Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional enzyme that is required for synaptic plasticity and has been proposed to be a primary molecular component of the etiology of alcohol addiction. Chronic alcohol intake upregulates CaMKIIα protein expression in reward-related brain regions including the amygdala and nucleus accumbens, and CaMKIIα activity in the amygdala is required for the positive reinforcing effects of alcohol, suggesting this system promotes consumption in the early stages of alcohol addiction. Alternatively, the medial prefrontal cortex (mPFC) is known to inhibit limbic activity via CaMKII-dependent excitatory projections and may, therefore, enable top-down regulation of motivation. Here we sought to remove that regulatory control by site-specifically inhibiting CaMKII activity in the mPFC, and measured effects on the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Infusion of the CAMKII inhibitor KN-93 (0–10.0 μg) in the mPFC primarily increased alcohol+sucrose reinforced response rate in a dose- and time-dependent manner. KN-93 infusion reduced response rate in behavior-matched sucrose-only controls. Importantly, potentiation of operant responding for sweetened alcohol occurred immediately after infusion, at a time during which effects on sucrose responding were not observed, and persisted through the session. These results suggest that endogenous CaMKII activity in the mPFC exerts inhibitory control over the positive reinforcing effects of alcohol. Downregulation of CaMKII signaling in the mPFC might contribute to escalated alcohol use.
Keywords: CaMKII, alcohol self-administration, positive reinforcement, prefrontal cortex, KN-93, addiction
Alcohol dependence is a complex neuropsychiatric disorder that afflicts more than 9% of the total US population. Epidemiological studies suggest that a majority of individuals (52%) actively engage in moderate, or sub-dependent, levels of alcohol drinking at amounts that are capable of inducing widespread changes in fundamental neural systems that normally regulate cognition, motivation, and other adaptive functions of the organism [1, 2]. For example, recent evidence indicates that moderate drinking launches a cascade of neuroadaptations in the amygdala proteome, linked to calcium/calmodulin-dependent protein kinase II (CaMKII), that mechanistically drive the positive reinforcing effects of alcohol and may serve, therefore, as a molecular pathway from use to abuse [3]. Thus, elucidating the neural mechanisms that regulate alcohol consumption in the non-dependent state is critical for a comprehensive understanding of the etiology of addiction.
CaMKII is a family of Ca2+-activated Ser/Thr protein kinases that mediates many intracellular responses in the brain including regulation of membrane current, neurotransmitter synthesis and release, cytoskeletal organization, and synaptic plasticity [4, 5]. CaMKII is activated when neuronal depolarization leads to Ca2+ entry into the cell through multiple sources including ionotropic glutamate receptors (calcium-permeable AMPA and NMDA receptors), L-type voltage-gated calcium channels, and via release from internal stores. Following activation, CaMKII translocate to the membrane and/or postsynaptic density where it regulates receptor (i.e., NMDA, AMPA) activity [6]. Importantly, a number of CaMKII interacting proteins, including NMDA and AMPA receptors, PSD proteins, CREB, and the MAPKs regulate alcohol-related behaviors including self-administration and relapse (e.g.,[7-10]). Thus, CaMKII may represent a molecular point of convergence in the regulation of maladaptive behaviors associated with alcohol abuse.
Emerging evidence indicates that CAMKII activity and function are altered by alcohol in reward-related brain regions. Increases in CaMKII protein expression and activation (e.g., phosphorylation) have been reported in the cortex, nucleus accumbens and amygdala following alcohol dependence as well as by moderate, or non-dependent, levels induced by voluntary drinking or operant self-administration [3, 11-13]. The functional importance of CAMKII on alcohol-associated behaviors has been recently studied using both pharmacological and genetic approaches. CaMKIIα autophosphorylation-deficient mice show reduced alcohol consumption and preference and have a blunted response to the locomotor-stimulating effects of alcohol [14]. We have shown that the positive reinforcing effects of sweetened alcohol require CaMKII activity in the amygdala [3] but the complete neural circuitry of CaMKII-dependent regulation of alcohol-seeking behavior remains to be fully characterized.
