Abstract
To date, there is a limited understanding of the role of the airway microbiome in the early-life development of respiratory diseases such as asthma, partly due to a lack of simple and minimally invasive sample collection methods. In order to characterize the baseline microbiome of the upper respiratory tract (URT) in infants, a comparatively non-invasive method for sampling the URT microbiome suitable for use in infants was developed. Microbiome samples were collected by placing filter paper in the nostrils of thirty-three healthy, term infants enrolled as part of the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) study. After bacterial genomic DNA was extracted from the filters, amplicons were generated with universal primers targeting the V1 – V3 region of the 16S rRNA gene. This method was capable of capturing a wide variety of taxa expected to inhabit the nasal cavity. Analyses stratifying subjects by demographic and environmental factors previously observed or predicted to influence microbial communities were performed. Microbial community richness was found to be higher in infants who had been delivered via Cesarean section and in those who had been formula-fed; an association was observed between diet and delivery, which confounds this analysis. We have established a baseline URT microbiome using a non-invasive filter paper nasal sampling for this population and future studies will be performed in this large observational cohort of infants to investigate the relationship between viral infections, the URT microbiota, and the development of childhood wheezing illnesses.
Keywords: microbiome, 16S rRNA, next-generation sequencing, upper respiratory tract
Introduction
The human microbiome (i.e., all the microbes colonizing the human body) is recognized as playing a fundamental role in maintaining host health [1]. Epidemiological studies have established an association between exposures that can lead to an abnormal microbiome in early life (e.g., mode of delivery, use of antibiotics in utero or during infancy, and non-farm living) and an increased risk of asthma [2,3]. The use of next-generation sequencing (NGS) technology to sequence conserved bacterial genes has led to a rapid increase in knowledge about the complex relationships among commensal microbial communities and host health [4,5].
While the intestinal microbiota has been extensively sampled [6], comparatively few studies have characterized the upper respiratory tract (URT) microbiome. Further research on the URT microbiome is warranted because this microbial community is thought to play a role in the development of allergy and asthma through modifying airway mucosal inflammation and as a stimulator of immune regulation [7]. Culture-based studies have found that colonization of the URT in neonates with known pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, may increase the risk of subsequent development of childhood wheezing illnesses [8,9], while culture-independent methods have demonstrated a perturbation of the microbial community in adult asthmatic patients [10-12]. Bacteria are not the only microorganisms that inhabit the URT. Respiratory viral infections during infancy have been linked to the development of subsequent childhood wheezing and asthma later in life [13-16]. Viruses are capable of disturbing the airway microbiome through several possible mechanisms; conversely, the microbiome can alter host immune response to viral infections [17]. Therefore, additional studies of the infant URT microbiome are needed to better characterize typical microbial communities and their colonization patterns over the first few months and years of life, as well as to elucidate the influence of interactions between respiratory viruses and the microbiome on the risk of childhood wheezing illness.
In order to characterize the microbiome of the infant URT, we utilized a minimally invasive sampling method suitable for use in infants, which we have used to establish the baseline nasal microbiome of a cohort of middle Tennessee infants enrolled as part of the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) study [18]. The use of filter paper is safer and less invasive than nasal lavage or swab in infants, and has been used in other early life studies [19]. The objective of this study was to determine if this sampling technique is capable of capturing the wide variety of microbes that inhabit the URT. The confirmation of a valid method of microbiome analyses using a minimally invasive sampling method will facilitate a greater understanding of the longitudinal changes in the respiratory microbiome by facilitating early time-point of sampling, and aid in furthering our understanding of the link between the airway microbiota and the subsequent development of asthma in children.
Materials and Methods
Study Population
INSPIRE is an observational population-based longitudinal birth cohort study of previously healthy, term infants enrolled near birth with surveillance and capture of respiratory illnesses during their first winter viral season. Yearly follow-up to assess the development of childhood wheezing illnesses (including asthma) is ongoing. Eligible infants were born between June and December so that they were on average ≤6 months of age during their first winter viral season. Informed consent was obtained from the legal guardians of each infant. All procedures were in accordance with the ethical standards of the Vanderbilt University Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Demographic and environmental data, including factors expected to influence the URT microbiota (such as age, sex, race and ethnicity, mode of delivery, breastfeeding, in utero or early-life exposure to antibiotics, and household pets), were recorded at the time of enrollment. Nasal filter paper samples were also obtained at enrollment in a subset of infants as described below. The first infants enrolled during the second year of INSPIRE (2013-2014) with available nasal filter paper samples (n = 33) were selected for this analysis.
DNA Sampling and Extraction
The nasal microbiome was sampled by placing a dry filter paper, a 3 × 15 mm synthetic absorptive matrix (SAM) (Leucosorb BTM, Pall Life Sciences, Port Washington, NY), approximately 5-7 mm into each of the infants’ nares laterally against the anterior portion of the inferior nasal turbinate and then pressing against the outside of the nose with a finger to push the paper laterally and absorb the nasal fluid. The filter paper was left in each nostril for two minutes and removed with forceps or a gloved hand and placed into a sterile container and stored immediately at −20 °C, and then transferred to −80 °C freezer within 24 hours. Microbial cells were lysed enzymatically and thermally. DNA was extracted from lysed cells through two phenol:chloroform:isoamyl alcohol extractions [20]. Genomic DNA was cleaned by isopropanol precipitation. Further details on the extraction protocol can be found in the Online Supplement.
