Abstract
Objective
While it is well established that adipose tissue derived inflammation plays an important role in the pathogenic mechanisms linking obesity with metabolic dysfunction, the inflammatory mediators involved have not been fully elucidated. Here, we explored IL-12 family cytokines with a focus on IL-27 during obesity-induced inflammation in mice and cultured adipocytes following exposure to inflammatory stimuli.
Methods
Relative mRNA abundance of IL-12 cytokines was assessed by RT-PCR in genetically obese B6-ob/ob mice as well as C57BL/6J mice fed a high fat diet and in adipocytes following exposure to inflammatory stimuli. Protein secretion of cytokines into culture media was assessed by ELISA and the biological outcome of IL-27 stimulation was assessed by RT-PCR and immunoblotting.
Results
Heterodimeric subunits constituting IL-27 were significantly induced in obese mice. While all IL-12 genes were markedly induced by inflammatory stress in cultured adipocytes, IL-27 protein was the only cytokine secreted into culture media in response to inflammatory stress. Cultured adipocytes also responded to IL-27 stimulation with divergent outcomes that were dependent on the inflammatory milieu of target cells.
Conclusions
These findings support the premise of autocrine/paracrine mechanisms involving IL-27 in adipocytes under conditions of inflammatory stress that may link obesity with inflammatory diseases.
Keywords: interleukins, adipocytes, adipokines, obesity, inflammation
Introduction
Obesity is a major risk factor for cardiovascular disease and type 2 diabetes (1, 2). Early studies have revealed that chronic inflammation, originating in adipose tissue (AT), is an important element of pathogenic mechanisms linking obesity and insulin resistance (IR) (3–5). Mounting evidence has demonstrated that levels of chemokines and cytokines are elevated during obesity, while ablation of these inflammatory molecules improves insulin signaling in adipocytes (6). Molecular mechanisms that underlie the initiation of AT inflammation during the onset of obesity include secretion of chemokines, such as monocyte chemoattractant protein-1 (MCP-1) (7), that enhance AT macrophage infiltration and elevate inflammatory processes mediating IR. While it is now well-established that chronic inflammation is highly associated with obesity-induced inflammation and IR, the inflammatory mediators involved have not been fully elucidated.
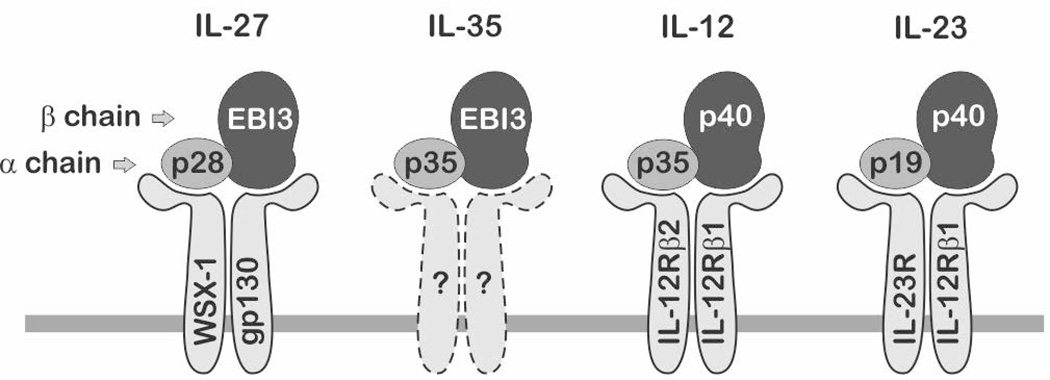
Recent evidence highlights a potential role for IL-12 family cytokines in obesity-related inflammatory diseases and metabolic dysfunctions (8, 9). Under conditions of obesity, elevated expression of IL-12 has been shown to be associated with IR and increased AT inflammation (10–15). While it has also been shown that plasma levels of several IL-12 family members are elevated with obesity, diabetes, and metabolic syndrome (10–12, 16), the cellular origins and underlying mechanisms have not been clearly elucidated. IL-12 family cytokines are mostly expressed in classic immune cells including macrophages and dendritic cells and known to play critical roles in linking innate and adaptive immunity. IL-12 family includes four heterodimeric cytokines: IL-12, IL-23, IL-27, and IL-35, where each cytokine is composed of shared alpha and beta chains (Fig.1).
Fig.1. IL-12 family cytokines.
IL-12 family cytokines are heterodimers composed of shared alpha and beta chain subunits that dimerize to form IL-27, IL-35, IL-12, and IL-23. Each cytokine signals through unique heterodimeric cell surface receptors. Receptor composition for the newest member of the family, IL-35, has not been determined.
Here we examined regulation of IL-12 family cytokines in AT with obesity as well as in adipocytes during differentiation and inflammatory stress. Our novel observations indicate that heterodimeric subunits of IL-27 are highly induced in AT with obesity and IL-27 protein secreted from preadipocytes (PAs) and adipocytes (ADs) during inflammatory stress. This study supports a role for IL-12 family members, particularly IL-27, as potential mediators linking obesity to inflammatory diseases.
Methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), calf bovine serum (CS), Trypsin-EDTA, and recombinant murine tumor necrosis factor (TNF) α were purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from HyClone. The following antibodies were used for immunoblot analysis: Phospho-STAT1 (Tyr701), phospho-STAT3 (Tyr705), phospho-ERK (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), and IκBα (Cell Signaling). Recombinant murine IL-27 was obtained from R&D Systems and lipopolysaccharide (LPS) was from Sigma.
Mice and experimental diets
Animals used for this study include genetically obese male B6.V-Lepob/J (B6-ob/ob) mice and their lean littermates as well as C57BL/6J mice rendered obese by diet and their lean controls. All mice were housed and treated by the supplier (Jackson Laboratories, Bar Harbor, Maine) until shipment 1 wk prior to tissue harvest. B6-ob/ob mice and lean littermates were purchased for experimentation at 10 wks of age and given free access to a standard laboratory chow diet. C57BL/6J mice subjected to diet-induced obesity (DIO) were fed a high fat diet consisting of 60% kcal from fat from 6 wks of age. Lean C57BL/6J control mice were fed a control low fat diet consisting of 10% kcal from fat from 6 wks of age. Both diets contained 10% kcal from protein with the balance in caloric value provided by differences in carbohydrate content. Mice were given free access to food and shipped for experimentation at 24 wks of age. All animals were euthanized by CO2 gas asphyxiation and epididymal AT collected and processed for preparation of total RNA. Animal care and use was in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee.
