Abstract
Background
The neural network mechanisms underlying visceral hypersensitivity in irritable bowel syndrome (IBS) are incompletely understood. It has been proposed that an intrinsic salience network plays an important role in chronic pain and IBS symptoms. Using neuroimaging, we examined brain responses to rectal distension in adolescent IBS patients, focusing on determining the alteration of salience network integrity in IBS and its functional implications in current theoretical frameworks. We hypothesized that (1) brain responses to visceral stimulation in adolescents are similar to those in adults, and (2) IBS is associated with an altered salience network interaction with other neurocognitive networks, particularly the default mode network (DMN) and executive control network (ECN), as predicted by the theoretical models.
Methods
IBS patients and controls received subliminal and liminal rectal distension during imaging. Stimulus-induced brain activations were determined. Salience network integrity was evaluated by functional connectivity of its seed regions activated by rectal distension in the insular and cingulate cortices.
Key Results
Compared with controls, IBS patients demonstrated greater activation to rectal distension in neural structures of the homeostatic afferent and emotional arousal networks, especially the anterior cingulate and insular cortices. Greater brain responses to liminal vs. subliminal distension were observed in both groups. Particularly, IBS is uniquely associated with an excessive coupling of the salience network with the DMN and ECN in their key frontal and parietal node areas.
Conclusions & Inferences
Our study provided consistent evidence supporting the theoretical predictions of altered salience network functioning as a neuropathological mechanism of IBS symptoms.
Keywords: Irritable bowel syndrome, Salience network, fMRI-guided functional connectivity analysis, Homeostatic afferent processing network, Emotional arousal network
Introduction
Over the past decade, neuroimaging studies have made significant progress in identifying key neural structures within the brain-gut axis involved in responding to experimental visceral stimulation and medication in irritable bowel syndrome (IBS) patients and healthy controls (1–4). However, alterations of the integrity of neural network circuitries in IBS and the abnormal interactions among them have yet to be elucidated for a further understanding of IBS neuropathophysiology from a network perspective of brain functioning (5).
The human brain intrinsically is organized into distinct functional networks supporting various sensory, motor, emotional and cognitive functions (6). Of particular relevance to the understanding of visceral hypersensitivity and altered brain-gut interaction in IBS is an intrinsic brain network, the salience network (7). With its two key anchor nodes located in the anterior insula (AI) and anterior cingulate cortex (ACC), the salience network has extensive connections with cortical and subcortical brain structures, including the medial prefrontal cortex, thalamus, amygdala, cerebellum, and midbrain structures. The salience network plays an important role in disparate attentional, cognitive, affective and regulatory functions (8). A striking finding from prior neuroimaging IBS studies is that brain structures with prominent and consistent activation to experimental gut or esophageal stimulation showed a significant overlap with the key node areas of the salience network, particularly the AI and ACC, as well as brain regions mediated by the salience network, such as the sensorimotor attention system (2–4, 8, 9). A recent review study explicitly proposed that abnormal functioning of salience circuitry is an important neural network mechanism for understanding a variety of clinical conditions involving chronic persistent pain, including IBS (10). Moreover, abnormal salience network interaction with the intrinsic default model network (DMN) and executive control network (ECN) has been proposed as a unifying parsimonious theoretical model for understanding a variety of psychiatric and neurological disorders including anxiety and depression (11), which are common comorbidities of IBS (12, 13). A handful of neuroimaging studies have examined altered neural network circuitries in IBS patients with regard to gender-related differences (14, 15), perceptual habituation (16), top-down corticolimbic modulation of pain suppression (17, 18), and influences of early adverse life events (19). However, the way in which salience network integrity and functioning are altered in IBS patients during experimental gut stimulation has yet to be understood.
The main purpose of this study is twofold. First, we aimed to identify, using functional imaging, brain areas activated by subliminal (below conscious perception threshold) and liminal (barely perceivable) rectal distension in adolescent IBS patients and age-matched healthy controls. Neuroimaging investigation of IBS in the pediatric patient population has been particularly scarce; we address this knowledge gap in this study. Second, we sought to detect and characterize potential alterations of salience network functioning in IBS patients compared with controls by examining functional connectivity of the salience network from its two key node areas in the insula and anterior cingulate cortices that were activated during liminal rectal distension. We anticipated that brain responses to rectal distension in adolescent IBS patients are similar to those in adults, given the existence of a unifying homeostatic afferent processing network (20). Based on the proposed models of altered salience network functioning in chronic pain and brain disorders (10, 11), we hypothesized that visceral hypersensitivity in IBS patients is associated with (1) elevated brain responses to experimental gut stimulation in neural structures included in or directly modulated by the salience network, and (2) an abnormal coupling or interaction of the salience network with major intrinsic neurocognitive networks, especially the DMN and ECN.
