analysis. Figure 1 illustrates schematically the inclusion and exclusion process at each phase of analysis.
Figure 1. Flow chart of exclusion and inclusion at sequential steps in the analysis.
86 patients met initial enrolment criteria (highest level trauma activation) and had blood collected at the time of their initial vascular access, usually in the field. Patients under 18, known pregnancies and transfers were excluded as well as patients with documented coagulopathy or hepatic disease. As the determination of trauma activation by the paramedics in the field occasionally enrolls patients who are not in fact traumatically injured (e.g. medical illness only or severe substance intoxication), patients who did not actually have a traumatic injury of significant severity were excluded ex post facto. Criteria for severity were defined as injury requiring any operative intervention (including resuscitative thoracotomy), any blood product transfusion, injuries necessitating an ICU stay of any length or death from injuries (N=72). Hyperfibrinolytic patients (N=25) were discriminated from non-hyperfibrinolytic patients (N=47) by TXA-reversibility of apparent clot lysis. The conventional definition of hyperfibrinolysis in trauma of a TEG LY30 ≥ 3% was used, with the additional criteria that the observed lysis must be TXA-reversible, to eliminate false positives from platelet retraction (N=9). Initial blood sample volumes were not always sufficient to run the extended TEG battery that includes the tPA-challenged TEG assay; therefore, only a nested subset of the patients included in the basic TEG and ELISA data analysis have the additional data from the tPA-challenged TEG (N=52).
Healthy volunteers (n=14) were used as controls. These subjects 50% male and between the ages of 19 and 50. They were required to be in general good health and not pregnant, with no chronic medical conditions, no illnesses or injuries within the past 6 weeks and no taking medications.
Sample Collection
Citrated blood samples from both trauma patients and healthy controls were subjected to a battery of viscoelastic hemostatic assays (VHAs) as well as conventional coagulation tests. Platelet free plasma for ELISAs was prepared, within 30 minutes of collection, from citrated samples by a two-step centrifugation process using unopened vacuum containers. Samples were spun at 1000× g, at 4 degrees centigrade for 15 minutes, the supernatant decanted and respun at 12,600× g for 6 minutes, at which point the supernatant plasma was immediately flash frozen in liquid nitrogen and then stored at −80° centigrade.
Screening for Hyperfibrinolysis
Blood from the 72 patients underwent a variety of VHAs including citrated Rapid Thrombelastography (TEG). The lysis at 30 minutes after maximum amplitude (LY30) is the basic parameter for defining hyperfibrinolysis. We have previously defined a threshold value of LY30 of 3% for clinically relevant hyperfibrinolysis in citrated kaolin-activated (CK) TEG. In Rapid TEG, the threshold is similar, but this modality is subject to both underreporting of LY30 when the maximum amplitude (MA) is low and to false positives for hyperfibrinolysis due to vigorous platelet retraction.(46, 47) Therefore, we confirmed all findings of hyperfibrinolysis on Rapid TEG by demonstrating ex vivo reversibility of fibrinolysis with 250 μg of tranexamic acid added to a 500 μL citrated blood sample run in parallel. Patients with fully tranexamic acid (TXA) reversible clot lysis on TEG were segregated into the hyperfibrinolytic cohort.
PAI-1 and tPA Level Determination
PAI-1 and tPA both exist in a number of isoforms and cleavage products. In their active forms they are mutually inhibitory and form a covalent complex of 1:1 stoichiometry that sequesters both of their active sites. This complex is rapidly cleared and the complexation reaction goes essentially to completion; therefore, capturing the instantaneous relative ratios of active tPA, active PAI-1 and their inactive complex requires rapid chilling, separation and freezing of plasma samples, as described above. ELISAs for active tPA, active PAI-1 and the inactive PAI-1/tPA complex (Molecular Innovations, Novi, MI, USA) were run on duplicate plasma samples for hyperfibrinolytic trauma patients and quadruplicate samples for healthy controls to allow normalization to identical controls across multiple 96-well reaction plates. Active tPA was determined by immobilization on a PAI-1 functionalized surface and probing with a sandwich antibody. Active PAI-1 was similarly determined on a urokinase functionalized surface. The PAI-1/tPA complex was immobilized with an anti-tPA antibody and detected with an anti-PAI-1 sandwich antibody. Concentrations are reported as molarities, with total tPA reported as the sum of the concentrations of active tPA plus the one-to-one complex and total PAI-1 as the sum of active PAI-1 plus complex, respectively.
tPA-Challenged TEG
The triple ELISA (active tPA, active PAI-1 and their inactive complex) reflects the balance between the chief activator of the plasminogen-plasmin system (tPA) and its cognate inhibitor, PAI-1, which exists in stoichiometric balance with tPA. This methodology is too time consuming and expensive for routine use as a clinical assay; therefore, we sought to develop a functionally equivalent assay. Native TEG, challenged with a low dose of exogenous tPA (75 ng/mL of human single-chain tPA in whole blood, Molecular Innovations, Novi, MI, USA), was used as a functional readout of PAI-1 reserve. The principle of this assay is that as PAI-1 levels drop, the TEG LY30 in response to the low dose tPA challenge increases.