Cell culture
The murine 3T3-L1 cell line was purchased from Howard Green, Harvard Medical School (17). Cells were propagated in DMEM supplemented with 10% CS until reaching density-induced arrest, as previously described (18). At 2 days post-confluence, growth medium was replaced with DMEM supplemented with 10% FBS, 0.5 mM 1-methy-3-isobutylxanthane, 1 µM dexamethasone, and 1.7 µM insulin for 2 days. Subsequently, cells were cultured in DMEM supplemented only with 10% FBS over the following 6 days as PAs differentiated into mature ADs. Experiments described herein were conducted in density-arrested day 0 preadipocytes (PA) or day 8 adipocytes (AD). All experiments were repeated 2–3 times to validate results and ensure reliability.
Immunoblotting
Relative protein abundance was quantified by immunoblotting as previously described (19). Briefly, cell monolayer were washed with phosphate-buffer saline (PBS) and scraped into ice-cold lysis buffer containing 0.1 M Tris, 150 mM NaCl, 10% sodium dodecyl sulfate, 1% Triton X, 0.5% Nonidet P-40 (NP40), 1 mM EDTA, 1 mM EGTA and protease/phosphatase inhibitors. Equal amounts of whole cell lysate protein were separated by electrophoresis, transferred to polyvinylidene fluoride membranes and probed with specified antibodies.
Real-Time RT-PCR
Relative mRNA abundance was quantified as previously described (15, 20). For animal experiments, total RNA was isolated from epididymal AT by utilizing Trizol reagent according to manufacturer’s protocol and processed as described by Qiagen RNA clean-up protocol. For cell experiments, total RNA was extracted and genomic DNA contamination was removed using the RNeasy Plus Mini Kit. Total RNA quality was reverse-transcribed using a high capacity cDNA reverse transcription kit (AB).
PCR amplification was run utilizing the AB 7500 real time PCR system. All TaqMan primer probes used in this study were also purchased from AB (Table 1). All data were presented as mean ± standard error of the mean and representative of duplicate determinations. Data were normalized to 18S and measured as relative differences using the 2−ΔΔCT method. For reference, average CT values were presented (Table 1) for lean white adipose tissue (WAT), confluent RAW 264.7 macrophage cells, confluent 3T3-L1 preadipocytes (PA) and differentiated 3T3-L1 adipocytes (AD). Differences in gene expression between lean and obese as well as between PAs and ADs were determined via student’s t-test where a p-value of <0.05 was considered significant. Other data were analyzed using analysis of variance (ANOVA), with Tukey's post-hoc analysis used when the p-value for the respective parameter was statistically significant (p < 0.05).
Table 1.
IL-12 family cytokine/receptor and inflammatory/adipocyte genes analyzed in this study.
| Name | Accession | ABI number | a WAT | b RAW | c PA | d AD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | CT | CT | CT | |||||||
| IL-12 cytokines | IL-27 | IL-35 | IL-12 | IL-23 | ||||||
| p28 | ● | NM_145636 | Mm00461164_m1 | 32 | 28 | 34 | 28 | |||
| EBI3 | ● | ● | NM_015766 | Mm00469294_m1 | 28 | 24 | 32 | 34 | ||
| p35 | ● | ● | NM_008351 | Mm01208555_m1 | 32 | 35 | 36 | 35 | ||
| p40 | ● | ● | NM_008352 | Mm00434174_m1 | 30 | 33 | 36 | 36 | ||
| p19 | ● | NM_031252 | Mm00518984_m1 | 27 | 25 | 30 | 31 | |||
| IL-12 receptors | ||||||||||
| WSX-1 | ● | NM_016671 | Mm00497259_m1 | 28 | 34 | 33 | 36 | |||
| gp130 | ● | NM_010560 | Mm00439665_m1 | 21 | 25 | 22 | 24 | |||
| IL-12Rβ2 | ● | NM_008354 | Mm00434200_m1 | 29 | 33 | 35 | 36 | |||
| IL-12Rβ1 | ● | ● | NM_008353 | Mm00434189_m1 | 29 | 29 | 30 | 34 | ||
| IL-23R | ● | NM_144548 | Mm00519943_m1 | 28 | 32 | 33 | 36 | |||
| inflammatory genes | ||||||||||
| MCP-1 | NM_011333 | Mm00441242_m1 | 27 | 17 | 23 | 27 | ||||
| IL-6 | NM_031168 | Mm99999064_m1 | 32 | 26 | 29 | 30 | ||||
| TNFα | NM_013693 | Mm99999068_m1 | 29 | 21 | 34 | 35 | ||||
| reference gene | ||||||||||
| 18S | X03205 | 4342930E | 9 | 7 | 7 | 8 |
Average threshold cycle (CT) for white adipose tissue (WAT) from 10 wk old lean C57BL/6J mice.
Average threshold cycle (CT) for confluent murine RAW 264.7 macrophage cells.
Average threshold cycle (CT) for confluent murine 3T3-L1 preadipocytes (PA).
Average threshold cycle (CT) for mature day 8 murine 3T3-L1 adipocytes (AD).
ELISA
PAs and ADs were treated with or without TNFα (100 pM) and LPS (100 ng/ml) for 12, 24, and 36 hrs. Supernatants were subjected to sandwich ELISA. IL-12 (p35/p40) and IL-27(p28) protein levels in culture media were determined by sandwich ELISA as described by the manufacturer (R & D systems). IL-23 (p19/p40) protein levels in culture media were also determined by sandwich ELISA as described by the manufacturer (Biolegend). Optical densities were determined using a PowerWave microplate spectrophotometer (BioTek) at 450 nm. Wavelength correction of 570 nm was used for optical imperfections in the plate. Data were analyzed using a four parameter logistic (4-PL) curve-fit.