Materials and Methods
Study Participants
Study participants included nine adolescent IBS patients aged 12–17 years (5 females and 4 males; mean age, 14.9 years; standard deviation [SD], 1.9 years) and eight age-matched healthy volunteers (controls) aged 12–16 years (three females and five males; mean age, 14.6 years; SD, 1.6 years). All patients and controls provided written informed consent to participate in this study. The Institutional Review Board of the Children’s Hospital of Wisconsin approved all experimental protocols. All IBS patients fulfilled the Rome III criteria for the diagnoses of IBS. Healthy controls reported no history of gastrointestinal diseases or pain-related illnesses.
Psychiatric Assessments
All participants were pre-screened for comorbid psychiatric conditions, as these conditions co-occur at higher rates in the patient population and are known to affect functional imaging results (12). These conditions were assessed using the Children’s Somatization Inventory, How-I-Feel Questionnaire, Multidimensional Anxiety Scale for Children 2nd Edition, and Children’s Depression Inventory. Participants who had clinically elevated scores on measures of mood disruption, anxiety, and/or somatic complaints were excluded from the study.
Rectal Distension Stimulation Protocol
Rectal distension stimulation was administered to all IBS patients and controls using a commercially available computer-controlled barostat (G and J Electronics, Inc., Willowdale, Ontario, Canada) that modulates the air pressure of a balloon placed in the rectum. The balloon was 4.5cm in width and 5cm in length. The volume of the balloon was restricted at 70 ml. The threshold for non-painful liminal rectal sensation was anticipated to be 25–30 mmHg. We therefore restricted the maximum pressure to 40 mmHg. After balloon placement, the pressure thresholds for generating subliminal and liminal stimulations were assessed individually with each participant. During this process, the barostat was programmed to deliver rapid phasic distention at a constant pressure for 10 seconds. At each minor step increase of pressure, the participant was asked whether he/she could feel anything. The process was carefully carried out to ensure that the pressure thresholds for eliciting the intended rectal distension stimulations were tailored precisely to each individual in each group (21). The balloon pressures that elicited subliminal and liminal stimulation were at 15±4 and 24±4 mmHg for IBS patients, and 16±6 and 26±5 for controls. During each functional magnetic resonance imaging (fMRI) scan, the balloon was inflated and deflated at a maximum flow rate of 60 mL/s to maintain a desired pressure level. During liminal stimulation, no participant reported feeling pain.
Subject exclusion criteria included major clinical depression, anxiety, claustrophobia, or fear of loud sounds. In order to acclimatize participants with fMRI, all participants were first tested in a mock scanner. Only with the confirmation that a study participant was able to receive fMRI, did the study team administer rectal distension procedures. The study participant was then transported to the scanner room, lying supine. During the preparatory processes and fMRI scans, all participants were accompanied and guided by the doctor with whom they became familiar during either the clinical diagnosis (for IBS patients) or the recruiting process (for controls). In addition to the doctor, every participant had a parent present in the scanner control room during the entire fMRI period. These steps were taken purposely to reduce study participants’ anxiety and fear related to the rectal distension and fMRI scanning.
Imaging Data Acquisition
Imaging acquisition was performed using a GE Signa 3T scanner (GE, Waukesha, Wisconsin, USA) with a standard 8-channel head coil at the Medical College of Wisconsin. Each fMRI run consisted of four repeating cycles, with each cycle comprising a 15-second pressure and a 25-second rest (Fig. 1A). Two scans were performed during each of the subliminal and liminal stimulation conditions. Participants were not informed of the type or level of rectal distension stimulation, be it subliminal or liminal, before the start of each scan in order to minimize the anticipatory effects in the acquired data. Functional blood-oxygen-level-dependent (BOLD) signals were acquired in the sagittal plane (repetition time, 2 s; echo time, 25 ms; slice thickness, 4 mm; in-plane resolution, 3.75 × 3.75 mm2; flip angle, 77°; field of view (FOV), 24 cm; matrix size, 64-by-64). The whole-brain high-resolution spoiled-gradient-recalled (SPGR) anatomical images were always obtained immediately after the completion of all fMRI runs (TR, 9.5 ms; TE, 3.9 ms; slices thickness, 1.2 mm; flip angle, 12°; FOV, 24 cm; matrix size, 256 × 224).
Figure 1.