Statistical Analysis
Statistical calculations and tests were performed using the Prism statistical software package (version 6.0d for Mac OS X, GraphPad Inc.). Comparisons of groups were by the non-parametric two-tailed Mann-Whitney U test, unless otherwise noted. The receiver operating characteristic was also plotted for the novel tPA-challenged TEG.
Results
Hyperfibrinolysis in Trauma Patients
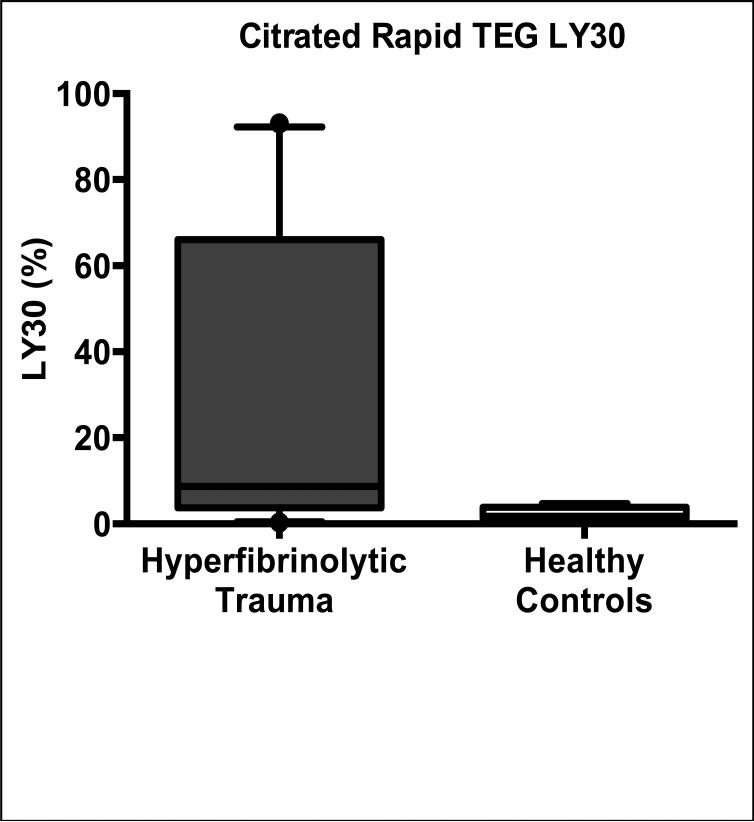
25 of 72 trauma patients with injuries severe enough to be included in our analysis had hyperfibrinolysis on citrated Rapid TEG, confirmed by ex vivo TXA reversibility (figure 1). The median degree of fibrinolysis in the hyperfibrinolytic trauma patients by TEG LY30 was 8.7% (IQR 3.7—66.0%) versus 1.8% (IQR 1.3—3.9%) for healthy controls (p=0.0009, two-tailed Mann-Whitney U test); see figure 2. These 25 hyperfibrinolytic (HF) patients were generally of much higher injury acuity than their non-fibrinolytic (NF) counterparts: Mortality in the HF group was 52% compared to 6% for NF patients. 11 HF patients required resuscitative thoracotomy, of which 4 survived, compared to zero thoracotomies in the NF group. Median (IQR) ISS, SBP and base excess were all worse in the HF group, 33 (22—41), 60 (0—86) mmHg and −9 (−7 – −17) mEq/L , respectively; compared to the NF group: 17 (9—32), 84 (66—118) mmHg, and −7 (−5.5 – −8.5) mEq/L.
Figure 2. Characteristics of hyperfibrinolytic HF trauma patients.