Behavioral pathologies in addiction, such as exacerbated drug-seeking behavior, represent a dynamic interplay between heightened motivation and cognitive regulatory processes that control goal-directed behaviors. Under normal conditions, the medial prefrontal cortex (mPFC) exerts “top-down” control over limbic motivational systems via CaMKII-positive glutamatergic projections to brain regions, such as the nucleus accumbens and amygdala. However, repeated drug use is thought to result in PFC dysregulation, loss of executive control, and exacerbated drug use due to impaired response inhibition [15]. Although evidence cited above implicates CaMKII signaling in the rewarding and reinforcing effects of alcohol, the role of this critical cell signaling molecule in the PFC remains unknown. To address this gap in knowledge, the objective of the current experiment was to determine if CaMKII activity in the mPFC mechanistically regulates the positive reinforcing effects of alcohol. If the mPFC exerts top-down regulatory control of behavioral pathologies in alcohol addiction via response inhibition, we predicted that blockade of CaMKII activity in the mPFC would increase, or exacerbate, sweetened alcohol self-administration via increased positive reinforcement function. Given the prominence of CaMKII signaling in the development and regulation of new behavior, these findings will provide novel information on how alcohol may gain control over behavior during the early stages of addiction when drug use is primarily driven by positive reinforcement.
Male C57BL/6J mice (10-wks old; n=40) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were group-housed and initially handled for 7 days (Figure 1A). Food and water were freely available unless noted. The vivarium was maintained on a reverse 12:12 light/dark schedule, with average temperature and humidity of 25°C and 40%, respectively. All protocols were conducted in accordance with the Institutional Animal Care and Use Committee of the University of North Carolina-Chapel Hill and the Guide for the Care and Use of Laboratory Animals.
FIGURE 1.
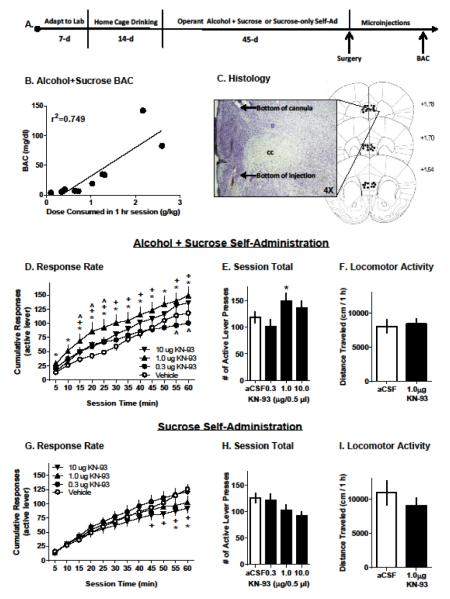
(A) Experimental timeline. (B) Significant correlation (R2=0.749) between individual alcohol dose consumed (g/kg;x-axis) and blood alcohol content (BAC,y-axis). (C) Photomicrograph showing the bottom of the guide cannula and injection site from a coronal mouse brain section. Schematic shows injection sites for all mice that were included in microinjection studies. (D&G) Response rate plotted as cumulative number of active lever presses per 5-min interval (Mean±SEM). Open circles represent vehicle performance and filled symbols represent each dosage of KN-93. Statistically significant dose-effects at each time point are indicated by the following symbols: * (1μg), ^ (0.3μg), and + (10 μg), p<0.05 versus vehicle. (E-F; H-I) Vertical bars represent total active lever presses in a 1-hr self-administration session, or horizontal distance traveled (cm/1-hr) in an open-field locomotor test, respectively. White bars reflect behavior after Veh infusion. Black bars reflect behavior after infusion of KN-93. * - indicates significantly different from vehicle, p<0.05. Data are plotted as Mean±SEM.