16S rRNA Gene Profiling by 454 Pyrosequencing
The V1-V3 hypervariable region of 16S rRNA was targeted using primers 27F/534R. Primer sequences can be found in the Online Supplement. Amplicons were generated with Platinum Taq polymerase (Life Technologies, Grand Island, NY) using the following cycling conditions: 95 °C for 5 minutes; 35 cycles of 95 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 30 seconds; and a final extension step at 72 °C for 7 minutes. Amplicons were cleaned using the QIAquick PCR Purification Kit (QIAGEN, Valencia, CA), as per the manufacturer’s instructions, with an additional drying step after the ethanol wash to ensure complete ethanol removal. Purified amplicons were quantified using SYBR Green (Life Technologies, Grand Island, NY) on a Synergy HT plate reader (BioTek, Winooski, VT), normalized, and pooled. The template was subjected to emulsion PCR, and pyrosequencing was performed at the J. Craig Venter Institute on a 454 sequencer using FLX-Titanium chemistry (Roche, Branford, CT).
Data Analysis
A mothur-based [21] automated 16S annotation pipeline, YAP, was used to perform the initial processing of the 16S rRNA gene datasets [22,23]. YAP is a distributed workflow wrapper designed at JCVI that executes a sequence of commands described in the mothur standard operating procedure for 454 reads [24] (http://www.mothur.org/wiki/454_SOP). For operational taxonomic unit (OTU) based analyses, OTUs were clustered at 97% sequence identity [25]. The outputs of this annotation are mothur-generated sequence count matrices describing the observed abundance of taxa at different ranks of the taxonomy in each sequencing sample as well as OTU abundance. OTUs are also labeled with the lowest level of the consensus taxonomy of their constituting sequences.
These count matrices were analyzed with our open-source analysis package MGSAT [26]. MGSAT is written in R. It applies several types of statistical tests, normalizations, and plotting routines to the abundance count matrices that are typically the output of annotating (meta) omics datasets, and it generates a structured HTML report that, in addition to results, shows method parameters and versions of the external packages. The user has fine-grained control over types of tests, parameters, and a description of a study design through a data structure that is provided as input to the top-level routine of the package.
We have applied several analysis methods at each level of the taxonomy and at the 97% OTU clustering level. Applying different statistical tests and ranking methods allowed us to assess their qualitative agreement, important for these multivariate datasets with likely correlated features. Each row of the abundance count matrix corresponds to a single microbiome sample and forms a multivariate observation (also addressed as “abundance profile”) that might be further transformed as described for a specific step in the analysis. Individual taxa or OTUs correspond to columns of the corresponding abundance count matrix and are referred to as “features” or “predictors.”
The following methods were used: ranking of features by differential abundance, comparing overall dissimilarity between taxonomic abundance profiles, analysis of richness to estimate OTU and genus counts and analysis of diversity to estimate richness and evenness, and independent filtering. They are discussed in detail in the Online Supplement.
Results
Use of filter paper to collect nasal microbiome
The microbiota of the URT of 33 infants enrolled in the INSPIRE study was profiled. Characteristics of these study subjects can be found in Table 1. Each infant was sampled at a single time point. Infants were evenly sampled by sex and the majority of infants were non-Hispanic and white, between 5 and 140 days old at the time of sample collection, and with a mean age of 51 days (standard deviation = 40.110). A small proportion (6.061%) had received antibiotics since birth; however, nearly 40% had been exposed to antibiotics in utero. Approximately a quarter had been delivered by Cesarean section and nearly two thirds had been breastfed. Infants who were breastfed were more likely to have been delivered vaginally (χ2 test, p-value = 0.044). Cesarean delivered infants were an average of 37 days old compared to vaginally delivered infants at 55 days. Formula-fed infants were 47 days old on average compared to breastfed infants, at 53 days old. The difference in age was not statistically significant when the cohort was stratified by either delivery mode or feeding type.
Table 1.