Patients with HF (demonstrated by tranexamic-reversible clot strength decay on their thrombelastogram (TEG)) have a median degree of clot lysis by Rapid TEG LY30 of 8.7% (IQR 3.7—66.0%) versus 1.8% (IQR 1.3—3.9%) for healthy controls (p=0.0009, two-tailed Mann-Whitney U test) as shown in this box-and-whisker plot. Note that none of the healthy controls had any degree of TXA-reversibility of their LY30. 25 of 72 trauma patients with injuries severe enough to be included in this analysis had HF. These 25 hyperfibrinolytic (HF) patients were generally of much higher acuity than their non-fibrinolytic (NF) counterparts: Mortality in the HF group was 52% compared to 6% for NF patients. 11 HF patients required resuscitative thoracotomy, of which 4 survived, compared to zero thoracotomies in the NF group. Median (IQR) ISS, SBP and base excess were all worse in the HF group, 33 (22—41), 60 (0—86) mmHg and −9 (−7 – −17) mEq/L , respectively; compared to the NF group: 17 (9—32), 84 (66—118) mmHg, and −7 (−5.5 – −8.5) mEq/L.
PAI-1 in Trauma and Controls
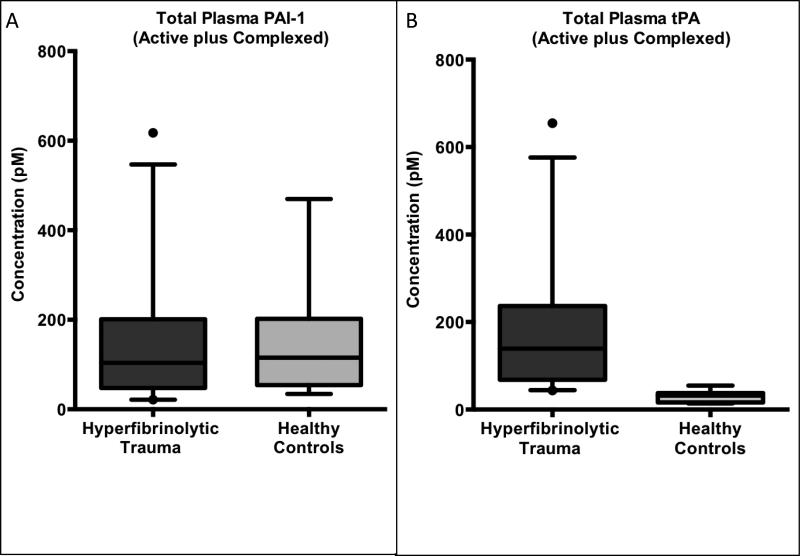
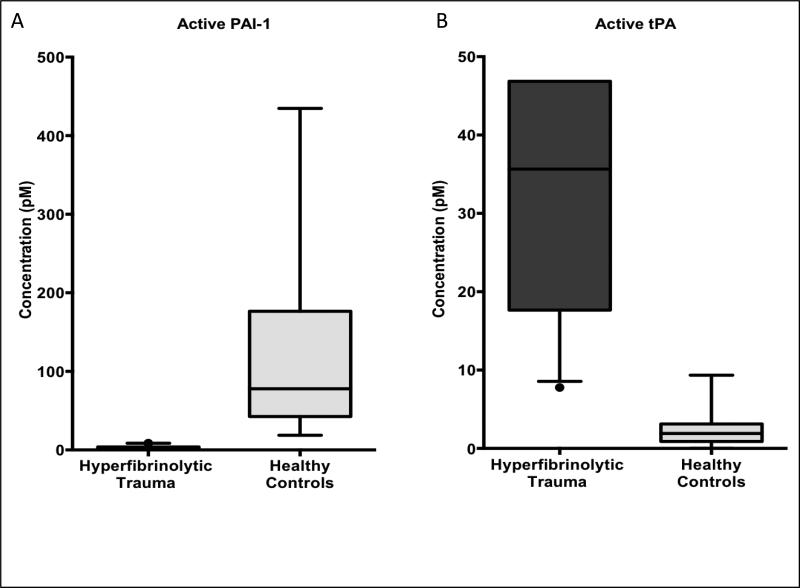
The total levels of PAI-1, the sum of the active PAI-1 species and its covalent complex with tPA, are not significantly different between hyperfibrinolytic trauma patients and healthy controls (figure 3A). Median total PAI-1 in hyperfibrinolytic trauma patients was 104 pM (IQR 48—201 pM) versus 115 pM (54—202 pM) for healthy controls (p=0.65, Mann-Whitney U test). However, the ratio of active to inactive (complexed) PAI-1 shifted downward two orders of magnitude from roughly 5:1 in healthy controls (median plasma PAI-1 activity 78 pM, IQR 42—176 pM) to 0.015:1 in hyperfibrinolytic trauma patients (median plasma PAI-1 activity 1.1 pM, IQR 0.0—3.8), p <0.0001, figure 4A.
Figure 3. Total circulating levels of PAI-1 is unchanged and tPA is elevated in hyperfibrinolytic (HF) trauma patients compared to healthy volunteer controls.