Self-administration studies were conducted as described [3]. One week after arrival, separate groups of mice were presented with a 9% alcohol (v/v)/2% sucrose (w/v) (Alcohol + Sucrose; Pharmco-AAPER, Shelbyville, KY) or 2% Sucrose (w/v; Sucrose-only) solution, along with water, in their home-cage for 2 weeks (Figure 1A). Home-cage exposure facilitates operant self-administration training [16]. After home-cage self-administration, mice were water restricted for 20-hrs and then placed into an operant conditioning chamber for a 16-hr overnight session during which lever press responses were reinforced with delivery of either Alcohol+Sucrose or sucrose-only. All conditioning occurred in sound-attenuating chambers (Med Associates; St.Albans, VT) containing retractable levers. Only responding on the active lever was reinforced. After 3 overnight sessions, mice were no longer water restricted and training was shortened to 1-hr sessions (Figure 1A). Throughout the remainder of the experiment, the response requirement was maintained at a fixed-ratio 4 schedule of reinforcement. Blood was collected after surgery on the 87th self-administration session via submandibular bleed and plasma alcohol levels were measured using an AM1 Alcohol Analyzer (Analox Instruments, Ltd., Lunenburg, MA;[7];Figure 1A).
After 45 sessions (Figure 1A), mice were anesthetized with ketamine (120 mg/kg,i.p.) and xylazine (9 mg/kg,i.p.), placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA), and implanted with a 26-gauge guide cannula (Plastics One, Roanoke, VA) unilaterally aimed at either the right or the left mPFC (AP +1.7mm; ML ±0.4mm; DV −1.2mm, from skull surface [3, 17]. The guide cannula was secured to the skull with dental cement (Durelon, Butler Schein, Dublin, OH) and a 33-gauge obturator was inserted. After 1 week of recovery, alcohol+sucrose and sucrose-only self-administration sessions resumed. During baseline, alcohol+sucrose self-administering mice consumed pharmacologically significant doses of sweetened alcohol (Mean±SEM = 0.97±0.15 g/kg). Dose consumed on DAY 87 (Mean±SEM = 1.06 ± 0.26 g/kg) was positively correlated with BAC (34.7mg/dl±14.2) at the end of the 1-hr session (r2=0.749;Figure 1B). Alcohol+sucrose and sucrose-only groups were behavior-matched on total responses during self-administration sessions before (data not shown) and after surgery (Figure 1E and 1H, aCSF bars) indicating that KN-93 drug effects were not related to basal performance.
The selective CAMKII inhibitor KN-93 ( N-[2-[[[3-(4-Chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide phosphate; Tocris Bioscience, Bristol, UK) was administered via site-specific microinjection. Mice were habituated to handing, and sham microinjections were conducted prior to sessions until stable levels of responding were observed (ca. 3 sham injections). Next, a within-subjects dose-effect curve for the CAMKII inhibitor, KN-93, was conducted in a counterbalanced design (n=9 for alcohol + sucrose; n=11 for sucrose-only mice). KN-93 (artificial cerebrospinal fluid, aCSF; 0.3-10 μg/injection; 4 injections/mouse) was infused into the mPFC of unrestrained mice (0.5 μl/injection; 0.125μl/min; 33-gauge injector extended 2mm beyond the tip of the guide cannula). After the infusion, mice were immediately placed into the operant chamber for a 1-hr self-administration session. Injections occurred 2x/week. Immediately after the final session, mice were deeply anesthetized with sodium pentobarbital (150mg/kg) and intracardially perfused with 0.9% phosphate buffered saline and 4% paraformaldehyde for histological verification of injection site (Figure 1C). Three mice were excluded from analysis due to missed cannula placement.