Demographic and environmental characteristics of the 33 sampled infants.
| Total (n = 33) | |
|---|---|
|
| |
| Sex | |
|
| |
| Male | 17 (51.5%) |
| Female | 16 (48.5%) |
|
| |
| Age at samplinga (days) | |
|
| |
| 5 - 17 | 9 (27.3%) |
| 18 - 35 | 8 (24.2%) |
| 36 - 67 | 8 (24.2%) |
| 68 - 140 | 8 (24.2%) |
|
| |
| Race | |
|
| |
| White | 24 (72.7%) |
| Black | 8 (24.2%) |
| Othera | 1 (3.0%) |
|
| |
| Ethnicity | |
|
| |
| Hispanic | 3 (9.1%) |
| Non-Hispanic | 30 (90.9%) |
|
| |
| Gestational age (weeks) | |
|
| |
| Mean [Range] | 39.3 [37 – 41] |
|
| |
| Mode of delivery | |
|
| |
| Cesarean | 8 (24.2%) |
| Vaginal | 25 (75.8%) |
|
| |
| Breastfed | |
|
| |
| Yes | 22 (66.7%) |
| No | 11 (33.3%) |
|
| |
| Infant received antibiotics since birth | |
|
| |
| Yes | 2 (6.1%) |
| No | 31 (93.9%) |
|
| |
| Mother received antibiotics during pregnancy | |
|
| |
| Yes | 13 (39.4%) |
| No | 20 (60.6%) |
|
| |
| Pet in household | |
|
| |
| Yes | 19 (57.6%) |
| No | 14 (42.4%) |
Infants were on average 51 days old at the time of sampling.
Category includes subjects of mixed race.
After extracting bacterial genomic DNA from the nasal filters, samples were pooled and sequenced using pyrosequencing. A total of 809,854 raw reads were obtained, with an average read length of 387 bp, and a total of 477,553 sequences, with an average read length of 258 bp were retained after quality filtering and trimming. On average, each sample was represented by 14,471 sequences, with a range of 2,617 – 21,098 sequences per sample (Table S1). All the OTUs were clustered at 97% similarity; the Good’s coverage for all samples was greater than 98%. Based on the high Good’s coverage scores and the minimum sequence per sample count, all samples were considered to be sequenced to an acceptable depth and therefore were retained for further analysis. On average, each sample was represented by an estimated 62 OTUs at 97% similarity clustering, with a range of 21 – 135 OTUs per sample when rarefying each sample to the lowest sample sequence count (2,617) to control for uneven sequencing depth.
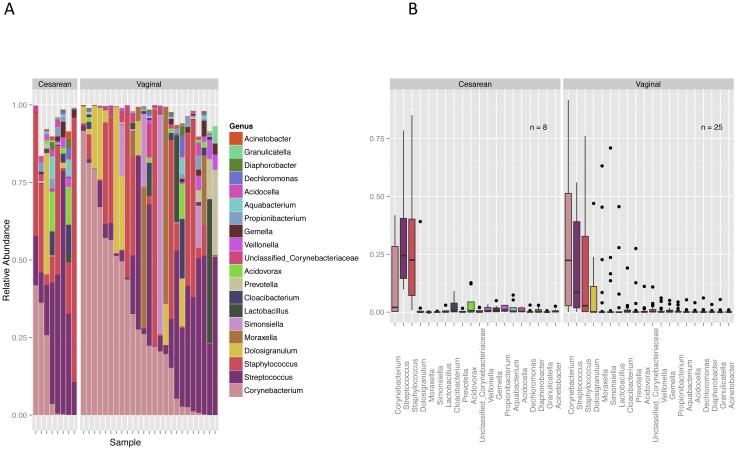
Overall, the nasal microbial community of this population was dominated by the Firmicutes (53.501%), Actinobacteria (30.608%), Proteobacteria (12.221%) and Bacteroidetes (3.510%) lineages, with all other phyla represented at <1% relative abundance. A total of 328 different genera were identified, with the most highly represented genera being Corynebacterium (28.430%), Streptococcus (21.912%), Staphylococcus (20.426%), Dolosigranulum (5.548%), and Moraxella (4.380%) (Figure 1).
Fig. 1.
a Stacked bar graph showing genus level taxonomic composition for each individual sample, expressed as a proportion of reads. The 20 genera with the highest average relative abundance are shown. Samples are stratified by mode of delivery and sorted by Corynebacterium abundance. b. Box plots showing relative abundance of the top 20 most highly represented genera, averaged over all samples for each delivery mode.
Effect of sex, age, race, and ethnicity on the infant nasal microbiome
Infant sex or ethnicity was not significantly associated with the nasal microbial community in this population. Both abundance-based (computed within each microbiome sample) and incidence-based (computed based on presence across samples) OTU richness tended to decrease with age, based on abundance-based inverted Shannon index, inverted Simpson index, Chao1, and observed OTUs and the incidence-based Chao, first order jackknife, and bootstrap indices, although this trend was not statistically significant for any calculated index. The abundance of Granulicatella (base mean = 40.765, log2 fold change = 0.050, q-value = <0.001) and Porphyromonas (base mean = 30.889, log2 fold change = 0.051, q-value = 0.002) were found to increase with age using the DESeq2 test. Overall abundance of both of these genera was low, and the observed increase in age appears to be mostly due to two of the older infants within the cohort having relatively high abundances of Granulicatella and Porphyromonas. Granulicatella was ranked first, tied with Moraxella, and Porphyromonas was ranked third by the feature selection probability protocol, with probabilities of selection into the model at 0.55 and 0.53, respectively. Incidence- and abundance-based richness indices tended to be lower in white enrolled infants compared to non-white enrolled infants; however, this trend was not found to be statistically significant.