(A) Total plasma PAI-1 (the sum of the free and tPA-complexed species) is unchanged in HF compared to healthy controls, indicating that enzymatic degradation of PAI-1 is not a prominent feature HF. (B) Conversely, total tPA (the sum of the free and PAI-1-complexed species) was elevated more than four-fold in HF compared to healthy controls, indicating that tPA upregulation is an early and necessary step in the evolution of HF in TIC.
Figure 4. Circulating levels of active PAI-1 are suppressed and active tPA levels elevated in hyperfibrinolytic (HF) trauma patients compared to healthy volunteer controls.
(A) Active (i.e. unbound to tPA) PAI-1 is suppressed to near zero in HF compared to healthy controls. (B) Conversely, active tPA (i.e. unbound to PAI-1) levels rise nearly 20-fold in HF compared to healthy volunteers. Taken together these findings demonstrate a sharp enzymatic switching behavior between the hyperfibrinolytic and the baseline physiologic state, which hinges on the relative abundance of the active forms of the mutually inhibitor species tPA and PAI-1.
tPA in Trauma and Controls
Conversely, total tPA levels (active plus complex) were significantly higher in hyperfibrinolytic trauma patients than controls 139 pM (IQR 68—237 pM) versus 32 pM (IQR 16—37 pM), p< 0,0001, Mann-Whitney U test (figure 3B). For the active tPA species, the ratio to inactive complex shifted upward in the hyperfibrinolytic trauma group (the opposite direction of PAI-1) from 0.09:1 to 0.22:1. While the shift in active:inactive ratio was a modest at slightly more than two-fold, as the total tPA level was increased by nearly five-fold in hyperfibrinolytic trauma over healthy controls, the net effect on active tPA levels was a nearly 20-fold increase in hyperfibrinolysis compared to controls at 36 pM (IQR 18–49 pM) versus 1.9 pM (IQR 0.9—3.1 pM), p<0.0001, see figure 4B.
PAI-1/tPA Covalent Complex in Trauma and Controls
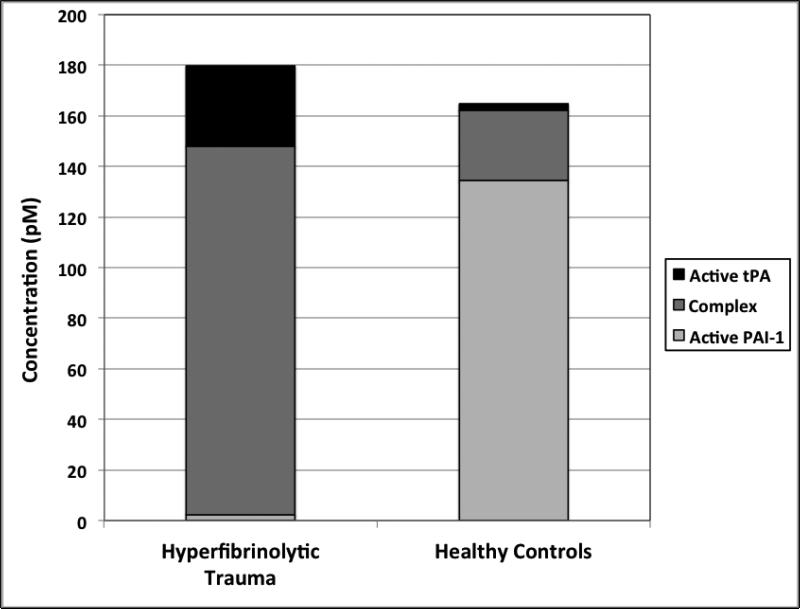
The level of PAI-1/tPA complex was increased more than three-fold in hyperfibrinolytic trauma patients (104 pM, IQR 48—98 pM) compared to controls (27 pM, IQR 14—37 pM), p<0.0001. The relative levels of active tPA, active PAI-1 and the covalent complex in hyperfibrinolytic patients and healthy controls are shown in figure 5. The shift in PAI-1from its free, active form to the inactive complex is seen to parallel a massive upregulation in total tPA levels in hyperfibrinolytic traumas compared to controls.
Figure 5. Relative levels of active tPA, active PAI-1 and the covalent complex of these two enzymes in hyperfibrinolytic (HF) patients and healthy controls illustrates the shifting balance of active versus complexed PAI-1, without change in its total concentration.
