Abstract
The fission yeast Schizosaccharomyces pombe has become a powerful model organism for cytokinesis studies, propelled by pioneering genetic screens in the 1980s and 1990s. S. pombe cells are rod-shaped and divide similarly to mammalian cells, utilizing a medially-placed actin- and myosin-based contractile ring. A cell wall division septum is deposited behind the constricting ring, forming the new ends of each daughter cell. Here we discuss recent advances in our understanding of the regulation of contractile ring formation through formin proteins and the role of the division septum in S. pombe cell division.
Introduction
Cytokinesis is the final stage of the cell cycle that physically divides a cell into two daughter cells. Spatial and temporal coordination of mitosis with cytokinesis ensures that each daughter cell inherits exactly one genomic complement. Over the past 30 years, Schizosaccharomyces pombe has been established as a leading model organism to study cytokinesis, due to its facile genetic manipulation and conserved mechanisms of division through an actin- and myosin-based contractile ring. Here we discuss advances made in the last three years, concentrating on discoveries about contractile ring formation and the regulation of formin proteins, in addition to the role of septation in driving cytokinesis.
Formation of the contractile ring
Contractile ring precursors
The S. pombe contractile ring (CR) forms from cortical precursor nodes that assemble in the cell middle [1]. Detailed study of these precursors revealed two distinct populations of cortical nodes, termed type 1 and type 2. Type 1 nodes (containing mitosis-promoting kinases Cdr1 and Cdr2) assemble during interphase and merge with type 2 nodes (containing Blt1, kinesin Klp8p, RhoGEF Gef2 and binding partner Nod1) during G2 [2]. Late in G2, the anillin-like protein Mid1 is exported from the nucleus and associates with the merged nodes nearby, thereby linking the position of the nucleus to the site of cell division [3]. Upon mitotic entry, core CR components are recruited by Mid1 to the cell division site, including Myosin II heavy and light chains (Myo2, Rlc1 and Cdc4), IQGAP Rng2, the F-BAR protein Cdc15, and the formin Cdc12 (reviewed in [4]). However, mid1 is not essential; in its absence, precursor nodes are still formed, but are not tightly restricted to the center of the cell [5]. In the absence of Mid1, CR formation is reliant on the Septation Initiation Network (SIN, reviewed in [6]), and occurs later during anaphase [7,8]. Both mechanisms can lead to CR formation; however, in the absence of mid1 CRs are often obliquely oriented and/or off-center [5,7]. In S. pombe, Mid1 also recruits the essential polo-like kinase Plo1 and phosphatase Clp1/Cdc14, which may help promote CR assembly before anaphase [9]. In a related fission yeast, Schizosaccharomyces japonicus, Mid1 also forms cortical nodes that anchor CR components, but it is not required for positioning the CR in the cell middle [10]. Outside of fission yeasts, CR precursor nodes have not been conclusively observed. Despite this, the molecules and mechanisms used in S. pombe CR formation appear to be widely conserved throughout eukaryotes [4].
An ordered assembly of components?
During CR formation, proteins are recruited to the cell division site in precise order, suggesting a hierarchical mechanism of ring assembly [1,11]. Mid1 localizes to the cell middle in G2, Rng2 and Myo2 localize to nodes ∼10 minutes prior to spindle pole body separation, and formin Cdc12 and F-BAR Cdc15 accumulate in nodes as spindle pole bodies separate. Precursor nodes condense into a ring ∼10 minutes after spindle pole body separation in mitosis [1,3,11,12]. The importance of ordered assembly was tested by ectopically localizing CR proteins to medial precursor nodes in the absence of Mid1 [13]. Interestingly, Rng2, Myo2 and Cdc12, but not Cdc15, were each able to initiate CR formation when they were the first to arrive at the medial cortex. Therefore, an invariant order of recruitment is not strictly required; instead, these core proteins appear to collaborate to build the CR. Future mechanistic studies are needed to clarify why some core proteins (Cdc12, Myo2 and Rng2) are competent for promoting ectopic CR formation while others (Cdc15) are not.
Regulation of formins during contractile ring assembly
After amassing core CR machinery at the cell middle, precursor nodes condense into a contiguous ring prior to anaphase, a process which can be modeled by a search-capture-pull-release mechanism [14]. A primary driver of this process is actin nucleation and elongation by the formin Cdc12. Thus, how the formin Cdc12 is targeted to and regulated at the site of division has been the subject of several recent studies.