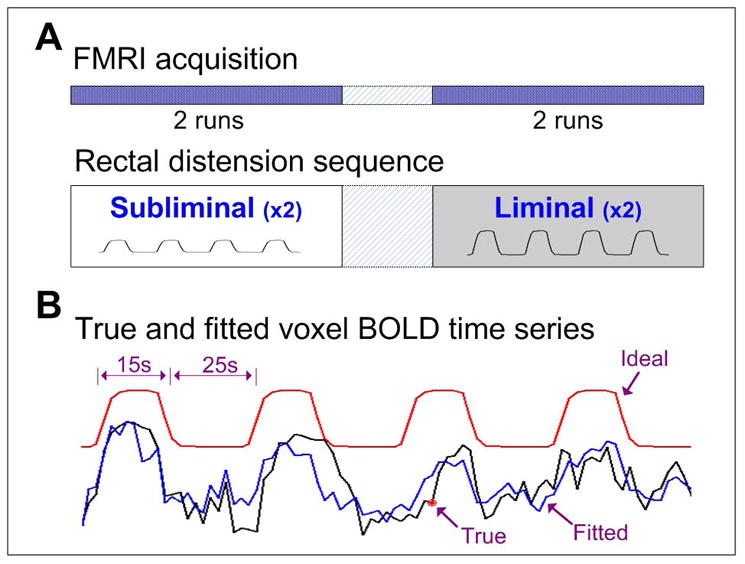
(A) Experimental paradigm. Adolescent IBS patients and age-matched healthy controls underwent two runs of fMRI during each of the subliminal and liminal rectal distension conditions. (B) Sample hemodynamical response to subliminal rectal distension at one activated voxel in the ACC area of an IBS patient. The red line denotes the ideal response to the rectal distension sequence, the black line is the actual BOLD response, and the blue line is the fitted response generated by the regression analysis.
Data Preprocessing
Imaging data analysis was performed using Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/afni) and MATLAB (The MathWorks, Natick, MA) software. First, anatomical images in the native space were manually transformed into the Talairach space; then functional data of each run were coregistered into the standard space with a resampling to a 3.5-mm cubic voxel size. The first four data points in each run were discarded to reduce the initial transient effects in data acquisition. Imaging data preprocessing procedures included despiking, detrending (3dDetrend in AFNI, using the Legendre polynomials with an order of 3), and motion correction to the volume image closest in time to the anatomical image acquisition (3dvolreg in AFNI, producing three translational and three rotational parameters for each volume image). White matter (WM) and cerebrospinal fluid (CSF) segmentation was performed using an automated tool in SPM (www.fil.ion.ucl.ac.uk/spm) across each individual’s high-resolution anatomical images. The average BOLD time series of all voxels contained in the WM and CSF regions were then computed separately.
Determining Rectal Distension-Induced Activation
Rectal distension-induced brain activation was quantified in a voxel wise manner by measuring the percent change of BOLD signal relative to the mean of voxel time course. Each voxel’s BOLD time series was scaled to have a new mean of 100. Then, BOLD signal changes relative to the new mean were evaluated by a general linear regression model (3dDeconvolve in AFNI) that estimates the goodness of fit of the voxel BOLD time series to the ideal hemodynamic response constructed based on the actual rectal distension sequence. Eight additional participating regressors included those representing noise artifacts from the six head motion parameters and average voxel BOLD time series of the WM and CSF. The obtained weight coefficients that quantify percent change of the BOLD signal in response to rectal distension were smoothed by a 6-mm full-width-half-maximum (FWHM) Gaussian kernel filter to compensate for inter-subject variability for computing group statistics. Sample rectal distension-induced BOLD activation was shown with one voxel in the ACC area of an IBS patient during subliminal stimulation (Fig. 1B). The actual BOLD response of the activated voxel (black line) matches closely in shape with the ideal hemodynamic response (red line) and the full-model fitted time series by the regression analysis (blue line).
The group-level activation maps during subliminal and liminal stimulations were obtained by voxelwise one-sample t-tests of weight coefficients against zero, followed by the correction of multiple comparisons using a probability and cluster thresholding technique (AlphaSim in AFNI). The AlphaSim program considers both voxel probability thresholding and minimum cluster-size thresholding to estimate the probability of false-positive occurrence from the frequency count of cluster sizes in each image. The rationale of this technique is that true regions of activation or connection tend to occur over contiguous voxels, whereas noise has much less of a tendency to form clusters. We used a minimum cluster threshold of 179 (with 2-mm isotropic voxels resampling) estimated from our previous imaging studies to suppress false-positive occurrence. Comparisons between the different stimulation and group conditions were evaluated by two-sample t-tests. We report the results at P < 0.05 after the correction of multiple comparisons.
fMRI-Guided Functional Connectivity Analysis
In this study, seed regions for functional connectivity analysis were defined based on rectal distension-induced activation in specific brain areas. This type of connectivity analysis performed with the presence of a task or stimulus was also called fMRI-guided functional connectivity analysis (22). In this study, the first-stage activation analysis showed that during liminal stimulation, both groups demonstrated prominent activation to rectal stimulation in the anterior cingulate and insular cortices, the key node areas of the salience network. Specifically, the activations in the insula were located primarily in the anterior portion, bilaterally for IBS and more prominently left for controls. Distension-induced activation in the anterior cingulate cortex, however, showed subtle locational differences between the two groups, with areas of activation located mainly in the anterior mid-cingulate cortex (aMCC) for controls and the more frontal part of the ACC, also known as the pregenual ACC (pACC) (23), for IBS patients. We thus picked the activated regions in the insular and cingulate cortices as the seed regions in the subsequence connectivity analysis. To increase computational power, we applied more strict P-values to determine the final formation of seed voxel clusters in these areas.