In HF (compared to healthy controls), the balance of PAI-1 shifts from its free, active form (light gray portion of each bar) predominating, to the inactive complex (dark gray portion of each bar). This shift parallels a massive upregulation in total tPA levels (dark gray plus black portions of each bar) with a resultant overflow of active tPA (black portion of each bar) as PAI-1 reserves are saturated and overwhelmed.
tPA-Challenged TEG
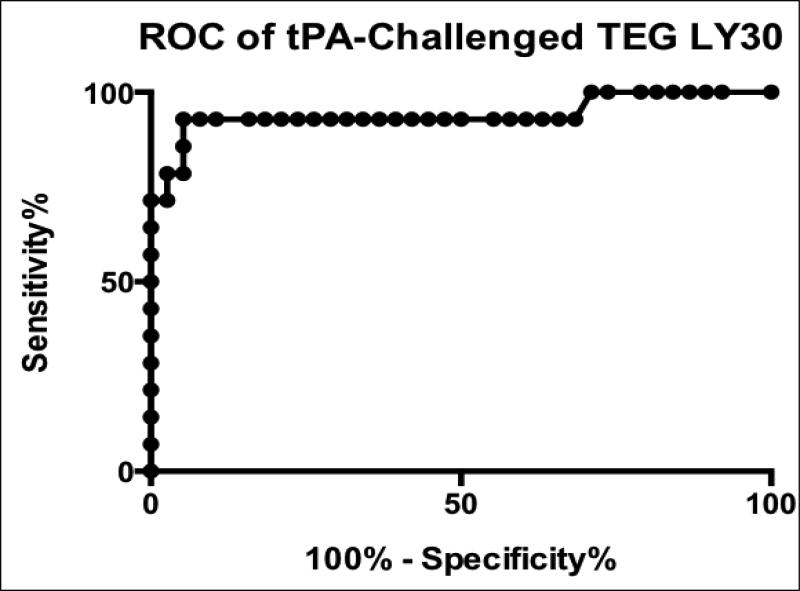
Hyperfibrinolytic trauma patients displayed increased whole blood fibrinolysis on TEG in response to an exogenous challenge with 75 ng/mL of tPA. Median LY30 with tPA challenge in hyperfibrinolytic trauma blood was 66.8% (IQR 31.0—87.3%) compared to 9.6% (IQR 8.3—10.2%) for non-fibrinolytic patients and 8.2% (IQR 4.8-14.8) for healthy volunteers. LY30 data from tPA-challenged TEGs was taken from a nested subset of proven (by TEG with TXA-reversibility) hyperfibrinolytic trauma patients (n=14) and non-fibrinolytic patients (n=38) for which sufficient blood was available for the additional TEG assay. The relative ratio of disease positives to controls was not qualitatively different from the overall population in this convenience sample, therefore selection biasing was deemed minimal (see figure 1). A receiver operating characteristic (ROC) curve for prediction of hyperfibrinolysis by tPA-challenged TEG was constructed (figure 6). The area under the ROC curve was 0.94, with sensitivity and specificity optimized at 92.9% and 94.7% respectively with a threshold value of LY30 >20.8%.
Figure 6. Receiver operating characteristic (ROC) curve for tPA-challenged thrombelastography (TEG) prediction of TXA-reversible hyperfibrinolysis.
Clot lysis in response to exogenous tPA added to the blood sample acts as a functional measure of PAI-1 reserves. The lower the levels of free PAI-1, the more exogenous tPA is left uncomplexed to cause measurable fibrinolysis, after ex vivo addition to whole blood. The area under the ROC curve was 0.94 (p <0.0005), with sensitivity and specificity optimized at 92.9% and 94.7% respectively with a threshold value of LY30 >20.8%.
Discussion
Massive tPA Release Overwhelms Free PAI-1 in TIC with Hyperfibrinolysis
A decrease in PAI-1 activity to nearly undetectable levels in TIC with overt hyperfibrinolysis has not only been demonstrated by other authors, but is to be expected. As the cognate inhibitor of tPA, PAI-1 not only interacts with tPA with very high affinity, blocking both enzyme's active sites, but the two react completely, forming a covalent complex that is rapidly cleared. Thus, for any degree of tPA activity (and thus plasmin-mediated fibrinolytic activity) to be detectable, tPA must exist in exces to PAI-1 and all available PAI-1 should be sequestered in the PAI-1/tPAcomplex. Conversely, the presence of any measurable level of excess active PAI-1 implies that virtually all available tPA is sequestered in this complex and fibrinolytic activity via the plasminogen-plasmin system should be minimal.
What has been unclear until this point is whether the shift in the PAI-1/tPA balance was the result of depletion of PAI-1 by enzymatic degradation or another mechanism. While in vitro studies show that, for example, the serine protease aPC can efficiently inactivate PAI-1 by proteolysis (particularly in the presence of potentiating cofactors such as vitronectin) no direct evidence exists for the neutralization of PAI-1 in TIC by this or any other enzyme in injured patients.