One study shed light on how Cdc12 finds the cell middle. Specifically, a binding interaction between a short motif in Cdc12's N terminus and the F-BAR domain of the scaffolding protein Cdc15 was shown to contribute to Cdc12 recruitment [15,16]. In the absence of this interaction, F-actin accumulation and CR formation was delayed [16]. This and previous studies point to the existence of additional targeting cues within the Cdc12 protein, the nature of which are currently being investigated.
Two other recent studies provided insight into Cdc12 regulation. One study reported that formin function is limited simply through competition with other F-actin nucleators for G-actin monomers, based on the observation that inhibition of the branched actin nucleator Arp2/3 increased the abundance of formin-nucleated actin structures and vice versa [17]. Although profilin binds all G-actin in the cell, it also directly binds the formin homology 1 domain, favoring formin-mediated F-actin nucleation over Arp2/3-mediated F-actin nucleation, even though the Arp2/3 complex is more abundant [18]. Thus, modulation of profilin levels or its interaction with Cdc12 could be means of regulating Cdc12 nucleation and elongation activities at the division site.
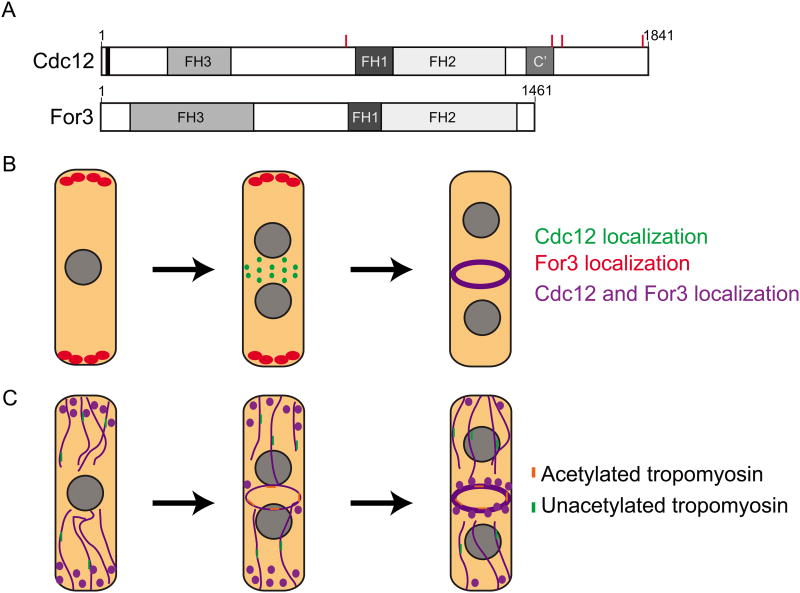
In another study tackling the question of Cdc12 regulation, a multimerization domain was identified in Cdc12's C-terminus (Figure 1, C′), which during interphase mediates oligomerization to form puncta of different sizes on the cortex. In mitosis, when the SIN becomes active, Cdc12 is phosphorylated on 4 residues by the SIN kinase Sid2 (Figure 1 A), inhibiting multimerization and consequent F-actin bundling [19]. Without Sid2-mediated inhibition of Cdc12 multimerization, the CR cannot be formed in the absence of Mid1 or maintained in a cytokinetic arrest, establishing multimerization as one layer of Cdc12 regulation which is essential for proper CR assembly and maintenance. Thus far, purified formins have been found to be strictly dimeric so it remains to be determined if other formins multimerize in a regulated manner in cells. Also, whether multimerization and F-actin bundling impact the F-actin nucleation and elongation activities of Cdc12's catalytic core awaits further study.
Figure 1.