Effect of mode of delivery on the infant nasal microbiome
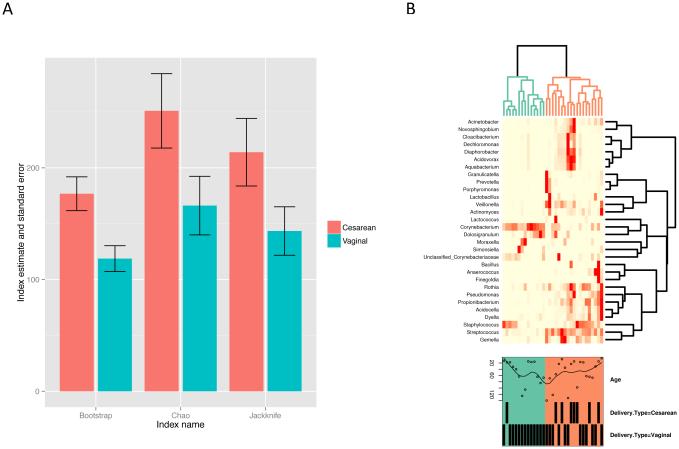
At the 97% OTU clustering level, the infant nasal microbiome was found to be of higher richness for those that had been delivered by Cesarean section. An abundance-based estimate of the number of observed OTUs (after rarefaction as described in Methods) was significantly different between groups defined by the delivery mode (p-value = 0.029, coefficient = −25.57) but Chao1 was not (p-value = 0.079, coefficient = −32.72). Both Shannon and inverted Simpson diversity indices (expressed as Hill numbers N1 and N2) were significantly lower for the vaginally delivered group (p-values = 0.006, 0.013; coefficients = −6.709, −3.419).
At the OTU level, incidence-based richness was higher for the Cesarean delivery mode (Figure 2a). The Adonis test for the association between beta-diversity and delivery mode was significant (p-value = 0.021, R2 = 0.057), while the betadisper test was not significant (p-value = 0.292); together, these data indicate an overall compositional dissimilarity between groups defined by the mode of delivery when only the presence of OTUs was considered. However, when using the abundance-based Bray-Curtis index for profiles composed of more abundant OTUs that passed independent filtering as described in Methods (71 OTUs in total) and normalized to proportions, the Adonis test was not significant (p-value = 0.147, R2 = 0.044), indicating that the estimate of compositional difference is sensitive to the contribution of low-abundant species.
Fig. 2.
a Incidence-based richness estimates at the genus level and corresponding standard errors for samples grouped by delivery type. The Chao, first-order jackknife (jack1), and bootstrap (boot) indices were calculated after rarefying each sample to the minimum sequence count multiple times (n = 400) and averaging the results. Taxonomic richness at the genus level was consistently higher in the nasal microbiota of infants who had been delivered via Cesarean section. b. Heatmap of abundance profiles at the genus level. Infant age, in days, and delivery mode are labeled for each sample. Two clades emerge, colored green and orange. Clustering due to delivery mode can be observed.
Infants that had been delivered by Cesarean section had a larger relative proportion of Firmicutes (base mean = 10039.72, log2 fold change = −0.207) and a lower relative abundance of Actinobacteria (base mean = 9409.75, log2 fold change = 1.674) compared to the vaginally delivered group. Corynebacterium made up a large proportion of the Actinobacteria and were of higher relative abundance in the vaginally delivered group (base mean = 11000, log2 fold change = 2.047); within the Firmicutes, the highly represented Staphylococcus were of higher relative abundance in the Cesarean-section group (base mean = 2746, log2 fold change = −0.755) (Figure 1b). However, the differences in relative abundance of these taxonomic groups by delivery mode were not statistically significant according to both the DESeq2 Wald test and the Wilcoxon rank-sum test after multiple testing adjustments. While overall Corynebacterium abundance did not differ by delivery mode, the abundance of two OTUs identified as Corynebacterium was significantly different according to the DESeq2 Wald test (OTU 0114 base mean = 501.7, log2 fold change = 6.847, q-value < 0.001; OTU 0993 base mean = 2.614, log2 fold change = 3.335, q-value = 0.002). The genus Novosphingobium, although not passing the significance q-value cutoff in the DESeq2 analysis, was ranked second, after Bacillus, according to the test statistic value; it was also ranked first by the feature stability selection protocol (with Bacillus ranked second), having a probability of selection into the model of 0.68.
Clustering based on the genus abundance profile was observed based on delivery mode (Figure 2b). Two main clusters can be seen that correlated with richness; where the microbiome of Cesarean-delivered infants clustered into the group with higher richness. Majority of vaginally-delivered infants clustered to the group with lower richness, however, a significant number of vaginally-delivered infants also clustered with the higher richness group. The higher abundance of Corynebacterium found in vaginally-delivered infants can be observed and appears to be a factor in the cluster pattern. High abundance of either Streptococcus or Staphylococcus, were found in a subgroup of vaginally-delivered infants, with a relatively high genus richness, and clustered with the Cesarean-delivered infants.