Results
Relative IL-12 family cytokine gene expression in AT of genetic and diet-induced obesity
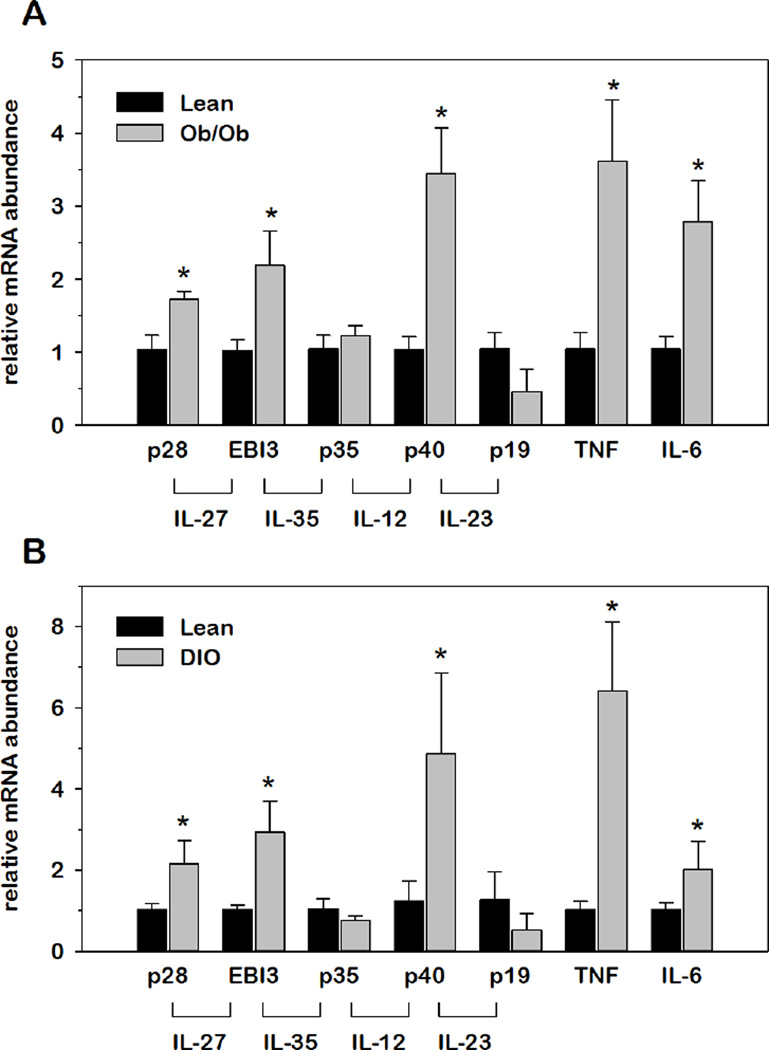
Adipose tissue is a key site regarding the origin of obesity-induced inflammation that contributes to systemic IR (21, 22). Therefore, we first examined the impact of obesity on IL-12 family cytokines in AT with genetic- and diet-induced obesity (DIO). Using these distinct models of obesity, relative mRNA abundance for each IL-12 family cytokine in AT was normalized to 18S and expressed as fold-differences relative to lean controls. To confirm obesity-induced inflammation, we also examined TNFα and IL-6 which are known to contribute to obesity-induced inflammation where expression originates in AT. As shown in Fig.2, relative mRNA abundance of p28, EBI3, and p40 was significantly induced in genetic-induced obese (ob/ob) mice, paralleling the induction of TNFα and IL-6 mRNA expression. Interestingly, the same three subunits were also elevated in AT with DIO consistent with other pro-inflammatory genes. These changes were also confirmed in retroperitoneal AT from the ob/ob experiment (data not shown). Collectively, these data demonstrated that p28, EBI3 and p40 are induced at the level of mRNA expression in AT concurrent with development of obesity-induced inflammation.
Fig.2. Relative IL-12 family cytokine gene expression in AT of genetic and diet-induced obesity.
Relative mRNA abundance of IL-12 family cytokine and inflammatory genes was determined by qRT-PCR from total RNA extracted from AT from (A) ob/ob mice as well as (B) mice subject to DIO. Data were normalized to 18S rRNA and relative abundance determined for each cytokine where obese values were expressed as fold-differences relative to lean within each group. Differences in gene expression between lean and obese animals were determined via student’s t-test where *p < 0.05 was considered significant.
Relative IL-12 family gene expression in 3T3-L1 adipocytes comparing undifferentiated preadipocyte (PA) and mature adipocyte (AD) mRNA abundance
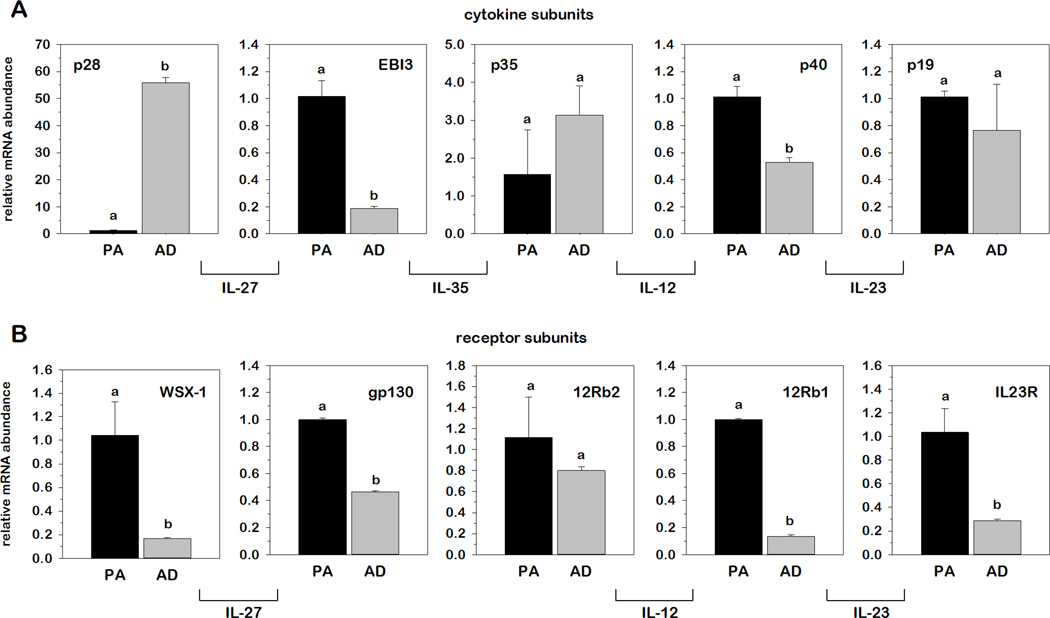
To investigate a potential role for adipocytes in IL-12 family gene expression, we next examined relative mRNA abundance for each cytokine and receptor in 3T3-L1 murine adipocytes where values observed in mature ADs were expressed as fold-differences relative to undifferentiated PAs. As shown in Fig.3, differentiation had dramatic and opposite effects on the relative mRNA abundance of p28 and EBI3, where p28 mRNA was markedly more abundant and EBI3 markedly less abundant in ADs compared to PAs. Interestingly, p28 was the only gene of the IL-12 family cytokine and receptor subunits examined that increased with differentiation. More to this point, differentiation actually resulted in a decrease in mRNA of WSX-1 and gp130 as well as IL-12Rβ1 and IL-23R, the receptor subunits of IL-27 and IL-23, respectively, suggesting the possibility that PAs may be more responsive than ADs to IL-12 family stimulation.