Two S. pombe formins, Cdc12 and For3, are important for contractile ring formation. (A) A schematic of the domain layout of Cdc12 and For3 drawn to scale. The red lines on Cdc12 represent the residues that are phosphorylated by Sid2. (B) A schematic of the localization of Cdc12 and For3 throughout the cell cycle. During interphase For3 localizes to the cell tips in a punctate pattern (Red). In early mitosis Cdc12 localizes to medial cortical nodes as the CR forms (Green). Once the CR is fully formed both Cdc12 and For3 co-localize in the CR (Purple). (C) A schematic representing the F-actin cytoskeleton throughout different cell cycle stages (Purple). In interphase For3 nucleates actin cables emanating from the cell tips. These actin structures are associated with unacetylated tropomyosin (Green). During CR formation Cdc12 forms de-novo F-actin in the cell middle. In addition For3-nucleated cables are pulled into the forming CR. The F-actin nucleated by Cdc12 is bound by acetylated tropomyosin (Orange).
Interestingly, once the CR has formed, Cdc12 function appears to become dispensable for the completion of cytokinesis. Although F-actin turns over quickly in the CR, Cdc12 is not required for CR constriction in vitro [20]. In light of this finding, it will be interesting to learn whether Cdc12 activity is purposefully terminated to ensure efficient CR constriction and the associated reduction in F-actin.
While Cdc12's function is restricted to CR formation [21,22], formin For3 forms longitudinal actin cables important for polarized cell growth [23] (Figure 1). Johnson et al. ectopically localized each formin to cell tips or the cell middle in the absence of the other formin [24]. Intriguingly, each formin formed F-actin with specific properties: Cdc12 formed F-actin with a high growth rate bound by acetylated tropomyosin, while For3 nucleated F-actin with a slow growth rate bound by unacetylated tropomyosin (Figure 1C). Tropomyosin is important for stabilizing F-actin structures, especially the CR, and acetylated tropomyosin is a more stable conformer [25-27]. Despite forming F-actin without acetylated tropomyosin, For3 was able to form a CR, but these rings often collapsed, demonstrating that Cdc12's unique properties are important for CR stability. It will be interesting to determine if other actin binding proteins exhibit a preference for Factin nucleated by certain formins and the mechanism of this specificity.
Although For3 and Cdc12 have distinct properties and Cdc12 is the only formin required for cytokinesis, For3-nucleated F-actin cables are also pulled into the CR [28,29] (Figure 1C). Cells lacking for3 have synthetic lethal genetic interactions with many essential players in CR formation, further supporting a role for this actin network during the initial stages of cytokinesis [30]. Cdc12 alleles lacking an N-terminus are also synthetically lethal in combination with for3Δ, suggesting that when Cdc12 function is compromised, For3's function becomes essential for cell division. The assembly of preexisting actin cables into the cleavage furrow is also evident in animal cells [31]. Thus, while both of these formins play a role in CR formation, their roles are distinct. Cdc12 is essential for de-novo F-actin formation at the cell division site whereas For3 makes actin cables that also incorporate into the CR.
Formation of a division septum
Function of glucans in the division septa
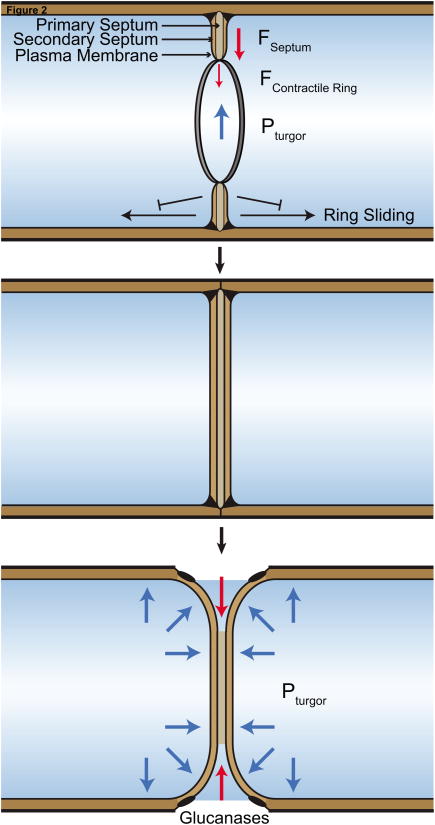
Once the CR is formed, it constricts in a myosin-dependent manner [32,33]. However, as cell-walled organisms, cytokinesis in S. pombe also requires the synthesis of a new cell wall between daughter cells. A tri-layer division septum, composed of an inner primary septum flanked by secondary septa, is deposited behind the constricting actomyosin ring to maintain a contiguous cell wall (Figure 2). After septum formation, glucanases are secreted to break down the inner primary septum which splits the daughter cells. Degradation of the primary septum in combination with outward turgor pressure facilitates gentle separation and rounding of each daughter cell's new end [34].