To perform connectivity analysis, the preprocessed fMRI data were analyzed again by a general linear regression model with only eight participating regressors representing the six head motion parameters and average voxel BOLD time series of the WM and CSF to suppress noise artifacts. The residual BOLD signals from the regression analysis were considered to have potential noise and artifact contaminations minimized. However, we did not apply conventionally adopted band-pass filtering (e.g., 0.01–0.1 Hz) to the functional imaging data because our experiment involved rectal distention stimulation and was not a pure resting-state fMRI. Functional connectivity networks were then assessed by calculating the Pearson cross-correlation (3dfim+ in AFNI) of the average voxel BOLD time series of a defined seed region with the rest of brain voxels. Spatial smoothing was performed using a 3.5-mm FWHM Gaussian kernel filter. Group connectivity maps and condition comparisons between IBS patients and controls were determined by one- and two-sample t-tests (P < 0.05), respectively, followed by the correction of multiple comparisons.
Results
Rectal-Distension-Stimuli-Induced Brain Activation in Control and IBS
Group comparisons of rectal-distension-stimuli-induced brain activation revealed significant differences between control and IBS patient groups during subliminal and liminal stimulations. Specifically, IBS patients demonstrated a greater extent and magnitude of activation than healthy controls in a number of keys areas of the cingulate and insular cortices that are involved in visceral afferent and emotional arousal processing (2, 20).
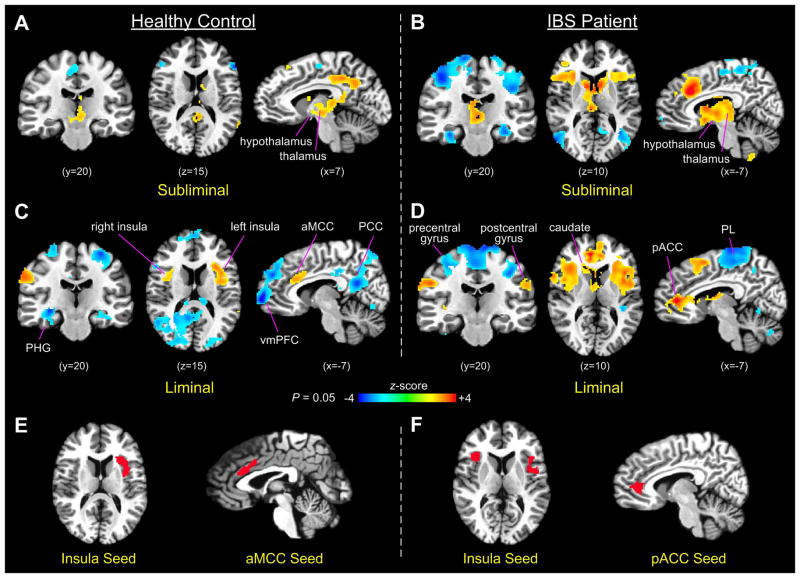
During subliminal stimulation, rectal-distension-induced brain activation in controls was present in large areas of the posterior cingulate cortex (PCC) and retrosplenial cortex, as well as contiguous areas in subcortical regions of the thalamus, hypothalamus, caudate, red nucleus, and periaqueductal gray (PAG). Brain responses during subliminal stimulation also included clustered areas of activation and deactivation in the anterior and posterior sections of the supplementary motor area (SMA), and deactivation in the bilateral inferior frontal gyrus. However, no stimuli-induced activation was detected in the insular cortex in controls during subliminal stimulation (Fig. 2A). In comparison, during subliminal stimulation, IBS patients showed prominent activations in a large clustered area of the aMCC, the bilateral insula (especially the anterior portion), dorsal medial prefrontal cortex (dmPFC), IFG, and large contiguous subcortical areas in the thalamus, hypothalamus, caudate, red nucleus, and PAG. The IBS patients also demonstrated deactivation in large areas of the bilateral sensorimotor cortices, left ventral medial prefrontal cortex (vmPFC), bilateral middle occipital gyrus, and extended bilateral hippocampal and parahippocampal areas (Fig. 2B).
Figure 2.
(A–D) Brain activation in controls and IBS patients during subliminal and liminal rectal distensions (PL, parietal lobule; PHG, parahippocampal gyrus). (E) Seed regions defined in the anterior insula (left) and aMCC (right) based on rectal-distension-induced activation in the control group. (F) Seed regions defined similarly in the bilateral anterior insula (left) and pACC (right) in the IBS patient group.
During liminal stimulation, both patients and controls demonstrated prominently increased activation in the anterior cingulate and insula cortices but decreased extent of activation in the subcortical structures, compared with subliminal stimulation. The control group showed significant activations in the bilateral insular cortices (more extensively in the left), aMCC, and right motor cortex (BA 2), and deactivations in significantly large areas of the PCC, precuneus, vmPFC, bilateral SMA (BA 4), and parahippocampal gyrus (Fig. 2C). Notably, the areas of deactivation in the parietal and frontal lobes are the typical member regions of the DMN, which were consistently deactivated during performing attention or cognitively demanding tasks in comparison with the resting state (6). Compared with subliminal stimulation, brain activations of the patient group during liminal stimulation significantly enlarged in the bilateral insula cortices. Particularly, activated areas in the cingulate cortex included both the aMCC and pACC, revealing a greater extent and magnitude of activation in the pACC (Fig. 2D). Activations in IBS patients were also present in clustered areas of the thalamus, caudate, SMA, and bilateral inferior parietal lobule, whereas significant deactivations were present in large areas of the bilateral sensorimotor cortex and scattered areas of the cerebellum.