Our data demonstrate that, in fact, circulating plasma concentrations of total PAI-1 in TIC patients with hyperfibrinolysis remain near their baseline levels found in healthy controls. Active (free) PAI-1 indeed drops to nearly undetectable levels in these patients, but rather than undergoing degradation, PAI-1 is sequestered into an inactive complex with tPA. This shift in PAI-1 from the free to complexed form is near total (>300-fold) and is driven by mass action due to an overwhelming increase in the total amount of tPA. This excess of tPA, having bound all available PAI-1, effectively overflows this first, critical regulatory checkpoint of the plasminogen-plasmin system and active tPA becomes detectable in the plasma of these hyperfibrinolytic patients at levels exceeding 20 times normal.
Development of a Novel Functional Assay for tPA/PAI-1 Balance
This insight allows us to target our future investigations at the regulators of tPA release, rather than inhibitors of PAI-1. The response of the microvascular endothelium to ischemic stress may play an important role in the upregulation of circulating tPA.(48) It is likely that this response is chiefly in the form of release of preformed tPA, as we detect the upregulation of tPA consistently in hyperfibrinolytic trauma patients even in blood drawn at the scene of injury by the responding paramedics.
This very early shift in the tPA balance may have significant clinical consequences, not yet appreciated. We have observed several cases of trauma patients who initially present without evidence of hyperfibrinolysis suddenly decompensate and become profoundly coagulopathic due to hyperfibrinolysis. These patients can be difficult to manage and may bleed massively and unexpectedly. Having a means of detection of this latent hyperfibrinolysis would therefore be of great clinical utility as it could provide an indication for early, prophylactic therapy with antifibrinolytics such as TXA. Moreover, worsening PAI-1 sequestration and total tPA levels may be a biomarker for occult, compensated shock with concomitant tPA release from ischemic endothelium in vulnerable circulatory beds such as the gut, kidney and liver. Unfortunately, our “triple ELISA” methodology is not practical for use in the setting of trauma, as it takes several hours to run these assays. We therefore sought to follow up on our finding related to the tPA/PAI-1 balance by developing a rapid, inexpensive and convenient functional assay for the shifting of this balance toward the hyperfibrinolytic end of the spectrum.
We have demonstrated that the primary driver in depletion of the circulating PAI-1 reserve in TIC is an increase in tPA, we designed an assay based on this principle: the tPA-challenged TEG. When a low dose (75 ng/mL) of recombinant single-chain tPA is added ex vivo to whole blood and a native TEG performed, only a very modest degree of fibrinolysis (by LY30) is observed in healthy controls. A spectrum of response is observed in trauma patients without overt fibrinolysis and marked increases (roughly 6-fold greater) LY30 are observed in cases of frank hyperfibrinolysis. This is an encouraging preliminary finding for the utility of this assay, but it remains to be determined if the tPA-challenged TEG has predictive value for delayed-onset or latent hyperfibrinolysis.
Summary
Our study has clearly demonstrated that early elevation of tPA is necessary for the development of hyperfibrinolysis, and that enzymatic degradation of PAI-1 does not play a significant role in the pathogenesis of systemic hyperfibrinolysis in TIC. An important limitation in the scope of these findings, however, is the methodology of highly selective protein analysis. There are several known (and likely many unknown) regulators of plasmin-mediated fibrinolysis. Further exploration of the mechanism of upregulation of tPA, and the downstream effector protein plasmin, is critical to developing an understanding of how fibrinolysis becomes uncontrolled in trauma.
Lastly, it is important to note that while this study indicates that aPC-mediated degradation of PAI-1 has little role to play in the direct induction of hyperfibrinolysis, aPC is likely a key player in other aspects of TIC.(40, 49, 50) Several publications have shown distinct phenotypes among patients with coagulation abnormalities after trauma, suggesting that fibrinolysis abnormalities are a distinct form of dysfunction from other components of TIC, an observation with important implications for treatment. (9, 32, 51-54) Our novel tPA-challenged TEG assay, though not currently approved for clinical use, may be of utility in trauma and other acute settings for revealing systemic hyperfibrinolysis in its earliest (latent) stage, when the balance of endogenous circulating tPA and PAI-1 is beginning to shift, but active tPA does not yet exist in excess in the systemic circulation and overt fibrinolysis is not yet detectable by conventional assays.