Figure 2.
Roles of septation in S. pombe cytokinesis. Top) Primary and secondary septa are formed simultaneously as the contractile ring constricts. Formation of the septum “locks” the ring into place and prevents sliding from the cell middle. Contractile ring constriction (FContractile Ring) and septum formation (FSeptum) oppose the cell's high turgor pressure (PTurgor) to constrict the division apparatus. Middle) A contiguous septum is formed and matures after the contractile ring completely constricts. Bottom) Glucanases are secreted to break down the inner primary septum which allows separation of daughter cells. Each new cell end rounds due to internal turgor pressure as separation occurs.
The S. pombe primary septum is predominantly composed of linear ß(1,3)glucans, while the secondary septum contains α(1,3)glucans, branched ß(1,3)glucans, linear ß(1,3)glucans, and galactomannans [35]. S. pombe septa do not contain chitin, unlike the prominent chitin ring present at S. cerevisiae septa [36]. At least three essential glucan synthases form the primary and secondary septa; catalytic subunits of these complexes are Bgs1, Bgs4, and Ags1. Bgs1 forms linear ß(1,3)glucans and is essential for primary septum formation. Bgs4 forms branched ß(1,3)glucans, while Ags1 forms α(1,3)glucans.
Recent work has defined the functions of Bgs4-derived branched ß-glucans and α-glucans produced by Ags1. When Ags1 function was compromised, the primary septum was improperly anchored to the cell wall and grew in an uncoordinated manner toward the cell middle [34]. In the absence of efficient anchoring, the primary septum often tore and cells lysed during separation. Thus, α-glucans are required to anchor the primary septum to the cell wall. Branched ß-glucans, on the other hand, are an essential component of the secondary septa. Cells lacking branched ß-glucans never formed a secondary septum, and only formed a twisted and unsupported primary septum [37]. If cells attempted to separate without a secondary septum, over-degradation of cell wall material occurred, resulting in lysis. Therefore, linear ß-glucans, branched ß-glucans, and α-glucans formed by Bgs1, Bgs4, and Ags1, are all essential, cooperating to build the division septum.
Septum formation forces
Septum formation has been recognized for years as necessary for CR constriction and completion of cytokinesis. Certain mutations compromising Bgs1 (cps1-191) lead to cell cycle arrest and the maintenance of CRs without constriction [38,39]. In the absence of microtubules (presumably impacting the cell polarity machinery) CRs in cps1-191 cells slid toward cell tips, suggesting they were dynamic and not locked into their medial position [40].
More information has emerged recently to explain how septum formation contributes to CR stabilization and constriction. First, with more advanced microscopy techniques, it has been confirmed that compromising glucan synthase activity permits CRs to slide and unravel [37,41]. This has also been evident in spheroplast studies; when cell walls were removed by digestion and prevented from re-forming, CRs slid from the cell middle [33]. However, when digesting enzymes were removed from spheroplasts, CRs formed and pinched the cell [32]. Formation of the septum, therefore, is capable of “locking” the CR in place (Figure 2).
In addition to preventing CR sliding, recent measurements of the forces necessary for cell division indicate septum formation contributes the predominant constriction force, in accord with its requirement for cell division. The CR and septum must overcome turgor pressure, which in S. pombe cells is ∼1 MPa [42]. Calculations of the maximum force myosin motors in the CR can exert (∼15 nN, 10 kPa) indicate myosins within the CR are not sufficient [42]. Synthesis of extracellular glucans may be able to exert a “ratchet” force as glucan synthases add subunits to glucan chains, which push against the plasma membrane, similar to models proposed for actin at the leading edge of animal cells [42,43]. Further mechanistic study of glucan synthases and glucan chains will clarify how this force is generated. The dependence on septum synthesis for CR constriction is similar in S. cerevisiae and possibly other cell walled organisms [36,44]. Animal cells, on the other hand, do not need to overcome large turgor pressures and can constrict with the force of myosin alone; however, there are contexts where extracellular elements contribute to cytokinesis in animal cells as well [45,46]
These conclusions have led to speculation about the purpose of the CR in fungi, if not to provide force for ingression. In S. cerevisiae, cytokinesis can complete in the absence of myosin-II through the over-synthesis of chitin to physically separate the daughter from mother bud [47]. In S. pombe, cells are able to complete cell division even when the F-actin in their CRs is depolymerized after constriction has begun [42,48]. However, F-actin depolymerization does not remove all components of the CR. Presumably the remaining proteins keep the glucan synthases active to complete division, similar to the S. cerevisiae chitin synthesis mechanism. CR contractility (through Myo2) has also been shown (historically and recently [32]) to be essential for CR constriction. The CR, therefore, remains essential for each step in cytokinesis: positioning the division plane, recruiting cytokinetic proteins, and activating constriction and septum synthesis. Furthermore, clearance of CR remnants is required to reinitiate polarized growth at new daughter cell ends [49].