Effects of diet, antibiotic use, and the presence of a pet in the household on the infant nasal microbiome
The infant nasal microbiome was found to be of higher richness at the genus level for those that had been formula fed. However, no significant associations between richness or diversity and breastfeeding status were found at the 97% OTU clustering or genus levels. Because there was an association between vaginal delivery and breastfeeding, either one or both of these factors may be contributing to the observed decrease in richness for the breastfed, vaginally-delivered group. Moraxella was of higher relative abundance in the formula-fed group and was the only genus that was significantly associated with breastfeeding when tested using the DESeq2 analysis (q-value = 0.003, base mean = 18.57, log2 fold change = 4.946); however, there was no difference in Moraxella relative abundance by diet according to the Wilcoxon rank-sum test.
At the genus level, maternal use of antibiotics during pregnancy was not found to correlate with richness or diversity. At the 97% OTU level, neither incidence- nor abundance-based richness was associated with maternal antibiotic use. The betadisper test was significant (p-value = 0.020), while the Adonis test was not (p-value = 0.785, R2 = 0.024), indicating that, while the taxonomic profile of the two groups was not significantly different, the variance within each group was not homogenous, with the microbiome appearing to be more variable in infants whose mothers had not received antibiotics. Although the overall taxonomic profile between the two groups did not appear to differ, Moraxella abundance was higher in infants whose mothers had received antibiotics during pregnancy when tested using DESeq2 (base mean = 34.4966, log2 fold change = −6.411, q-value < 0.001), but its abundance was not significantly different by the Wilcoxon rank-sum test.
Further, the abundance-based estimate Chao1 was significantly different between groups defined by the presence of a pet in the household (p-value = 0.050, coefficient = −31.86), but the number of observed OTUs after rarefaction was not (p-value = 0.421, coefficient = −8.449). Neither Shannon nor inverted Simpson diversity indices (expressed as Hill numbers N1 and N2) were significantly different by the presence of a pet (p-value = 0.585, 0.616; coefficient = 1.234, 0.632. This could be due to smaller sample size.
Discussion
At the phylum level, the taxonomic profiles in the present study with nasal sampling by filter paper largely corroborate previous research of the human nasopharyngeal microbiome [27-30], although differences in the population studied, sampling methods, DNA extraction protocols, amplification primers, and data processing pipelines preclude direct comparisons. Over 300 genera were identified using this method, suggesting that filters are an adequate replacement for nasal washes or swabs because they are able to both broadly replicate the findings of previous studies and are also capable of capturing a wide variety of bacterial taxa. We were able to detect taxa previously reported to commonly inhabit the nasal cavity, including Propionibacterineae, Corynebacterineae, Micrococcineae, and Staphylococcus [27-29].
To further validate our method, we attempted to replicate previously observed trends at a finer taxonomic level. A difference in the nasopharyngeal microbiomes of six-week-old infants who were breast- or formula-fed has been previously described: Dolosigranulum and Corynebacterium were of higher abundance and Staphylococcus, Prevotella, and Veillonella were of lower abundance in breastfed infants [31]. An association of Dolosigranulum and Corynebacterium colonization with breastfeeding has been confirmed in a second study [32]. With the exception of Staphylococcus being observed at a slightly higher abundance in the breastfed group, we observed similar trends in genus-level abundances as described above by Biesbroek, Bosch, et al. (2014) [31]. Although none of the differences in relative abundances of genera were of statistical significance in our study, this may be attributed to relatively low power due to the small sample size in this analysis. Future research with a large cohort will be necessary to determine the strength and/or validity of these diet-based trends in this population.
The observed higher abundance of Moraxella in the formula-fed group in our study may have implications for infant health because M. catarrhalis has been established as an important respiratory pathogen [33]. However, an association between colonization by M. catarrhalis and diet was not discovered in a cohort of Dutch children [34]. The length of sequences obtained in the present study does not allow us to identify taxa down to the species level; therefore, we cannot determine what proportion, if any, of the Moraxella we observed in this cohort are potential pathogens.
A number of studies have established that mode of delivery affects the taxonomic composition of the microbiota of infants [35-38], with the newborn microbiota reflecting the mother’s skin microbiome for Cesarean-section deliveries and the mother’s vaginal microbiome for vaginal deliveries [35]. Infants delivered via Cesarean section have been found to have a lower diversity [36,37] and richness [38] of their gut microbiota than those delivered vaginally. In contrast to what has been observed in the gut, we identified a higher taxonomic richness and diversity of the nasal microbiome in infants that had been delivered by Cesarean section. This difference could be due to the site of sample collection. The nasal microbiome may be more likely to be biased by transient bacteria compared to the gut microbiome; the total bacterial load in the airway is orders of magnitudes lower than that of the gut [39,40]. Thus the potential bias of transient bacteria is expected to be more pronounced within a lower biomass environment. This bias may be especially pronounced in the Cesarean section delivered subjects, as the increased richness in infants delivered by Cesarean section could be due to PCR amplification of these transient air-borne non-colonized bacteria from the nostrils. In contrast, the nasal microbiome may be more stable in the vaginally-delivered group, if colonization by commensals occurs earlier. At the phylum level, the taxonomic profile of the nasal microbiome of infants delivered vaginally more closely matched the previously observed nasal microbiome profile of adults [28,29] than did the microbiome of those that had been delivered by Cesarean section, supporting the hypothesis that the nasal microbiome of vaginally-delivered infants may be more representative of an environment that has been successfully colonized by stable commensals. Further gene expression, metagenomic sequencing, or sampling the same subject at multiple timepoints would be needed to discriminate between transient and commensal bacteria within the nasal passage of this cohort.