Mice that did not reliably self-administer alcohol+sucrose or sucrose-only after surgery were used to test the effects of KN-93 on locomotor activity as an index of non-specific effects (n=8 alcohol+sucrose;n=9 sucrose-only mice). Open-field activity was measured in Plexiglas chambers (27.9 cm2; ENV-510, Med Associates) that recorded X-Y ambulatory movements. Mice habituated to the open-field for 2-hrs. One week later, mice were given a sham injection and placed in the open-field for 1-hr. Subsequently, KN-93 (0 or 1.0 μg) was microinjected in a counterbalanced design, with one week separating each test (2 injections/mouse).
Two-way RM ANOVA (DOSE × TIME) was used to assess dose- and time-dependent effects of KN-93 (0 – 10 μg) microinjection on operant response rate and dose consumed expressed as cumulative responses per 5-min. Total response data were analyzed by one-way RM ANOVA. Dunnett’s multiple-comparison test was conducted when appropriate. A Pearson product-moment correlation was run to analyze the BAC data. The α level was set at 0.05 for all statistical tests.
Infusion of the CAMKII inhibitor KN-93 altered alcohol+sucrose reinforced responding in a time- and dose-dependent manner, and an interaction between the two factors was also evident. Specifically, cumulative alcohol+sucrose responses increased as a function of time (F11,88=118.5;p<0.0001;Figure 1D) indicating continued self-administration throughout the session. The effects of KN-93 depended on time as indicated by a significant dose × time interaction (F33,264=3.656;p<0.001;Figure 1D). Post-hoc analysis indicated that KN-93 (1.0μg) infusion in the PFC significantly increased the rate of alcohol+sucrose reinforced responding at all time points (0–60 min) as compared to vehicle. KN-93 (0.3μg) transiently increased alcohol+sucrose reinforced response rate (15-25 min) as compared to vehicle, but showed a reduction later in the session (55-60 min). Finally, KN-93 (10μg) increased response rate during most of the session (15-45 and 55-60 min). Summary analysis of total number of alcohol+sucrose reinforced responses showed a main effect of KN-93 dose (F3,24=2.957;p=0.05) with a significant overall increase evident following the 1.0 μg dose (Figure 1E). Alcohol consumption followed a similar pattern after infusion of vehicle and multiple doses of KN-93. The average alcohol dose consumed after aCSF injection was 1.0±0.09 g/kg/1-hr. Statistically, the dosage of alcohol (g/kg) consumed increased as the session progressed (F11,88=151.6;p<0.0001). There was a main effect of KN-93 on dose consumed (F3,24=3.525;p=0.03) and the effects of KN-93 on dose consumed changed as a function of time (F33,264=5.099;p<0.0001). The effect of KN-93 infusion into the PFC for this measurement paralleled what was observed for response rate. Namely, intra-PFC KN-93 (1.0μg) increased the dose that was consumed within the first 5 min of the self-administration session and persisted throughout the session whereas increased consumption after KN-93 (10μg) emerged and persisted from min 15-60. The lowest dose of KN-93 (0.3μg) reduced consumption during the last 15 min of the session. Importantly, inactive lever responding was not altered by KN-93 and most responses (87.9±2.5%) occurred on the active lever.
For sucrose-only self-administering mice, infusion of KN-93 into the mPFC of led to a significant main effect of time, and an interaction between time and dose. Specifically, responding on the active lever significantly increased as the session progressed (F11,110=104.2;p<0.0001;Figure 1G) and an interaction (F33,330=3.494;p<0.001;Figure 1G) revealed that KN-93 (10 μg) significantly reduced active lever responding as compared to vehicle (5-60 min). KN-93 (1.0 μg) infusion in the mPFC reduced responding sucrose-only reinforced responding during the last ten min of the session (50-60 min). Inactive lever responses were not affected and the majority of responses occurred on the active lever (85.3 ± 4.0% active lever responding). Summary analysis of total active lever responding showed that KN-93 did not alter sucrose-only reinforced responding (Figure 1H).