Signaling to glucan synthases
Septum formation begins only when the CR starts to constrict, but it is not yet known molecularly how these two processes are coordinated. Small GTPase Rho proteins control many aspects of cell division, including glucan synthase activation. In S. pombe, Bgs1 and Bgs4 are activated by Rho1, while Ags1 is activated by Rho2 [50,51]. Recent work has identified physical links between protein components of the contractile ring and Rho GTPase activators (RhoGEFs) that may explain this activation.
Cdc15 and Imp2, two membrane-binding F-BAR proteins in the CR, were found to recruit Rgf3, an essential RhoGEF that specifically activates Rho1 [52]. Localization of Rho1's RhoGEF is an attractive model for activation of Bgs1 and Bgs4 for septum synthesis at the division site. It has also been proposed that Cdc15 participates directly in trafficking Bgs1 to the division site from the Golgi [41], since Bgs1 recruitment is slowed when Cdc15 levels are repressed. However, slowed accumulation can be explained by a general delay in CR formation when Cdc15 is compromised [53]. In addition, recent work identified paxillin Pxl1, also recruited by Cdc15 to the CR, to cooperate with Bgs1 to ensure proper septum formation [54]. Further work will clarify the exact mechanistic link(s) between Cdc15 and septum synthase enzymes.
Additional physical links between the CR and Rho GTPase activators in the later stages of cell separation have also been discovered. Gef3 was identified as a specific RhoGEF for Rho4, which activates the secretion of Agn1 and Egn1 to degrade the PS and split the daughter cells [55,56]. Anillin Mid2 and septin proteins bind and localize Gef3 to a non-constricting ring flanking the septum, positioning this Rho4 activator at the appropriate place for exocyst activation and glucanase secretion [56].
Concluding remarks
Multiple advances in our understanding of S. pombe cytokinesis have been reported in the last few years, but key questions remain. We do not understand how Factin in the CR is organized, how other CR proteins are organized in this structure, or how the CR is disassembled during constriction. Mechanisms of glucan synthase regulation are becoming clearer, but how these enzymes are coordinated with CR constriction and produce force to divide the cell remain to be discovered.
Highlights.