Those that had been delivered via Cesarean section tended to have a higher proportion of Firmicutes, at the expense of Actinobacteria, compared to adults [28,29] and to the vaginally delivered infants in this study. A large proportion of the Actinobacteria in this cohort was classified within the genus Corynebacterium, and indeed, the relative abundance of Corynebacterium was found to be higher in the nasal microbiota of vaginally delivered infants compared to Cesarean delivered infants. In healthy adults, the Corynebacterium tend to dominate the nasal microbiota and may play a role in maintaining host health through the inhibition of Staphylococcus aureus growth [42] and colonization [43]. We observed a higher abundance of Staphylococcus in the Cesarean-delivered group (Figure 1b), supporting the hypothesis that Corynebacterium species may be capable of suppressing Staphylococcus growth as observed by others. Although the differences in relative abundance of Staphylococcus and Corynebacterium by delivery mode were not found to be statistically significant in this study, we did observe two Corynebacterium OTUs at statistically higher relative abundance within the vaginally delivered group. Our reads were of insufficient length to identify at the species level; it is possible that the delivery mode affects species within the Corynebacterium differently. The possible inhibitory effect of Corynebacterium on Staphylococccus warrants further examination within a larger cohort to better understand how interactions between members of the microbiota influence host health.
We also found that Novosphingobium was ranked as the second or first differentially represented genus depending on the ranking method. Representatives of genus Novosphingobium, which is often found in the external environment, have been recently found to be differentially more abundant in the lungs of patients with advanced stages of chronic obstructive pulmonary diseases (COPD), and the species have been shown to elicit inflammation in COPD mouse models [44]. Cesarean-section delivery is known to be associated with a significantly higher risk of developing asthma [45]. Therefore, the possibility of early Novosphingobium colonization to be either a causative factor or prognostic marker for lung-related diseases later in life is worth further investigation.
In this study population, we observed that infants who had been delivered by Cesarean section were less likely to have been breastfed, an association that has been documented previously [46-48]. The impact of formula feeding and a Cesarean-section delivery on the infant’s microbiome is an active area of research, and each has been linked to alterations in the microbial community profile [31,38,49] and to an increased risk in immune-mediated diseases [45,50]; as such, they are potential targets for early disease prevention interventions [51-53]. Given the apparent confounding relationship between these two variables, it should be considered that any observations in changes to microbial profiles associated with diet or delivery mode may be due to one, both, or an interaction between these two factors.
Initial colonization of the respiratory tract by commensal microbes and the temporal dynamics of the microbiome, especially in early childhood, are not yet fully understood but are of interest in understanding how alterations to the microbial community may predispose individuals to the development of immune-mediated diseases. The level of temporal variability of the nasal microbiome is not completely understood: in adults, a longitudinal study has shown the nostril microbiota to be relatively stable over time around a baseline mean [29], while another longitudinal study of two time points found the anterior nares to be highly variable between time points [41]. The respiratory microbiome was found to be moderately stable in young children, especially those with high abundances of Moraxella [32]. Our results suggest a gradual increase in stability of the nasal microbiome with age: the observed apparent decrease in richness with older age may be the result of stable commensal groups successfully colonizing the nasal environment, thus allowing fewer transient air-borne bacteria to be randomly captured during sampling efforts. However, it should be noted that for this analysis, while we sampled infants of various ages, each subject was sampled at only a single point in time; therefore, we are limited in what we can infer about typical colonization and succession patterns within this population. Further studies of the infant microbiome, especially longitudinal studies that sample the same infant at multiple time points, will be necessary to more fully understand typical bacterial succession patterns that occur after birth and how environmental factors and respiratory viruses may disturb that microbial community.
With this analysis, we aimed to first confirm the validity of an alternate, less invasive sampling method that is suitable for use in very young children to measure the nasal microbiota, and secondly to establish a baseline of the infant nasal microbiota within the INSPIRE cohort. This filter paper method of sampling the nares will be useful for characterizing the infant nasal microbiota to investigate patterns related to health and disease and for better understanding the effect of diet, delivery method, and other environmental and demographic factors on the airway microbiome. The observed increase in taxonomic richness in infants who had been formula-fed and delivered via Cesarean section may have implications for host health. We will use these methods to examine the relationship between the infant nasal microbiome, viral respiratory illnesses, and the subsequent development of wheezing illnesses within the large prospective INSPIRE birth cohort.