Fig.3. Relative IL-12 family cytokine and receptor mRNA expression in 3T3-L1 preadipocytes and adipocytes.
Relative mRNA abundance of IL-12 family cytokine (A) and receptor subunits (B) was determined by qRT-PCR from total RNA extracted for undifferentiated 3T3-L1 PAs and mature ADs. Data were normalized to 18S rRNA and relative abundance determined for each phenotype where AD values were expressed as fold-differences relative to PA. Differences in gene expression between cell phenotypes were determined via student’s t-test. Means not sharing a common superscript were considered significantly different (p < 0.05). IL-35 receptor subunits are currently unknown.
Relative IL-12 family cytokine gene expression in PAs and ADs under conditions of inflammatory stress
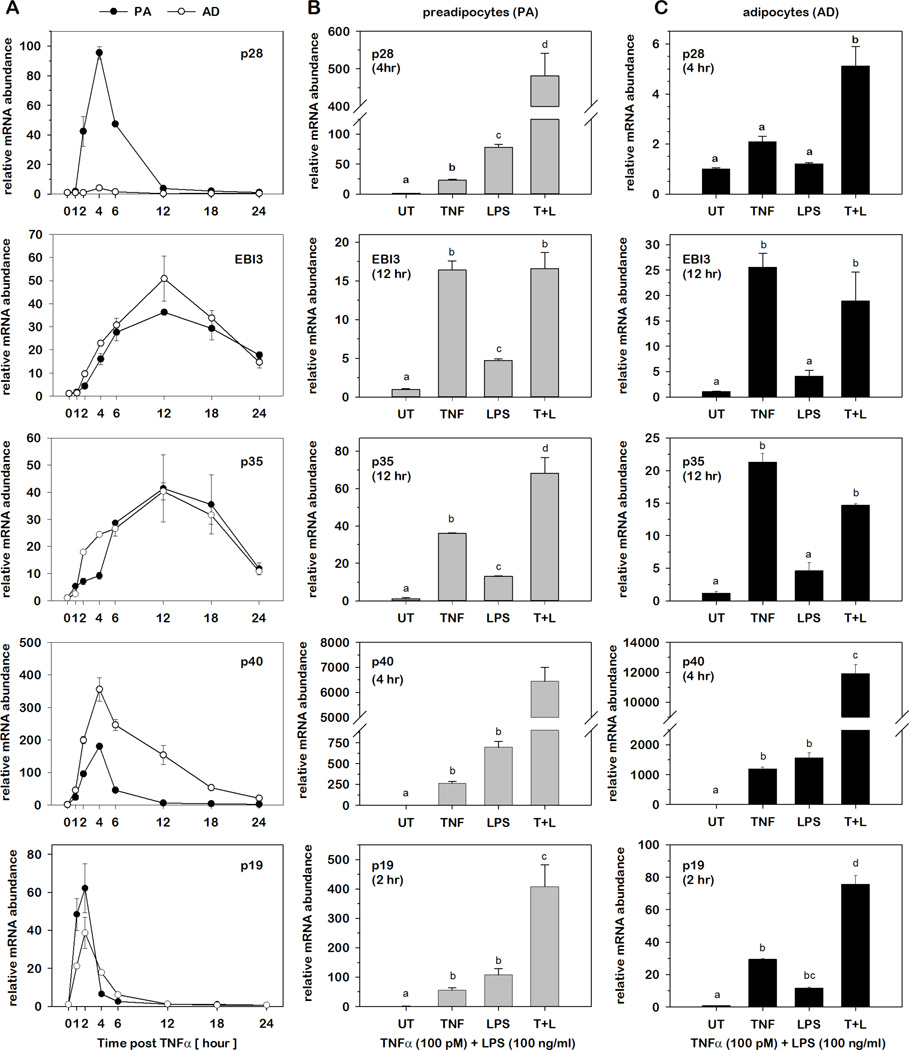
As we observed a greater impact of obesity on several IL-12 family gene expression in AT (Fig.2), we next determined the impact of inflammatory stress on relative IL-12 cytokine mRNA abundance in PAs and ADs as described above. For these determinations, total RNA was initially harvested from cells over time following stimulated with TNFα (100 pM) to determine the kinetic profile of each IL-12 family gene in both PAs and ADs. As shown in Fig.4A, the five subunit genes fell into two groups where p28, p40 and p19 presented with rapid and transient changes to peak induction (2–4 hrs) while EBI-3 and p35 presented with slower and prolonged changes to peak induction (12–18 hrs). This was followed by harvesting total RNA at times of peak induction for each gene following stimulation with TNFα (100 pM) or LPS (100 ng/ml) or the combination of these two potent inflammatory mediators. Relative mRNA abundance was determined for each cytokine where the impact of each agonist was expressed as fold-differences relative to untreated (UT) controls. As shown in Fig.4B–C, stimulation with TNFα or LPS markedly increased the mRNA abundance of all five IL-12 family cytokine subunits in a surprisingly similar pattern between PAs and ADs with one exception. While p28 mRNA increased nearly 500-fold when stimulated with both TNFα and LPS in PAs, the same inflammatory agonists elicited only a 5-fold increase in mRNA in ADs. This observation is particularly interesting as we demonstrated that p28 mRNA was elevated in ADs compared to PAs suggesting that this cytokine subunit becomes refractory to inflammatory stimuli with differentiation. These determinations also demonstrated a markedly synergistic effect of both inflammatory mediators in combination with the exception of EBI3 and p35 where TNFα stimulation alone was nearly equivalent to stimulation with both TNFα and LPS.