-Hierarchical protein recruitment is not essential for forming the contractile ring
-Profilin allows formins to compete with Arp2/3 for G-actin
-Septum formation prevents contractile ring sliding
-Septum formation contributes an essential force for cell division
Acknowledgments
The authors thank Dr. Janel Beckley for comments on the manuscript. We apologize to those whose work was not cited here due to space constraints. A.H.W was supported by AHA fellowship 14PRE19740000. N.A.M. was supported by AHA fellowship 15PRE21780003. This work was supported by NIH grant GM101035 to K.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
*of special interest
**of outstanding interest
- 1.Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Developmental Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu M, Berro J, Pu KM, Tebbs IR, Pollard TD. Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. The Journal of cell biology. 2014;204:977–988. doi: 10.1083/jcb.201307174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohrmann M, Fankhauser C, Brodbeck C, Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes and Development. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- 4.Pollard TD, Wu JQ. Understanding cytokinesis: lessons from fission yeast. Nature Reviews Molecular Cell Biology. 2010;11:149–155. doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha S, Pollard TD. Anillin-related protein Mid1p coordinates the assembly of the cytokinetic contractile ring in fission yeast. Molecular Biology of the Cell. 2012;23:3982–3992. doi: 10.1091/mbc.E12-07-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simanis V. Pombe's thirteen - control of fission yeast cell division by the septation initiation network. Journal of Cell Science. 2015:1–10. doi: 10.1242/jcs.094821. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Yan H, Balasubramanian MK. Assembly of normal actomyosin rings in the absence of Mid1p and cortical nodes in fission yeast. The Journal of Cell Biology. 2008;183:979–988. doi: 10.1083/jcb.200806151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachet O, Simanis V. Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes & Development. 2008;22:3205–3216. doi: 10.1101/gad.1697208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bähler J, Steever aB, Wheatley S, Wang YL, Pringle JR, Gould KL, McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. The Journal of Cell Biology. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Yam C, Oliferenko S. Rewiring of Cellular Division Site Selection in Evolution of Fission Yeasts. Current Biology. 2015;25:1187–1194. doi: 10.1016/j.cub.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laporte D, Coffman VC, Lee IJ, Wu JQ. Assembly and architecture of precursor nodes during fission yeast cytokinesis. The Journal of Cell Biology. 2011;192:1005–1021. doi: 10.1083/jcb.201008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padmanabhan A, Bakka K, Sevugan M, Naqvi NI, D'Souza V, Tang X, Mishra M, Balasubramanian MK. IQGAP-related Rng2p organizes cortical nodes and ensures position of cell division in fission yeast. Curr Biol. 2011;21:467–472. doi: 10.1016/j.cub.2011.01.059. [DOI] [PubMed] [Google Scholar]
- *13.Tao EY, Calvert M, Balasubramanian MK. Rewiring Mid1p-Independent Medial Division in Fission Yeast. Curr Biol. 2014 doi: 10.1016/j.cub.2014.07.074. This study determined that hierarchical recruitment of core contractile ring components is not essential, and defines Mid1's nonessential roles in early ring assembly. [DOI] [PubMed] [Google Scholar]
- 14.Vavylonis D, Wu JQ, Hao S, O'Shaughnessy B, Pollard TD. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 2008;319:97–100. doi: 10.1126/science.1151086. [DOI] [PubMed] [Google Scholar]
- 15.Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willet AH, McDonald NA, Bohnert KA, Baird MA, Allen JR, Davidson MW, Gould KL. The F-BAR Cdc15 promotes contractile ring formation through the direct recruitment of the formin Cdc12. J Cell Biol. 2015;208:391–399. doi: 10.1083/jcb.201411097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Burke TA, Christensen JR, Barone E, Suarez C, Sirotkin V, Kovar DR. Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr Biol. 2014;24:579–585. doi: 10.1016/j.cub.2014.01.072. These authors determined that formin and Arp2/3 actin networks compete for G-actin monomers bound by profilin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Suarez C, Carroll RT, Burke TA, Christensen JR, Bestul AJ, Sees JA, James ML, Sirotkin V, Kovar DR. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev Cell. 2015;32:43–53. doi: 10.1016/j.devcel.2014.10.027. This study determined that profilin modulates formin and Arp2/3 competition by inhibiting Arp2/3 nucleation and activating formin elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Bohnert KA, Grzegorzewska AP, Willet AH, Vander Kooi CW, Kovar DR, Gould KL. SIN-dependent phosphoinhibition of formin multimerization controls fission yeast cytokinesis. Genes Dev. 2013;27:2164–2177. doi: 10.1101/gad.224154.113. This study identified a new regulatory mechanism for a cytokinetic formin through multimerization. Control of multimerization by a cytokinetic signalling network was important for contractile ring formation and maintenence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra M, Kashiwazaki J, Takagi T, Srinivasan R, Huang Y, Balasubramanian MK, Mabuchi I. In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics. Nature Cell Biology. 2013 doi: 10.1038/ncb2781. [DOI] [PubMed] [Google Scholar]
- 21.Yonetani A, Lustig RJ, Moseley JB, Takeda T, Goode BL, Chang F. Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol Biol Cell. 2008;19:2208–2219. doi: 10.1091/mbc.E07-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feierbach B, Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol. 2001;11:1656–1665. doi: 10.1016/s0960-9822(01)00525-5. [DOI] [PubMed] [Google Scholar]
- *24.Johnson M, East DA, Mulvihill DP. Formins determine the functional properties of actin filaments in yeast. Curr Biol. 2014;24:1525–1530. doi: 10.1016/j.cub.2014.05.034. These authors determined that different formin molecules dictate the type of tropomyosin associated with F-actin filaments they produced. [DOI] [PubMed] [Google Scholar]
- 25.Skoumpla K, Coulton AT, Lehman W, Geeves Ma, Mulvihill DP. Acetylation regulates tropomyosin function in the fission yeast Schizosaccharomyces pombe. Journal of cell science. 2007;120:1635–1645. doi: 10.1242/jcs.001115. [DOI] [PubMed] [Google Scholar]
- 26.Skau CT, Neidt EM, Kovar DR. Role of Tropomyosin in Formin-mediated Contractile Ring Assembly in Fission Yeast. Molecular Biology of the Cell. 2009;20:2160–2173. doi: 10.1091/mbc.E08-12-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark BC, Sladewski TE, Pollard LW, Lord M. Tropomyosin and Myosin-II Cellular Levels Promote Actomyosin Ring Assembly in Fission Yeast. Molecular biology of the cell. 2010;21:989–1000. doi: 10.1091/mbc.E09-10-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Huang J, Huang Y, Yu H, Subramanian D, Padmanabhan A, Thadani R, Tao Y, Tang X, Wedlich-Soldner R, Balasubramanian MK. Nonmedially assembled F-actin cables incorporate into the actomyosin ring in fission yeast. J Cell Biol. 2012;199:831–847. doi: 10.1083/jcb.201209044. These authors visualized the incorporation of pre-existing longitudinal F-actin cables into the forming contractile ring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai R, Mabuchi I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2002;115:887–898. doi: 10.1242/jcs.115.5.887. [DOI] [PubMed] [Google Scholar]
- 30.Coffman VC, Sees JA, Kovar DR, Wu JQ. The formins Cdc12 and For3 cooperate during contractile ring assembly in cytokinesis. J Cell Biol. 2013;203:101–114. doi: 10.1083/jcb.201305022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green Ra, Paluch E, Oegema K. Cytokinesis in Animal Cells. Annual Review of Cell and Developmental Biology. 2012:1–30. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 32.Mishra M, Kashiwazaki J, Takagi T, Srinivasan R, Huang Y, Balasubramanian MK, Mabuchi I. In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics. Nature Cell Biology. 2013;15:853–859. doi: 10.1038/ncb2781. [DOI] [PubMed] [Google Scholar]
- 33.Stachowiak MR, Laplante C, Chin HF, Guirao B, Karatekin E, Pollard TD, O'Shaughnessy B. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev Cell. 2014;29:547–561. doi: 10.1016/j.devcel.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Cortes JC, Sato M, Munoz J, Moreno MB, Clemente-Ramos JA, Ramos M, Okada H, Osumi M, Duran A, Ribas JC. Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J Cell Biol. 2012;198:637–656. doi: 10.1083/jcb.201202015. This study identified α-glucans formed by Ags1 to be essential for the structural strength of the primary septum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humbel BM, Konomi M, Takagi T, Kamasawa N, Ishijima Sa, Osumi M. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast (Chichester, England) 2001;18:433–444. doi: 10.1002/yea.694. [DOI] [PubMed] [Google Scholar]
- 36.Roncero C, Sanchez Y. Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum. Yeast. 2010;27:521–530. doi: 10.1002/yea.1779. [DOI] [PubMed] [Google Scholar]