Supplementary Material
Acknowledgments
We thank Theresa Rodger for providing outstanding technical assistance and Dr. Karla M. Stucker for her critical review and editing of the manuscript. The clinical sample and data collection for this study was supported by a National Institute of Allergy and Infectious Diseases grant (AI U19-AI-095277) and a Vanderbilt Institute for Clinical and Translational Research Grant (UL1 TR000445) from NCATS/NIH. The sequencing work was generously supported by the NIAID/NIH Genomic Centers for Infectious Diseases (GCID) program (U19-AI-110819). MHS and SRD are supported by U19AI095227-supplement. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authors’ Contributions
MHS, RSP, MLM, LJA, KEN, TVH, and SRD conceived and designed the study. CRS, RSP and TH collected clinical samples, MHS, MT, RH, and SRD performed sample processing and 16S rRNA gene sequencing. MHS, AT, KEN, and SRD performed the 16S rRNA gene sequence data analysis. MHS, CRS, AT, MLM, LJA, KEN, TVH, and SRD wrote the manuscript, and all authors reviewed and approved the final version.
Conflicts of Interest
The authors have no significant conflicts of interest to declare.
References
- 1.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. doi:10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. doi:10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–1372. doi: 10.1016/S0140-6736(13)61536-6. doi:10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 4.Cho I, Blaser MJ. The Human Microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. doi:10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madupu R, Szpakowski S, Nelson KE. Microbiome in human health and disease. Sci Prog. 2013;96:153–170. doi: 10.3184/003685013X13683759820813. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv in Gastroenterol. 2013;6(4):295–308. doi: 10.1177/1756283X13482996. doi:10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legatzki A, Rosler B, von Mutius E. Microbiome diversity and asthma and allergy risk. Curr Allergy Asthma Rep. 2014;14(10):466. doi: 10.1007/s11882-014-0466-0. doi:10.1007/s11882-014-0466-0. [DOI] [PubMed] [Google Scholar]
- 8.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. doi:10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 9.von Linstow M-L, Schønning K, Hoegh AM, Sevelsted A, Vissing NH, Bisgaard H. Neonatal airway colonization is associated with troublesome lung symptoms in infants. Am J Respir Crit Care Med. 2013;188(8):1041–1042. doi: 10.1164/rccm.201302-0395LE. doi:10.1164/rccm.201302-0395LE. [DOI] [PubMed] [Google Scholar]
- 10.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WOC. Disordered microbial communities in asthmatic airways. PloS One. 2010;5(1):e8578–e8578. doi: 10.1371/journal.pone.0008578. doi:10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, Dimango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV, National Heart, Lung, and Blood Institute's Asthma Clinical Research Network Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381. doi: 10.1016/j.jaci.2010.10.048. e371-373. doi:10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–352. doi: 10.1016/j.jaci.2012.11.013. e341-343. doi:10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grad R, Morgan WJ. Long-term outcomes of early-onset wheeze and asthma. J Allergy Clin Immunol. 2012;130(2):299–307. doi: 10.1016/j.jaci.2012.05.022. doi:10.1016/j.jaci.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. doi:10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 15.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. doi: 10.1136/thx.2009.121582. doi:10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 16.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141. doi: 10.1164/rccm.200406-730OC. doi:10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 17.Lynch SV. Viruses and microbiome alterations. Ann Am Thoracic Soc. 2014;11(Suppl 1):S57–60. doi: 10.1513/AnnalsATS.201306-158MG. doi:10.1513/AnnalsATS.201306-158MG. [DOI] [PubMed] [Google Scholar]
- 18.Larkin EK, Gebretsadik T, Moore ML, Anderson LJ, Dupont WD, Chappell JD, Minton PA, Peebles RS, Jr., Moore PE, Valet RS, Arnold DH, Rosas-Salazar C, Das SR, Polack FP, Hartert TV. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE) BMC Pulm Med. 2015;15(1):45. doi: 10.1186/s12890-015-0040-0. doi:10.1186/s12890-015-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folsgaard NV, Schjorring S, Chawes BL, Rasmussen MA, Krogfelt KA, Brix S, Bisgaard H. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 2013;187(6):589–595. doi: 10.1164/rccm.201207-1297OC. doi:10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 20.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh M-J, Huang S-T, Ljungberg I, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10(1):174. doi: 10.1186/1479-5876-10-174. doi:info:pmid/22929533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schloss P, Westcott S, Ryabin T, Hall J, Hartmann M, Hollister E, Lesniewski R, Oakley B, Parks D, Robinson C, Sahl JWSB, Thallinger G, Van Horn D, Weber C. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009 Dec;75(23):7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian S YAP https://github.com/shpakoo/YAP.