Acknowledgements
The authors wish to thank our clinical research assistants: Sarah Ammons, Andrea Emard, Courtney Fleming and Raymond Shepherd-Singh for their invaluable efforts in obtaining samples for this study. Research reported in this publication was supported in part by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institute of General Medical Sciences and National Heart, Lung, and Blood Institutes of the National Institutes of Health under Award Numbers P50GM049222, T32GM008315 and UMHL120877 and we receive research support from Haemonetics LLC and TEM GmbH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Department of Defense or any of our industry sponsors.
Footnotes
Conflict of Interest Statement: We receive research support from the Haemonetics Corporation, Niles, IL and Tem GmbH
Author Contribution:
M.P.C., E.E.M., H.B.M., E.G., T.L.C., A.S., A.B., and C.C.C. designed the study. A.G. and J.G.C. managed the study and databases and collected and processed patient samples. F.G. and S.M. performed experiments and conducted data analysis. M.P.C., E.E.M., H.B.M., and E.G. prepared and edited the manuscript.
References
- 1.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–31. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 2.Blackbourne LH, Baer DG, Cestero RF, Inaba K, Rasmussen TE. Exsanguination shock: the next frontier in prevention of battlefield mortality. J Trauma. 2011;71(1 Suppl):S1–3. doi: 10.1097/TA.0b013e3182211286. [DOI] [PubMed] [Google Scholar]
- 3.Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. 2010;24(1):15–25. doi: 10.1016/j.bpa.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 5.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 6.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–14. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 7.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–7. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73(2):365–70. doi: 10.1097/TA.0b013e31825c1234. discussion 70. [DOI] [PubMed] [Google Scholar]
- 9.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: Clotting factor depletion versus fibrinolysis. Surgery. 2014;156(3):570–7. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gando S, Tedo I, Kubota M. Posttrauma coagulation and fibrinolysis. Crit Care Med. 1992 May;20(5):594–600. doi: 10.1097/00003246-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CRASH-2 collaborators. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–101. 101, e1–2. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 13.CRASH-2 trial collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, West B, Banerjee A, Silliman CC. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43(1):39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urano T, Wu K, Ihara H, Takada Y, Takada A. Novel mechanism to enhance tPA-induced fibrinolysis: effect of limited proteolysis of PAI-1 by neutrophil elastase. Pol J Pharmacol. 1996;48(2):209–13. [PubMed] [Google Scholar]
- 16.Wu K, Urano T, Ihara H, Takada Y, Fujie M, Shikimori M, Hashimoto K, Takada A. The cleavage and inactivation of plasminogen activator inhibitor type 1 by neutrophil elastase: the evaluation of its physiologic relevance in fibrinolysis. Blood. 1995;86(3):1056–61. [PubMed] [Google Scholar]
- 17.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, Namias N, Schulman CI, Proctor KG. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76(6):1373–8. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 18.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015;261(2):390–4. doi: 10.1097/SLA.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 19.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, Holcomb JB, Cotton BA. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg. 2015;78(5):905–11. doi: 10.1097/TA.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 20.Schochl H, Cadamuro J, Seidl S, Franz A, Solomon C, Schlimp CJ, Ziegler B. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013;84(4):454–9. doi: 10.1016/j.resuscitation.2012.08.318. [DOI] [PubMed] [Google Scholar]
- 21.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 22.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 23.Morton AP, Moore EE, Wohlauer MV, Lo K, Silliman CC, Burlew CC, Banerjee A. Revisiting early postinjury mortality: Are they bleeding because they are dying or dying because they are bleeding? J Surg Res. 2013;179(1):5–9. doi: 10.1016/j.jss.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorquist P, Brohlin M, Ehnebom J, Ericsson M, Kristiansen C, Pohl G, Deinum J. Plasminogen activator inhibitor type-1 interacts exclusively with the proteinase domain of tissue plasminogen activator. Biochim Biophys Acta. 1994;1209(2):191–202. doi: 10.1016/0167-4838(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 25.Stromqvist M, Karlsson KE, Björquist P, Andersson JO, Byström M, Hansson L, Johansson T, Deinum J. Characterisation of the complex of plasminogen activator inhibitor type 1 with tissue-type plasminogen activator by mass spectrometry and size-exclusion chromatography. Biochim Biophys Acta. 1996;1295(1):103–9. doi: 10.1016/0167-4838(96)00035-0. [DOI] [PubMed] [Google Scholar]