Fig.4. Relative IL-12 family cytokine gene expression in PAs and ADs under conditions of inflammatory stress.
Relative mRNA abundance of IL-12 family cytokines was determined by qRT-PCR from total RNA extracted for 3T3-L1 PAs and ADs overtime following stimulation with 100 pM TNFα (A). Subsequent studies were performed in PAs (B) and ADs (C) at the time of peak induction for each gene post-stimulation with 100 pM TNFα, 100 ng/ml LPS, or in combination. Data were normalized to 18S rRNA and expressed as fold differences relative to UT controls. Statistical differences were determined by ANOVA, with Tukey's post-hoc analysis performed when the p-value for the respective parameter was statistically significant. Means not sharing a common superscript were considered significantly different (p < 0.05).
Accumulation of IL-12 family cytokines in culture media of PAs and ADs under conditions of inflammatory stress
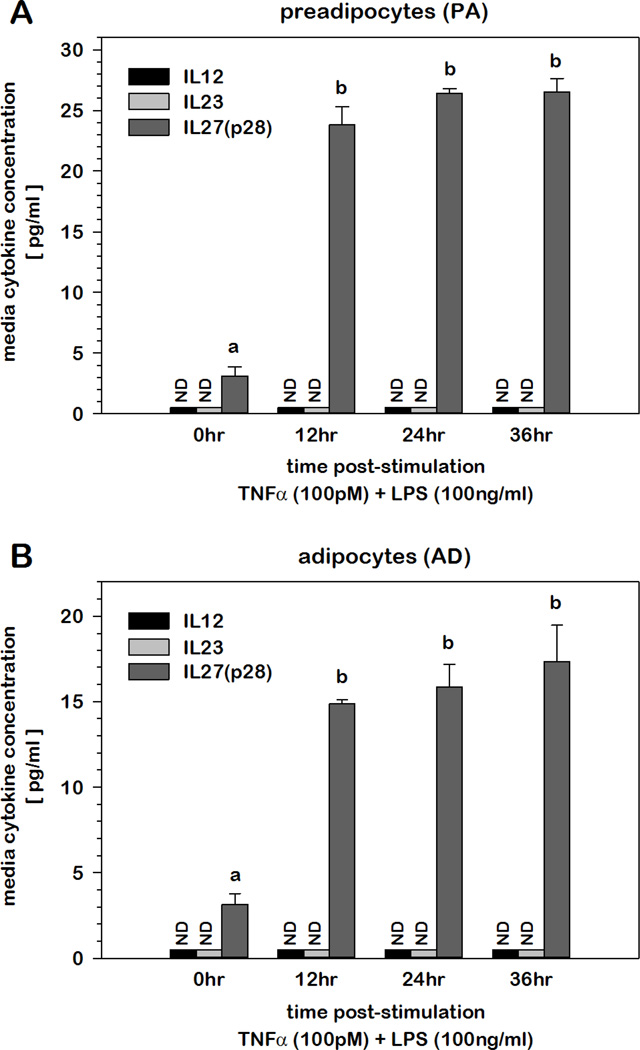
As inflammatory stimuli elevated the mRNA abundance of all five IL-12 family cytokine subunit genes, we next assessed the impact of inflammatory stress on the protein accumulation of IL-12, IL-23, and IL-27 in the culture media by ELISA. As functionality of these cytokines depends on heterodimeric partnering of specific alpha and beta chain subunits, ELISAs measuring both proteins were used for IL-12 and IL-23. As this co-detection technique was not commercially available for IL-27, sandwich ELISA measuring only p28 protein was utilized instead. As shown in Fig.5, IL-27 as measured by p28 protein detection accumulated in the culture media in a time-dependent manner following stimulation with TNFα and LPS. As others have demonstrated that secretion is dependent on prior dimer formation, these data likely represented secretion of functional IL-27 protein comprised of p28 and EBI3 heterodimers. Neither IL-12 nor IL-23 was detected in the culture media over a 36 hr period following inflammatory insult.
Fig.5. Accumulation of IL-12 family cytokines in culture media of PAs and ADs under conditions of inflammatory stress.
Concentration of IL-12, IL-23 and IL-27 was determined by ELISA in cell media over time following TNFα (100 pM) and LPS (100 ng/ml) stimulation of 3T3-L1 (A) PAs and (B) ADs. Values not detected (ND) were indicated. Means not sharing a common superscript were considered significantly different (p < 0.05).
Effect of IL-27 stimulation on signaling pathways and inflammatory gene expression
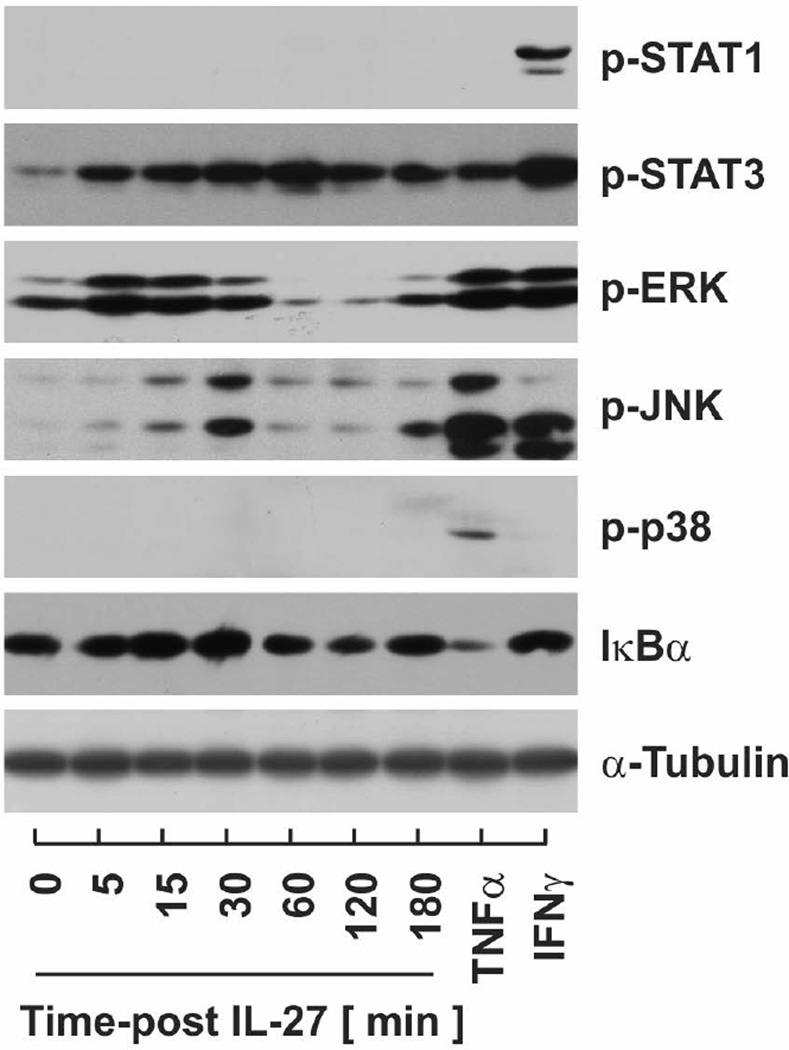
As we demonstrated that IL-27 was secreted and IL-27 receptor subunit mRNA was expressed in adipocytes, we next examined the effects of IL-27 stimulation on signaling pathways known to be regulated by this cytokine. For these determinations, cell lysates were collected over time from IL-27 stimulated PAs. The phosphorylated and activated forms of STAT and MAPK proteins as well as the degradation of IκBα as an indirect indicator of NF-κB signaling. As shown in Fig.6, IL-27 stimulation resulted in transient accumulation of p-ERK, p-JNK, and p-STAT3 with peak accumulation at 15, 30, and 60 mins, respectively. Phosphorylation of STAT1 and p38 was not detected and a minimal decrease in IκBα from IL-27 stimulation was observed.