- **37.Munoz J, Cortes JC, Sipiczki M, Ramos M, Clemente-Ramos JA, Moreno MB, Martins IM, Perez P, Ribas JC. Extracellular cell wall beta(1,3)glucan is required to couple septation to actomyosin ring contraction. J Cell Biol. 2013;203:265–282. doi: 10.1083/jcb.201304132. This paper identified Bgs4 and branched ß-glucans as essential for formation of the secondary septum and stabilization of the contractile ring in the cell middle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Wang H, Balasubramanian MK. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. Journal of cell science. 2000;113(Pt 7):1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Wang H, McCollum D, Balasubramanian MK. Drc1p/Cps1p, a 1,3-β-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics. 1999;153:1193–1203. doi: 10.1093/genetics/153.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardo M, Nurse P. Science. Vol. 300. New York, N.Y: 2003. Equatorial retention of the contractile actin ring by microtubules during cytokinesis; pp. 1569–1574. [DOI] [PubMed] [Google Scholar]
- 41.Arasada R, Pollard TD. Contractile Ring Stability in S. pombe Depends on FBAR Protein Cdc15p and Bgs1p Transport from the Golgi Complex. Cell Reports. 2014 doi: 10.1016/j.celrep.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of Turgor Pressure, the Contractile Ring, and Septum Assembly to Forces in Cytokinesis in Fission Yeast. Current Biology. 2012;22:1–8. doi: 10.1016/j.cub.2012.06.042. This study identified the high turgor pressure of S. pombe as a force that contractile ring constriction must overcome, implicating septum assembly as the primary force of ingression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophysical journal. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt M, Bowers B, Varma A, Roh DH, Cabib E. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. Journal of cell science. 2002;115:293–302. doi: 10.1242/jcs.115.2.293. [DOI] [PubMed] [Google Scholar]
- 45.Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, Mitani S, Sugahara K, Nomura K. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature. 2003;423:443–448. doi: 10.1038/nature01635. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Vogel BE. A secreted protein promotes cleavage furrow maturation during cytokinesis. Current Biology. 2011;21:114–119. doi: 10.1016/j.cub.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolliday N, Pitcher M, Li R. Direct Evidence for a Critical Role of Myosin II in Budding Yeast Cytokinesis and the Evolvability of New Cytokinetic Mechanisms in the Absence of Myosin II. Molecular Biology of the Cell. 2003;14:798–809. doi: 10.1091/mbc.E02-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Munteanu EL, He J, Ursell T, Bathe M, Huang KC, Chang F. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol Biol Cell. 2015;26:78–90. doi: 10.1091/mbc.E14-10-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bohnert KA, Gould KL. Cytokinesis-Based Constraints on Polarized Cell Growth in Fission Yeast. PLoS Genetics. 2012;8:e1003004–e1003004. doi: 10.1371/journal.pgen.1003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arellano M, Durán a, Pérez P. Rho 1 GTPase activates the (1-3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. The EMBO journal. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- 51.Calonge TM, Nakano K, Arellano M, Arai R, Katayama S, Toda T, Mabuchi I, Perez P. Schizosaccharomyces pombe rho2p GTPase regulates cell wall alpha-glucan biosynthesis through the protein kinase pck2p. Molecular biology of the cell. 2000;11:4393–4401. doi: 10.1091/mbc.11.12.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren L, Willet AH, Roberts-Galbraith RH, McDonald NA, Feoktistova A, Chen JS, Huang H, Guillen R, Boone C, Sidhu SS, et al. The Cdc15 and Imp2 SH3 domains cooperatively scaffold a network of proteins that redundantly ensure efficient cell division in fission yeast. Mol Biol Cell. 2015;26:256–269. doi: 10.1091/mbc.E14-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts-Galbraith RH, Chen JS, Wang J, Gould KL. The SH3 domains of two PCH family members cooperate in assembly of the schizosaccharomyces pombe contractile ring. Journal of Cell Biology. 2009;184:113–127. doi: 10.1083/jcb.200806044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.G. Cortés JC, Pujol N, Sato M, Pinar M, Ramos M, Moreno B, Osumi M, Ribas JC, Pérez P. Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast. PLOS Genetics. 2015;11:e1005358–e1005358. doi: 10.1371/journal.pgen.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos B, Martín-Cuadrado AB, Vázquez De Aldana CR, Del Rey F, Pérez P. Rho4 GTPase is involved in secretion of glucanases during fission yeast cytokinesis. Eukaryotic Cell. 2005;4:1639–1645. doi: 10.1128/EC.4.10.1639-1645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang N, Wang M, Zhu YH, Grosel TW, Sun D, Kudryashov DS, Wu JQ. The RhoGEF Gef3 interacts with the septin complex and activates the GTPase Rho4 during fission yeast cytokinesis. Molecular Biology of the Cell. 2014;26:238–255. doi: 10.1091/mbc.E14-07-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]