Importantly, neither experimental group (alcohol+sucrose or sucrose-only) showed altered locomotor activity after infusion of KN-93 (1.0 μg, Figure 1F&1I), indicating that drug effects on operant responding were not associated with reductions in motor ability.
CAMKII signaling is required for synaptic plasticity [5] and it is increasingly evident that this signaling pathway regulates the positive reinforcing effects of alcohol [3], which are prominent in the initial stages of addiction. In this study, we discovered that inhibition of CaMKII in the mPFC increased the positive reinforcing effects of sweetened alcohol versus parallel sucrose-only control. Timecourse data indicated that all doses of KN-93 increased alcohol+sucrose reinforced responding at some point during the 1-h session. Interestingly, increases in response rate after infusion of KN-93 (1.0 or 10 μg) emerged during the first 5-15 min of the operant session suggesting that CaMKII inhibition may have increased motivation, or conditioned reinforcement, prior to the direct CNS pharmacological effects of consumed alcohol. Alternatively, KN-93 may have inhibited the activation of CaMKII that occurs during alcohol self-administration (e.g., [3]), which could increase intake in an attempt to achieve a desired pharmacological effect. Both the initial increase and persistence of the drug effect over time are consistent with known functions of the mPFC, and suggests that CaMKII activity in this brain region may exert top-down inhibition, or dampening, of alcohol reinforcement in non-dependent mice. Importantly, these data are consistent with our recent finding that inhibition of extracellular-related protein kinase (ERK), a downstream target of CAMKII, in the mPFC significantly increased operant responding for alcohol+sucrose [16] suggesting similar dampening of alcohol reinforcement by ERK signaling. It will be important to evaluate regulation by this system following dependence, when inhibitory regulation may be diminished.
KN-93 (1 and 10 μg) infusion in the mPFC reduced sucrose-only reinforced response rate during the last 15 min of the self-administration session, which is consistent with blunted sucrose preference seen in global αCAMKIIT286A knockout mice [18]. This suggests that CaMKII activity in the mPFC may regulate motivation to consume sucrose. However, KN-93 (0.3 μg) also reduced alcohol+sucrose reinforced response rate during the last 10-min of the session after an initial increase during early time points. The delayed nature of these reductions in self-administration may reflect diffusion to adjacent nuclei or downstream effects of CaMKII inhibition that are not reinforcer specific.
CAMKII plays an integral role in glutamate neurotransmission throughout the brain [19, 20]. Growing evidence indicates that glutamate neurocircuitry regulates alcohol drinking, reinforcement and relapse in multiple species [3, 10, 11, 21, 22]. In vitro, chronic alcohol exposure increases CAMKII activity and glutamate reuptake in cortical astrocytes; this effect is blocked with KN-93 suggesting that cortical CAMKII activity is glutamate-dependent [23]. Moreover, CAMKII interacts with the GluA1 of the AMPA receptor to facilitate LTP thereby enhancing excitability and prolonging channel conductance [24]. In the amygdala, we previously found that alcohol drinking increases both GluA1 and CAMKII phosphorylation, and operant responding for alcohol is decreased by CAMKII inhibition and by AMPA receptor antagonism [3]. Likewise, we demonstrated a functional link between CAMKII and AMPA in the amygdala; positive modulation of the AMPA receptor activity potently increased operant responding for alcohol, which was blocked by CAMKII inhibition [25]. A natural extension of the present findings would be to determine if glutamate transmission in the mPFC regulates alcohol self-administration in a CaMKII-dependent manner.