Fig.6. Effect of IL-27 stimulation on inflammatory signaling pathways.
Signaling pathways were evaluated by immunoblotting cell lysates collected from 3T3-L1 PAs over time following 50 nM IL-27 stimulation. TNFα (100 pM) and IFNγ (20 ng/ml) were included as positive controls for individual pathways.
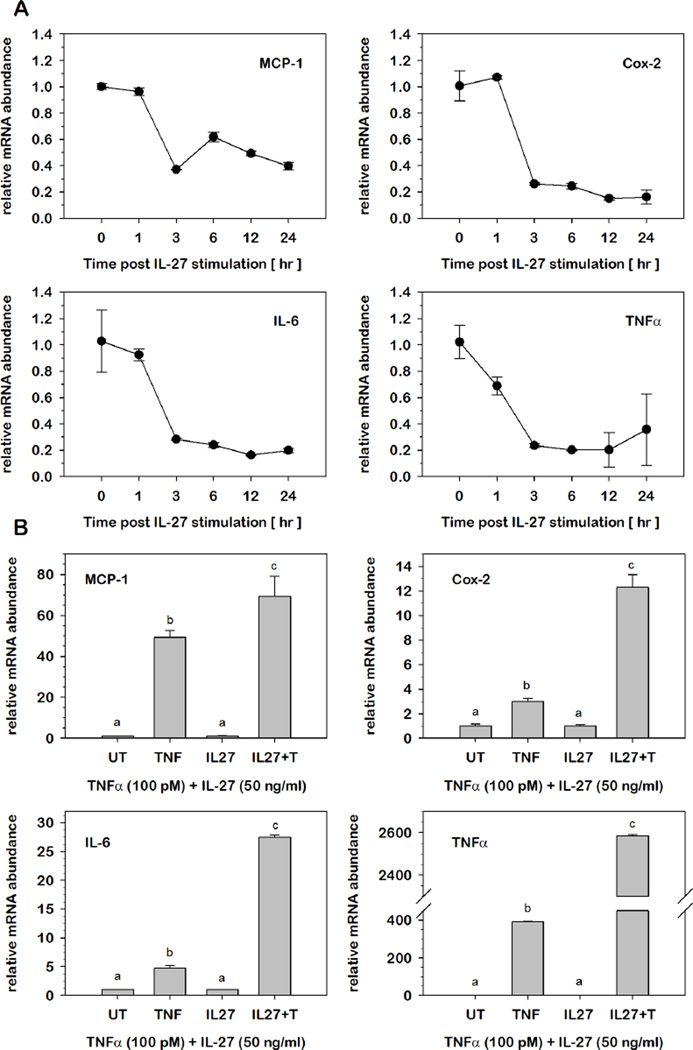
Since IL-27 can eliciting signaling pathways that link cytokine action to inflammatory gene expression, we examined the effects of IL-27 stimulation on basal inflammatory gene expression. Total RNA was extracted from PA following stimulation with IL-27. Relative mRNA abundance at each successive time point was expressed as fold-differences relative to levels observed prior to stimulation (0 hr). As shown in Fig.7A, IL-27 stimulation resulted in a decrease in mRNA abundance for each of four classic inflammatory genes of MCP-1, IL-6, COX-2, and TNFα. In each case, the effect was maximal within 3 hrs and was sustained throughout the 24 hrs period under study suggesting a potential anti-inflammatory role of IL-27 under these conditions.
Fig.7. Effect of IL-27 stimulation on inflammatory gene expression in preadipocytes.
(A) Relative mRNA abundance was determined by qRT-PCR over time for each inflammatory gene following 50 nM IL-27 stimulation of 3T3-L1 PAs. Data were normalized to 18S rRNA and relative abundance for each time point was expressed as fold-differences relative to levels observed prior to stimulation (0 hr). (B) Relative mRNA abundance of specified inflammatory genes was determined by qRT-PCR from total RNA extracted from 3T3-L1 PAs with or without IL-27 (50 nM) pretreatment prior to TNFα (100 pM) stimulation for 2 hrs. Data were normalized to 18S rRNA and expressed as fold differences relative to UT controls for TNFα stimulation and IL-27 for IL-27+TNFα stimulation. Statistical differences were determined by ANOVA, with Tukey's post-hoc analysis performed when the p-value for the respective parameter was statistically significant. Means not sharing a common superscript were considered significantly different (p < 0.05).