- 23.Fouts DE, Szpakowski S, Purushe J, Torralba M, Waterman RC, MacNeil MD, Alexander LJ, Nelson KE. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS One. 2012;7(11):e48289. doi: 10.1371/journal.pone.0048289. doi:10.1371/journal.pone.0048289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One. 2011;6(12):e27310. doi: 10.1371/journal.pone.0027310. doi:10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009;19(7):1141–1152. doi: 10.1101/gr.085464.108. doi:10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tovchigrechko A. MGSAT - Statistical analysis of microbiome and proteome abundance matrices with automated report generation. https://bitbucket.org/andreyto/mgsat. [Google Scholar]
- 27.Bassis CM, Tang AL, Young VB, Pynnonen MA. The nasal cavity microbiota of healthy adults. Microbiome. 2014;2(1):27–27. doi: 10.1186/2049-2618-2-27. doi:10.1186/2049-2618-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486. doi:10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5(5):e10598. doi: 10.1371/journal.pone.0010598. doi:10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio. 2010;1(3) doi: 10.1128/mBio.00129-10. doi:10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biesbroek G, Bosch AATM, Wang X, Keijser BJF, Veenhoven RH, Sanders EAM, Bogaert D. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med. 2014;190(3):298–308. doi: 10.1164/rccm.201401-0073OC. doi:10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 32.Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, Bogaert D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190(11):1283–1292. doi: 10.1164/rccm.201407-1240OC. doi:10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 33.Verduin CM, Hol C, Fleer A, van Dijk H, van Belkum A. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15(1):125–144. doi: 10.1128/CMR.15.1.125-144.2002. doi:10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhaegh SJC, Lebon A, Saarloos JA, Verbrugh HA, Jaddoe VWV, Hofman A, Hays JP, Moll HA, van Belkum A. Determinants of Moraxella catarrhalis colonization in healthy Dutch children during the first 14 months of life. Clin Microbiol Infec. 2010;16(7):992–997. doi: 10.1111/j.1469-0691.2009.03008.x. doi:10.1111/j.1469-0691.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. doi:10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138(9):1796S–1800. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 37.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. doi:10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 38.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Can Med Assoc J. 2013;185(5):385–394. doi: 10.1503/cmaj.121189. doi:10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tlaskalová-Hogenová H, Štěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Šrůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–120. doi: 10.1038/cmi.2010.67. doi:doi:10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006 doi: 10.1126/science.1124234. doi:10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Gao H, Mihindukulasuriya KA, La Rosa PS, Wylie KM, Vishnivetskaya T, Podar M, Warner B, Tarr PI, Nelson DE, Fortenberry JD, Holland MJ, Burr SE, Shannon WD, Sodergren E, Weinstock GM. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013;14(1):R1. doi: 10.1186/gb-2013-14-1-r1. doi:10.1186/gb-2013-14-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan M, Pamp SJ, Fukuyama J, Hwang PH, Cho D-Y, Holmes S, Relman DA. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013;14(6):631–640. doi: 10.1016/j.chom.2013.11.005. doi:10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. Bacterial Competition for Human Nasal Cavity Colonization: Role of Staphylococcal agr Alleles. Appl Environ Microbiol. 2003;69(1):18–23. doi: 10.1128/AEM.69.1.18-23.2003. doi:10.1128/aem.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutebemberwa A, Stevens MJ, Perez MJ, Smith LP, Sanders L, Cosgrove G, Robertson CE, Tuder RM, Harris JK. Novosphingobium and its potential role in chronic obstructive pulmonary diseases: insights from microbiome studies. PLoS One. 2014;9(10):e111150. doi: 10.1371/journal.pone.0111150. doi:10.1371/journal.pone.0111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, Wall MA. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35(11):1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. doi:10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 46.Hauck YL, Fenwick J, Dhaliwal SS, Butt J. A Western Australian survey of breastfeeding initiation, prevalence and early cessation patterns. Matern Child Health J. 2011;15(2):260–268. doi: 10.1007/s10995-009-0554-2. doi:10.1007/s10995-009-0554-2. [DOI] [PubMed] [Google Scholar]
- 47.Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr. 2012;95(5):1113–1135. doi: 10.3945/ajcn.111.030254. doi:10.3945/ajcn.111.030254. [DOI] [PubMed] [Google Scholar]
- 48.Zanardo V, Svegliado G, Cavallin F, Giustardi A, Cosmi E, Litta P, Trevisanuto D. Elective cesarean delivery: does it have a negative effect on breastfeeding? Birth. 2010;37(4):275–279. doi: 10.1111/j.1523-536X.2010.00421.x. doi:10.1111/j.1523-536X.2010.00421.x. [DOI] [PubMed] [Google Scholar]
- 49.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. doi:10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 50.Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I, Wichmann HE, Bolte G. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15(1):48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 51.Collado MC, Rautava S, Isolauri E, Salminen S. Gut microbiota: a source of novel tools to reduce the risk of human disease? Pediatr Res. 2015;77(1-2):182–188. doi: 10.1038/pr.2014.173. doi:10.1038/pr.2014.173. [DOI] [PubMed] [Google Scholar]
- 52.Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69(1):1–10. doi: 10.1016/j.phrs.2012.09.001. doi:10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. 2012;129(5):950–960. doi: 10.1542/peds.2011-2736. doi:10.1542/peds.2011-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.