- 26.de Fouw NJ, van Tilburg NH, Haverkate F, Bertina RM. Activated protein C accelerates clot lysis by virtue of its anticoagulant activity. Blood Coagul Fibrinolysis. 1993;4(2):201–10. doi: 10.1097/00001721-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Bertina RM, van Tilburg NH, de Fouw NJ, Haverkate F. Thrombin, a link between coagulation activation and fibrinolysis. Ann N Y Acad Sci. 1992;667:239–48. doi: 10.1111/j.1749-6632.1992.tb51621.x. [DOI] [PubMed] [Google Scholar]
- 28.de Fouw NJ, de Jong YF, Haverkate F, Bertina RM. Activated protein C increases fibrin clot lysis by neutralization of plasminogen activator inhibitor--no evidence for a cofactor role of protein S. Thromb Haemost. 1988;60(2):328–33. [PubMed] [Google Scholar]
- 29.de Fouw NJ, van Hinsbergh VW, de Jong YF, Haverkate F, Bertina RM. The interaction of activated protein C and thrombin with the plasminogen activator inhibitor released from human endothelial cells. Thromb Haemost. 1987;57(2):176–82. [PubMed] [Google Scholar]
- 30.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012 Aug;92(4):877–91. viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–31. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 33.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 34.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252(3):434–42. doi: 10.1097/SLA.0b013e3181f09191. discussion 43-4. [DOI] [PubMed] [Google Scholar]
- 35.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–9. doi: 10.1097/TA.0b013e31828b7fa1. discussion 9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: Clotting factor depletion versus fibrinolysis. Surgery. 2014;156(3):570–7. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordenhem A, Leander K, Hallqvist J, de Faire U, Sten-Linder M, Wiman B. The complex between tPA and PAI-1: risk factor for myocardial infarction as studied in the SHEEP project. Thromb Res. 2005;116(3):223–32. doi: 10.1016/j.thromres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Bounameaux H, Kruithof EK. On the association of elevated tPA/PAI-1 complex and von Willebrand factor with recurrent myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20(8):1857–9. doi: 10.1161/01.atv.20.8.1857. [DOI] [PubMed] [Google Scholar]
- 39.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg. 2012;72(2):428–36. doi: 10.1097/ta.0b013e31821e0f93. [DOI] [PubMed] [Google Scholar]
- 40.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 41.Hrafnkelsdottir T, Gudnason T, Wall U, Jern C, Jern S. Regulation of local availability of active tissue-type plasminogen activator in vivo in man. J Thromb Haemost. 2004;2(11):1960–8. doi: 10.1111/j.1538-7836.2004.00948.x. [DOI] [PubMed] [Google Scholar]
- 42.Hrafnkelsdottir T, Ottosson P, Gudnason T, Samuelsson O, Jern S. Impaired endothelial release of tissue-type plasminogen activator in patients with chronic kidney disease and hypertension. Hypertension. 2004;44(3):300–4. doi: 10.1161/01.HYP.0000137380.91476.fb. [DOI] [PubMed] [Google Scholar]
- 43.Burggraaf J, Schoemaker HC, Kroon JM, Huisman L, Kluft C, Cohen AF. Influence of 1-desamino-8-D-vasopressin on endogenous fibrinolysis, haemodynamics and liver blood flow in healthy subjects. Clin Sci (Lond) 1994;86(5):497–503. doi: 10.1042/cs0860497. [DOI] [PubMed] [Google Scholar]
- 44.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De'Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 45.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 46.Bontekoe IJ, van der Meer PF, de Korte D. Determination of thromboelastographic responsiveness in stored single-donor platelet concentrates. Transfusion. 2014;54(6):1610–8. doi: 10.1111/trf.12515. [DOI] [PubMed] [Google Scholar]
- 47.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005;100(6):1781–5. doi: 10.1213/01.ANE.0000149902.73689.64. [DOI] [PubMed] [Google Scholar]
- 48.Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32(6):1341–8. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- 49.MacLeod JB, Winkler AM, McCoy CC, Hillyer CD, Shaz BH. Early trauma induced coagulopathy (ETIC): prevalence across the injury spectrum. Injury. 2014;45(5):910–5. doi: 10.1016/j.injury.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Shaz BH, Winkler AM, James AB, Hillyer CD, MacLeod JB. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011;70(6):1401–7. doi: 10.1097/TA.0b013e31821266e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schochl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Y, Dua A, Matijevic N, Wang YW, Pati S, Wade CE, Ko TC, Holcomb JB. Never-frozen liquid plasma blocks endothelial permeability as effectively as thawed fresh frozen plasma. J Trauma Acute Care Surg. 2014;77(1):28–33. doi: 10.1097/TA.0000000000000276. discussion. [DOI] [PubMed] [Google Scholar]
- 54.Johansson PI, Sørensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, Ostrowski SR. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Crit Care. 2011;15(6):R272. doi: 10.1186/cc10553. [DOI] [PMC free article] [PubMed] [Google Scholar]