It is intriguing that pharmacological inhibition of CAMKII in the mPFC increased sweetened alcohol self-administration in this study but has the opposite effect in the amygdala [3]. Since both the amygdala and mPFC send CaMKII-positive glutamatergic projections to the nucleus accumbens, these results may reflect CaMKII involvement in the differential motivational and executive control over alcohol self-administration exerted by these converging neural circuits [15]. Thus, alcohol use upregulates CaMKII activity in the amygdala as previously shown [3], and we propose that this may lead to increased glutamate-dependent neural activity in the nucleus accumbens, which may increase alcohol-reinforced responding. By contrast, CaMKII-positive glutamatergic projections from the mPFC to nucleus accumbens may inhibit alcohol self-administration via reduced positive reinforcement. Although we did not measure the effects of alcohol on CAMKII activity (e.g., phosphorylation), we predict that CAMKII phosphorylation would be downregulated in the mPFC after alcohol self-administration. Since enhancement of motivational drive and blunted executive control are hallmark properties of alcohol dependence, it will be of critical importance to evaluate CaMKII activity in these brain regions and circuits following the development of dependence.
RESEARCH HIGHLIGHTS.
CAMKII activity is important for neural plasticity and development of drug addiction
Moderate alcohol drinking alters CaMKII expression and phosphorylation in the CNS
Pharmacological inhibition of CAMKII in the PFC increases lever-press responding reinforced by sweetened alcohol
The PFC may dampen drug use via top-down control of limbic motivational brain regions
ACKNOWLEDGEMENTS
This research was supported by grants R37AA014983 and P60AA011065 to CWH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Administration SAaMHS, editor. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2014. [Google Scholar]
- [2].Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- [3].Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, et al. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Molecular and cellular neurosciences. 2005;29:139–47. doi: 10.1016/j.mcn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [7].Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK(1/2), but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Menard C, Gaudreau P, Quirion R. Signaling pathways relevant to cognition-enhancing drug targets. Handb Exp Pharmacol. 2015;228:59–98. doi: 10.1007/978-3-319-16522-6_3. [DOI] [PubMed] [Google Scholar]
- [9].Pandey SC. The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacology & therapeutics. 2004;104:47–58. doi: 10.1016/j.pharmthera.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [10].Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–9. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mahadev K, Chetty CS, Vemuri MC. Effect of prenatal and postnatal ethanol exposure on Ca2+ /calmodulin-dependent protein kinase II in rat cerebral cortex. Alcohol. 2001;23:183–8. doi: 10.1016/s0741-8329(01)00133-1. [DOI] [PubMed] [Google Scholar]
- [13].McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, et al. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: a proteomics study. Pharmacol Biochem Behav. 2009;92:304–13. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Easton AC, Lucchesi W, Lourdusamy A, Lenz B, Solati J, Golub Y, et al. alphaCaMKII autophosphorylation controls the establishment of alcohol drinking behavior. Neuropsychopharmacology. 2013;38:1636–47. doi: 10.1038/npp.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- [16].Faccidomo S, Salling MC, Galunas C, Hodge CW. Operant ethanol self-administration increases extracellular-regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. submitted. [DOI] [PMC free article] [PubMed]
- [17].Franklin KBJ, Paxinos G. The Mouse Brain in Sterotaxic Coordinates. 2 ed Academic Press; New York: 2001. [Google Scholar]
- [18].Easton AC, Lucchesi W, Mizuno K, Fernandes C, Schumann G, Giese KP, et al. alphaCaMKII autophosphorylation controls the establishment of alcohol-induced conditioned place preference in mice. Behav Brain Res. 2013;252:72–6. doi: 10.1016/j.bbr.2013.05.045. [DOI] [PubMed] [Google Scholar]
- [19].Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–5. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- [20].Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–11. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW. Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol. 2013;18:54–65. doi: 10.1111/adb.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–38. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Smith TL, Navratilova E. Increased calcium/calmodulin protein kinase activity in astrocytes chronically exposed to ethanol: influences on glutamate transport. Neuroscience letters. 1999;269:145–8. doi: 10.1016/s0304-3940(99)00438-3. [DOI] [PubMed] [Google Scholar]
- [24].Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–9. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- [25].Cannady R, Fisher KR, Graham C, Crayle J, Besheer J, Hodge CW. Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. submitted. [DOI] [PMC free article] [PubMed]