Areas of activation in the insular and cingulate cortices during liminal stimulation were defined as seed regions for each group in the subsequent functional connectivity analysis. We applied more stringent P-values to determine seed clusters in the two areas. Specifically, seed regions for the control group were determined at P=0.02, resulting in an aMCC seed containing 367 voxels (2-mm isotropic) and a left insular seed containing 263 voxels (Fig. 2E). In comparison, seed regions for the IBS patient group were determined at P=0.01 resulting in a pACC seed containing 343 voxels and at P=0.005 resulting in a bilateral insular seed containing 206 voxels (Fig. 2F). IBS patients showed a greater extent and magnitude of brain activation in the insular and cingulate cortices during liminal stimulation than controls. Therefore, to ensure that a comparable number of voxels were included in the seed regions in the two groups, more stringent thresholds were required for the IBS group.
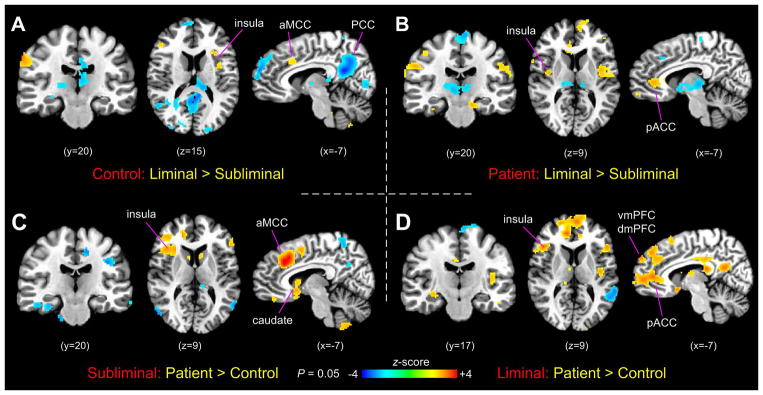
Two-sample t-test comparisons of rectal-distension-induced brain responses were performed between subliminal and liminal stimulation conditions in each group as well as between the patient and control groups at each stimulation condition. The comparisons revealed a trend of results similar to those observed in the activation maps. For the control group, in comparing the liminal and subliminal stimulations, rectal-distension-induced activity showed significant increases in the aMCC, left anterior and middle insula, middle frontal gyrus, and right motor cortex, and decreases in the PCC, retrosplenial cortex, vmPFC, thalamus, scattered clusters in the IFG, visual cortex, and cerebellum (Fig. 3A). For the IBS group, the same comparison showed increases in the bilateral insular cortices (mostly in the middle and posterior portions), pACC, clustered areas of the bilateral sensorimotor cortices, and bilateral hippocampal gyrus, and decreases in the dorsal medial frontal area, SMA, and thalamus (Fig. 3B). Group comparison during subliminal stimulation showed that the IBS patients had significantly increased activity in the right anterior insula, aMCC, pACC, subgenual ACC, right inferior and middle frontal gyrus, and clustered areas in the cerebellum, and decreased activity in the precuneus, bilateral temporal-occipital conjunction, and parahippocampal gyrus. Group comparison during liminal stimulation showed that IBS patients had significantly increased activity in the bilateral anterior insula, left posterior insula, aMCC, pACC, subgenual ACC, bilateral IFG (BA 13 and 45), PCC, left vmPFC, and right dmPFC, and decreased activity in clustered areas of the parietal lobule and cerebellum.
Figure 3.
(A) Comparison of brain activation between subliminal and liminal stimulation conditions in the control group. (B) The same in the IBS patient group. (C) Group comparison of brain activation between controls and IBS patients during subliminal stimulation. (D) The same during liminal stimulation.
In the supplemental information, we have provided detailed region-specific summaries of brain activation to rectal distension in both groups as well as group comparisons at each rectal distension stimulation condition (Supplemental Tables 1–6).