We next determined the effect of IL-27 stimulation on cytokine-mediate inflammatory gene expression. Total RNA was extracted from PAs at 2 hrs post-stimulation with TNFα in the presence or absence of IL-27. To examine effect of TNFα on IL-27-mediated inflammatory gene expression, relative mRNA abundance was determined for each inflammatory gene where the impact of each agonist was expressed as fold-differences relative to untreated (UT) controls or IL-27 alone prior to TNFα stimulation, respectively. As illustrated in Fig.7B, TNFα elicited a marked increase in mRNA for all four inflammatory genes previously determined to peak at 2 hrs post-stimulation. IL-27 pretreatment markedly increased the effect of TNFα on mRNA accumulation of four inflammatory genes of MCP-1, IL-6, COX-2, and TNFα suggesting a potential pro-inflammatory role of IL-27 under these conditions.
Discussion
Our report presents novel evidence demonstrating divergent regulation of select IL-12 family cytokines in AT with obesity and in adipocytes under conditions of inflammatory stress. Numerous reports have established key roles for IL-12 family cytokines in the host defense against microbial infections, consequently leading to studies examining the regulation of this cytokine family in immune cells such as macrophages, neutrophils and dendritic cells. While early literature suggested that expression of IL-12 family cytokines is restricted to classic immune cells (23), more recent studies demonstrate regulation of these cytokines in other, traditionally non-immune cell types as well. For instance, the heterodimeric subunits of IL-27, p28 and EBI3, have been shown to be expressed in trophoblasts (24) and highly induced in vascular smooth muscle cells and endothelial cells in response to pro-inflammatory stimuli (25, 26). Our study contributes novel findings to this point as we demonstrate that all IL-12 family cytokines are readily detectable at the mRNA level in murine AT and adipocytes. Of the four IL-12 family cytokines, we also show that both IL-27 cytokine subunit genes were significantly induced in AT with obesity paralleling the induction of other inflammatory cytokines and consistent with inflammatory mediators originating from AT that cause obesity-induced IR and systemic metabolic dysfunction (22, 27).
As AT macrophage infiltration is a well-established hallmark of obesity (3), AT mRNA levels reported here likely reflect contributions from both immune as well as non-immune cell types. To this point, however, we also demonstrate that all IL-12 family cytokines are expressed at the mRNA level in the murine 3T3-L1 adipocytes that are devoid of macrophages and other immune cells. However, ‘macrophage-like’ properties have been ascribed to adipocytes where undifferentiated PAs have been reported to be more sensitive to cytokine stimulation or more potent regarding cytokine secretion when compared to mature ADs (28). Consistent with this premise, we report here that four of five IL-12 family receptor subunits as well as two of five cytokine subunits were suppressed at the mRNA level in ADs compared to PAs under basal conditions. All five cytokine genes were induced in PAs as well as ADs under conditions of inflammatory stress to differing degrees highlighting the adipocyte as a novel, non-immune cell type for IL-12 family cytokine expression.
Data presented here highlight several points of interest regarding IL-12 family gene expression. First, we demonstrate that the beta chain subunits, EBI3 and p40 were divergently regulated in a similar fashion under diverse conditions. Both genes were induced with obesity, suppressed with adipocyte differentiation, and induced with inflammatory stress in both PAs and ADs. Conversely, and with the exception of p28, data presented here also demonstrate that alpha chain subunit expression is unchanged with obesity and differentiation. However, as all IL-12 family members were induced by TNFα, LPS, or a combination thereof, these data also demonstrate that inflammatory stress is a potent stimulus for both alpha and beta subunits of all family members. The second point of interest involves the regulation of IL-27 where differentiation induces p28 expression, but suppresses EBI3 expression. Although both subunits were markedly induced with inflammatory stress in PAs, only EBI3 retained that capacity following differentiation. While the refractory nature of p28 that accompanies differentiation might suggest that IL-27 only plays a role in PAs, we also demonstrate that constitutive, basal levels of p28 mRNA are elevated with differentiation and that IL-27 is secreted from both PAs as well as ADs under conditions of inflammatory stress.
With the observation that all five cytokine subunit genes were markedly induced under conditions of inflammatory stress in adipocytes, we examined the accumulation of IL-12, IL-23, and IL-27 protein in culture media over time following stimulation with TNFα and LPS. Surprisingly, IL-27(p28), but not IL-12 or IL-23, was detected where levels increased and plateaued with time following stimulation. It should be noted, however, that protein detection for each cytokine was performed with quantitative sandwich enzyme immunoassay techniques. The sandwich ELISAs detecting IL-12 and IL-23 employed antibodies to p35/p40 and p19/p40, respectively. The only sandwich ELISA currently available for IL-27 detection uses only p28 antibodies as opposed to p28/EBI3. Thus, while the ELISA used in this study clearly confirms the specificity of p28 accumulation in the culture media, it does not specifically distinguish whether this represents p28 monomers, dimers or p28/EBI3 heterodimeric complexes. However, with the exception of p40, there is extensive literature supporting the premise that formation of each heterodimeric complex within the cell is an obligatory prerequisite for secretion of the functional cytokine. Thus, the detection of p28 in the culture media likely represents functional p28/EBI3 heterodimers positioning IL-27 as a member of the growing list of adipokines that are secreted from adipocytes. This notion is supported by our observation that p28 protein increased in the culture media of ADs even though p28 mRNA was refractory to inflammatory stress suggesting that IL-27 secretion was modulated by changes in EBI3 gene expression. It should also be noted that while IL-12 and IL-23 were not detected under these conditions, these results do not rule out the possibility of localized, undetectable cytokine secretion that may be sufficient for stimulation of autocrine/paracrine functions through cell surface receptors.
The interaction between cytokine cascade networks within AT is an important mechanism linking obesity to inflammatory diseases. As we found secretion of IL-27 and expression of IL-27 receptors in both PAs and ADs, we hypothesize that IL-27 may act as an inflammatory mediator in a paracrine/autocrine manner. It has been reported that IL-27 has dual functions involved in pro-inflammatory and anti-inflammatory processes (23, 29, 30). While the mechanisms underlying its anti-inflammatory properties are not yet clear, it has been observed that IL-27 suppresses several key inflammatory cytokines, such as TNFα, IL-6, and IL-17 (31–34). Others show anti-inflammatory and anti-viral roles of IL-27 in various tissues including cardiac muscle and liver (35, 36). We present data in this report demonstrating diverging functions of IL-27 in adipocytes that appear to reflect the inflammatory milieu of target cells. Under basal conditions, IL-27 acts as an anti-inflammatory cytokine by reducing expression of a number of pro-inflammatory genes. In contrast, IL-27 appears to function as a pro-inflammatory cytokine by elevating the expression of pro-inflammatory cytokines under conditions for inflammatory stress. Together, these findings suggest that IL-27 may play a protective role in obesity-associated inflammatory diseases. However, once obesity-induced inflammation is established, IL-27 may exacerbate the inflammatory stress that negatively regulates AT metabolism and function. The pleiotropic nature of p27 presented here is consistent with most gp130 cytokines which are known for diverse functions based on the state of the target cell. Mechanisms responsible for this observation are under investigation.
In summary, this study provides the first comprehensive and systematic analysis of the IL-12 family cytokines in AT and adipocytes. Data here demonstrate that of the four IL-12 family cytokines, IL-27 is highly regulated in AT with obesity and in cultured adipocytes under conditions of inflammatory stress. As we confirmed IL-27 as an adipokine, we further report potential anti-inflammatory or pro-inflammatory roles for IL-27 that reflects the inflammatory environment of target cells. These data collectively highlight possible roles for IL-27 in adipocytes under conditions that link obesity with inflammatory diseases.
Bullet Points.
What is already known
IL-12 family cytokines have been reported as prospective regulators linking obesity to insulin resistance.
Plasma levels of select IL-12 family members are elevated with obesity, diabetes, and metabolic syndrome.
IL-27 has been shown to pro-inflammatory and anti-inflammatory functions.
What this study adds
IL-27 subunit genes are induced with pro-inflammatory cytokines in obese mice.
Alpha subunit of IL-27 (i.e., p28) is the only IL-12 family gene, ligand or receptor, whose mRNA level is elevated in adipocytes (AD) relative to preadipocytes (PA).
IL-27 protein is secreted from both PAs and ADs in response to inflammatory stress.
PAs respond to IL-27 stimulation with divergent biological outcomes that are dependent on the inflammatory milieu of target cells.
Acknowledgments
Authors are grateful for the superb technical assistance from Dr. Robin Hopkins.
Supported by National Institutes of Health:
R01-DK52968 (JMS)
R15-DK082799 (RFM)
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Caterson ID, Gill TP. Obesity: epidemiology and possible prevention. Best Pract Res Clin Endocrinol Metab. 2002;16(4):595–610. doi: 10.1053/beem.2002.0228. [DOI] [PubMed] [Google Scholar]
- 3.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 5.Maury E, Noel L, Detry R, Brichard SM. In vitro hyperresponsiveness to tumor necrosis factor-alpha contributes to adipokine dysregulation in omental adipocytes of obese subjects. J Clin Endocrinol Metab. 2009;94(4):1393–1400. doi: 10.1210/jc.2008-2196. [DOI] [PubMed] [Google Scholar]
- 6.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 7.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Oben JA, Yang S, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40(2):434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42(4):880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 10.Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147(5):2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- 11.Wegner M, Winiarska H, Bobkiewicz-Kozlowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine. 2008;42(3):312–316. doi: 10.1016/j.cyto.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Surendar J, Mohan V, Rao MM, Babu S, Aravindhan V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103) Diabetes Technol Ther. 2011;13(4):477–482. doi: 10.1089/dia.2010.0178. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Choi Y, Choi YH, Park T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Merino D, Drogou C, Guezennec CY, Chennaoui M. Effects of chronic exercise on cytokine production in white adipose tissue and skeletal muscle of rats. Cytokine. 2007;40(1):23–29. doi: 10.1016/j.cyto.2007.07.188. [DOI] [PubMed] [Google Scholar]
- 15.Nam H, Ferguson BS, Stephens JM, Morrison RF. Impact of obesity on IL-12 family gene expression in insulin responsive tissues. Biochim Biophys Acta. 2013;1832(1):11–19. doi: 10.1016/j.bbadis.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler G, Dworak O, Salamon F, Salamon D, Speer G, Cseh K. Increased interleukin-12 plasma concentrations in both, insulin-dependent and non-insulin-dependent diabetes mellitus. Diabetologia. 1998;41(4):488. doi: 10.1007/s001250050935. [DOI] [PubMed] [Google Scholar]
- 17.Djian P, Phillips M, Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985;124(3):554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- 18.Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999;274(24):17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 19.Auld CA, Fernandes KM, Morrison RF. Skp2-mediated p27(Kip1) degradation during S/G2 phase progression of adipocyte hyperplasia. J Cell Physiol. 2007;211(1):101–111. doi: 10.1002/jcp.20915. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson BS, Nam H, Hopkins RG, Morrison RF. Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS One. 2010;5(12):e15208. doi: 10.1371/journal.pone.0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Keller MP, Attie AD. Physiological insights gained from gene expression analysis in obesity and diabetes. Annu Rev Nutr. 2010;30:341–364. doi: 10.1146/annurev.nutr.012809.104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 24.Coulomb-L'Hermine A, Larousserie F, Pflanz S, Bardel E, Kastelein RA, Devergne O. Expression of interleukin-27 by human trophoblast cells. Placenta. 2007;28(11–12):1133–1140. doi: 10.1016/j.placenta.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Kempe S, Heinz P, Kokai E, Devergne O, Marx N, Wirth T. Epstein-barr virus-induced gene-3 is expressed in human atheroma plaques. Am J Pathol. 2009;175(1):440–447. doi: 10.2353/ajpath.2009.080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33(16):5308–5319. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulain-Godefroy O, Froguel P. Preadipocyte response and impairment of differentiation in an inflammatory environment. Biochem Biophys Res Commun. 2007;356(3):662–667. doi: 10.1016/j.bbrc.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 31.Hamano S, Himeno K, Miyazaki Y, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 32.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170(10):4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka A, Hamano S, Miyazaki Y, et al. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172(6):3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 34.Baker BJ, Park KW, Qin H, Ma X, Benveniste EN. IL-27 inhibits OSM-mediated TNF-alpha and iNOS gene expression in microglia. Glia. 2010;58(9):1082–1093. doi: 10.1002/glia.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yajima T, Yasukawa H, Jeon ES, et al. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114(22):2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. [DOI] [PubMed] [Google Scholar]
- 36.Bender H, Wiesinger MY, Nordhoff C, et al. Interleukin-27 displays interferon-gamma-like functions in human hepatoma cells and hepatocytes. Hepatologys. 2009;50(2):585–591. doi: 10.1002/hep.22988. [DOI] [PubMed] [Google Scholar]