Insular and Cingulate Seed Functional Connectivity in Patient and Control Groups
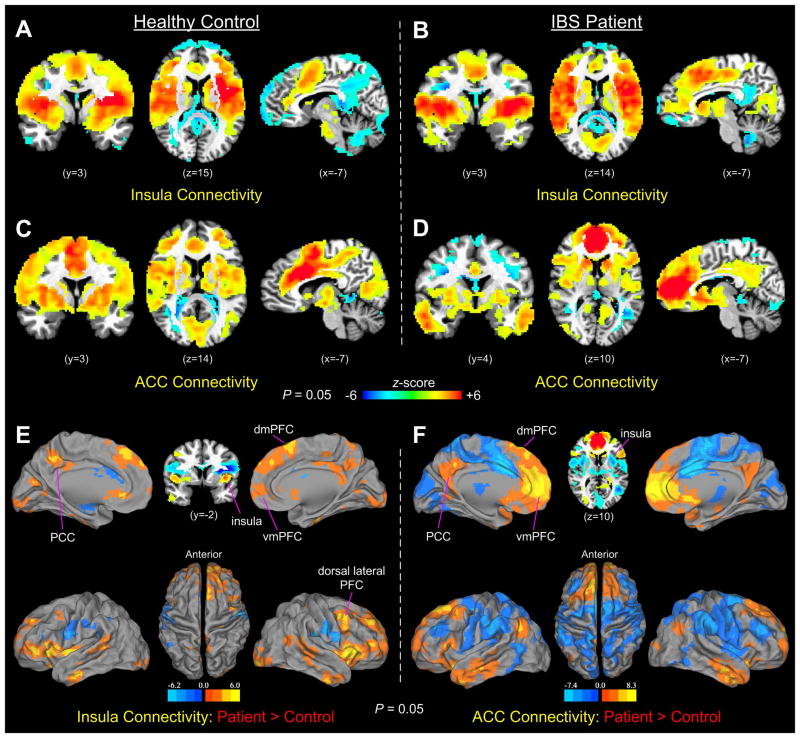
Functional connectivity networks from the seed regions in the insular and cingulate cortices showed prominent connectivity in all key cortical and subcortical structures of the salience network in both groups (Fig. 4A–D). The set of brain regions included primarily the anterior insula, ACC, SMA, amygdala, substantia nigra or ventral tegmental area, and thalamus (7). In addition to the common spatial patterns, there were, however, noticeable differences in functionally connected brain areas between the insular and cingulate seeds in each group, as well as between-group differences with each seed. Specifically, in controls, the insula seed showed a greater extent of negative connections in areas of the vmPFC, dmPFC, PCC, and cerebellum (Fig. 4A), while the aMCC seed showed more connections in the more posterior segment of the dorsal ACC, part of the precuneus, and the visual cortex (Fig. 4C). In comparison, the insula seed of IBS showed a greater extent of connectivity in the areas of the dmPFC, vmPFC, PCC, visual cortex, and subcortical areas of the caudate and thalamus (Fig. 4B), while the pACC seed showed significant positive connectivity in large areas of the dmPFC, vmPFC, and PCC, and the same set of subcortical structures (Fig. 4D). Further group comparisons indicated that functional connectivity of the insular and cingulate seeds both increased significantly in IBS patients in the vmPFC, dmPFC, and PCC compared with healthy controls. Notably, the vmPFC and PCC both are the key nodes of the DMN, and the dmPFC is a part of the ECN. In addition, IBS patients showed prominently increased connections in the right dorsal lateral PFC and bilateral IFG compared with controls.
Figure 4.
(A–B) Functional connectivity of the insula seeds in the control and IBS groups during liminal stimulation. (C–D) Functional connectivity of the aMCC (in controls) and pACC (in IBS patients) seeds during liminal stimulation. (E–F) Group comparisons of insular and cingulate functional connectivity between controls and IBS patients. In both comparisons, IBS patients showed significantly increased functional connections in the dmPFC, vmPFC, dorsal lateral PFC, and PCC, compared with controls.
To determine whether the observed group differences in functional connectivity were exclusively led by locational differences in the seed regions in the insular and cingulate cortices between the control and IBS patient groups, we conducted an additional set of connectivity analyses by simply switching the insular and cingulate seeds between the two groups. In this case, the results showed similar connectivity maps between IBS patients and controls during liminal stimulation, if an identical insula or cingulate seed was used for both groups (Supplemental Fig. 1). Similar results were replicated also with data acquired during subliminal stimulation (Supplemental Fig. 2). The results thus indicated that the observed functional connectivity differences between controls and IBS patients were introduced mainly by seed location differences in the insular and cingulate subareas that were activated during liminal rectal distension.
Discussion
Altered brain-gut communication in IBS is associated with abnormal changes of attentional, affective and regulatory processes in the central nervous system that involve distributed cortical and subcortical structures operating in large-scale brain networks (5, 20). Of the identified intrinsic brain networks, the neural structures of the salience network and the overall functions ascribed to it are particularly relevant to the investigation of neural underpinnings of IBS symptoms and comorbidities (10, 11, 19). The main findings of the study can be summarized in four aspects. First, brain responses to non-painful liminal rectal distension in adolescents are quite similar to those observed in adults in comparable stimulation conditions (2). Second, brain responses to liminal stimulation were more extensive compared with those to subliminal stimulation in both groups. Third, IBS patients, compared with controls, showed significantly increased brain activation to rectal distension in a collection of brain structures, mostly the limbic-system-related areas, in both stimulation conditions. Lastly, IBS patients were uniquely associated with significantly increased insula and anterior cingulate functional connectivities in the key frontal and parietal node areas of the DMN and ECN, suggesting an excessive coupling of the salience network with the major intrinsic neurocognitive networks.
With respect to rectal-distension-induced brain activation, our findings are highly consistent with the general conclusions of a recent quantitative meta-analysis of brain regions activated by supraliminal, non-painful rectal distension in adult IBS patients (2). In general, adolescent IBS patients, compared with controls, also demonstrated a greater engagement of brain regions of the homeostatic afferent processing and emotional arousal networks in response to subliminal and liminal stimulations, respectively. During subliminal stimulation, IBS patients showed significant activation in the bilateral insular cortices and aMCC, while controls did not. Activations in the thalamus and adjacent mid-brain structures were also stronger in IBS patients. The homeostatic afferent processing network that mediates visceral sensation and responses consists of the key nodes in the thalamus, insula, and aMCC (14, 20). Visceral afferents project via the thalamus to the primary interoceptive cortex (the posterior insula) and to the aMCC that modulates affective, motivational and motor aspects of brain response to visceral sensation (8, 23–25). The insula plays a significant role in visceral and somatic sensory processing and autonomic regulation of the gastrointestinal tract and heart (26, 27). The ACC plays a prominent role in decision-making, motor action, and response selection, potentially via its links to the midcingulate cortex, supplementary motor cortex, and other motor areas. (28–30). Functional roles ascribed to ACC also include emotion (31) and pain perception (32). The greater engagement of these brain areas during subliminal stimulation in IBS indicates that although rectal stimulation was below the perception threshold, neural structures involved in the homeostatic afferent processing had already been recruited to a greater extent in IBS patients than in controls to attend to rectal stimulus and initiate autonomic behavioral and background emotional responses. In a general sense, such findings offer direct supporting evidence of the presence of visceral hypersensitivity in the central processing networks of IBS patients.
During liminal stimulation, rectal distension invoked significant activation in the pACC in IBS patients but not in controls. The IBS patients also had greater activations in the SMA and ventral medial frontal and bilateral primary motor cortices. The emotional arousal network, which modulates emotional processes related to visceral sensation, such as fear and anxiety, comprises of a variety of cortical and subcortical structures including key nodes in the amygdala and pACC, and extended circuitry in the anterior insula, orbitofrontal cortex, hippocampus, and dorsal pons (14, 31, 33). Our findings indicate that when rectal distension pressure went beyond the perception threshold, a greater engagement of regions in the emotional arousal network occurred in IBS patients compared with controls. Such findings were less likely due to a stronger anticipatory effect in IBS patients than in controls, because study participants were not informed of the type of rectal distension before the start of a scan. Moreover, the liminal distension thresholds were individually tailored to generate a same kind of innocuous sensation (barely perceivable) in both groups. Therefore, the group differences were more likely due to alterations of homeostatic afferent processing within the brain-gut axis in IBS patients.
A primary finding of the current study is that functional connectivity networks from the insula and anterior cingulate seeds showed significantly different connectivity patterns between the control and IBS patient groups during liminal stimulation. In IBS patients, the insula and anterior cingulate functional connectivities significantly increased in the key node areas of the DMN, specifically the vmPFC and PCC, and key node areas of the ECN, specifically the medial and dorsolateral PFC, compared with controls (Fig. 4). With the ACC and insula as the two anchor nodes of the salience network and the presence of all key components of the salience network in the functional connectivity maps, the results indicate an excessive coupling of the salience network with the DMN and ECN in IBS patients. The additional set of functional connectivity analyses (Supplemental Figs. 1–2) further indicates that the observed group differences were introduced exclusively by seed location differences in the anterior insular and cingulate cortices between the two groups. Therefore, by activating subtly different subareas of the insular and cingulate cortices by rectal distension, profound modification of interaction between the salience network and the DMN and ECN can be introduced in response to visceral afferent input in IBS patients.
Considered in the context of psychological comorbidities of IBS, our connectivity findings support the triple network model of altered interaction of the salience network with the DMN and ECN as an underlying general mechanism of a variety of psychological disorders (8, 11). The model emphasizes the crucial role of the salience network in modulating the engagement of the ECN and disengagement of the DMN when attending to a salience event (e.g., rectal distension here), and its alteration in disorder conditions. For instance, hyperactivity of the salience network, especially the AI node, has been consistently implicated in anxiety disorders (34, 35). In depressed patients, excessive cross-network couplings between the salience network and the DMN were present, presumably reflecting the inability to cycle out internal mental processes (self-related rumination) when attending to salient events (11, 36, 37). Chronic pain patients also suffer from depression, anxiety, and impaired cognitive ability (38), as is often the case in IBS patients (12). By controlling for psychiatric comorbid by excluding children with suspected depression, anxiety, and somatic disorders, we begin to describe the unique contributions IBS may have on salience network functioning. Recent resting-state fMRI network connectivity studies using either the seed-based approach (15) or independent component analysis (ICA) (19) have also identified sex-related, disease-related and early-adverse-life-events-related network connectivity changes in several frontal and parietal regions, implicating particularly altered functional roles of the salience network and ECN in IBS. Although a direct comparison between our study and these recent studies is difficult to establish, because of differences in the experimental paradigm (e.g., task-related vs. resting state), analytical technique (e.g., fMRI-guided connectivity analysis vs. ICA or predefined seeds), and patient population, current network studies together suggest that altered salience network functioning underlies various symptoms and disease traits of IBS.
A few methodological concerns shall be noted. First, the number of participants in this study is relatively small. Recruiting young children as volunteers for an fMRI study is extremely challenging especially when the use of rectal distension is involved. However, we performed two independent fMRI runs with each participant in each stimulation condition. This significantly improved the power of our statistical analysis. Additionally, our activation findings bear much resemblance to those found in the meta-analysis of adult IBS studies (2), partially validating our results. To our knowledge, no prior studies reported on regional brain activation and connectivity in pediatric IBS patients. We hope our study provides a unique perspective on this subject and will lead to more pediatric studies in the future. Given that our study included only eight controls and nine IBS patients, the results should be considered preliminary. Future investigations shall test the findings in a larger sample size across different age groups. Salience network functioning may also be evaluated in responding to non-visceral sensation to pinpoint whether the observed excessive coupling in IBS patients is exclusively associated with visceral afferents only. Second, the fMRI-guided connectivity analysis is a novel approach used in the current study. It appeared to be crucial in revealing the functional connectivity differences between controls and IBS patients. It is a useful approach for determining network interaction within and between different levels of cognitive hierarchy when an actual cognitive task or a sensory stimulus is present (22). The use of this approach is justified in the context of the current study because a salience value is always associated with a sensory event or cognitive object, be it internal or external. Previous studies found that a novel stimulus invokes greater insular cortex responses (8). Thus, salience network functioning should be in a completely engaged state when rectal distension becomes perceivable (liminal). In our results, this was supported by the coexistence of activation to rectal distension in both the ACC and anterior insula, which appeared only during liminal stimulation in healthy controls.
In summary, our study supports altered salience network functioning as an explanatory central neural network mechanism to common IBS symptoms, in line with the theoretical models of the salience network to chronic pain and brain disorders (10, 11).
Supplementary Material
Key Messages.
Brain responses to subliminal and liminal rectal distensions in adolescent patients with irritable bowel syndrome and healthy controls are similar to those observed in adults. An excessive coupling of the salience network with key frontal and parietal node areas of the default mode network and executive control network was present in patients, consistent with the theoretical predictions of altered salience network functioning in chronic pain and symptoms of irritable bowel syndrome.
The excessive coupling of the salience network with the default mode and executive control networks was led mainly by activating subtly different subareas of the insular and cingulate cortices in response to rectal distension in patients with irritable bowel syndrome.
The engagement of neural structures in the homeostatic and emotional arousal networks in response to rectal distension was greater in patients with irritable bowel syndrome compared with healthy controls. We observed greater brain responses to liminal vs. subliminal rectal distension in both groups.
Acknowledgments
Research presented in this publication was supported in part by a grant from the Digestive Diseases Center, Medical College of Wisconsin, and National Institutes of Health (NIH) grants R01GM103894 and R01DK025731. We thank Carrie M. O’Connor, MA, and Lydia Washechek, BA, for editorial assistance.
Footnotes
Competing Interests: the authors have no competing interests.
Author Contributions
MRS, AS & RS designed and performed the research. XL & BDW analyzed the data. XL & MRS wrote the paper. SJL & MK contributed technical support or tools for the study.
References
- 1.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21(6):579–96. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140(1):91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18(4):663–80. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Jones MP, Dilley JB, Drossman D, Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. 2006;18(2):91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 5.Tillisch K, Labus JS. Advances in imaging the brain-gut axis: functional gastrointestinal disorders. Gastroenterology. 2011;140(2):407–411. e1. doi: 10.1053/j.gastro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME. The restless brain. Brain connectivity. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30(5):263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 10.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 (Suppl 8):28–36. discussion 37. [PubMed] [Google Scholar]
- 13.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17(2):131–9. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34(43):14252–9. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47(3):952–60. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38(4):720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Kilpatrick L, Labus J, Tillisch K, Braun A, Hong JY, Ashe-McNalley C, Naliboff B, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76(6):404–12. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130(1):26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33(10):2487–98. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 25.Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138(2):362–74. doi: 10.1016/j.pain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 27.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 28.Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28(51):13775–85. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt BA. Cingulate neurobiology and disease. New York: Oxford University Press; 2009. p. xxxiv.p. 829. [Google Scholar]
- 30.Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- 31.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 32.Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30(6):855–63. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 35.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 36.Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, Gonzalez R, Demiralp E, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11(1):85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10(4):470–8. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1–2):129–36. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.