Abstract
Posterior cortical atrophy (PCA) is an understudied visual impairment syndrome most often due to “posterior Alzheimer’s disease (AD)” pathology. Case studies detected mutations in PSEN1, PSEN2, GRN, MAPT and PRNP in subjects with clinical PCA. To detect the frequency and spectrum of mutations in known dementia genes in PCA, we screened 124 European-American subjects with clinical PCA (n=67) or posterior AD neuropathology (n=57) for variants in genes implicated in AD, frontotemporal dementia, and prion disease using NeuroX, a customized exome array. Frequencies in PCA of the variants annotated as pathogenic or potentially pathogenic were compared against ~4,300 European-American population controls from the NHLBI Exome Sequencing Project (ESP). We identified two rare variants not previously reported in PCA, TREM2 Arg47His and PSEN2 Ser130Leu. No other pathogenic or potentially pathogenic variants were detected in the screened dementia genes. In this first systematic variant screen of a PCA cohort, we report two rare mutations in TREM2 and PSEN2, validate our previously reported APOE ε4 association, and demonstrate the utility of NeuroX.
Keywords: PCA, posterior Alzheimer’s disease, dementia, APOE, TREM2, PSEN2, NeuroX
1. INTRODUCTION
Posterior cortical atrophy (PCA) was first described by Benson et al. (Benson et al., 1988) as a syndrome characterized by predominant visual deficits in the absence of primary ocular disease, and relative preservation of episodic memory and insight early in the disorder (Crutch et al., 2012). Its features often reflect involvement of posterior brain regions, and include some or all of these clinical features: Components of Bálint’s or Gerstmann syndromes, alexia, constructional dyspraxia, environmental disorientation, ideomotor apraxia, prosopagnosia or visual field defects. Neuropathologic series of PCA revealed AD pathology in 80–100% of cases (Ala et al., 1996; Alladi et al., 2007; Renner et al., 2004; Tang-Wai et al., 2004; Victoroff et al., 1994). Additionally, amyloid neuroimaging (de Souza et al., 2011; Lehmann et al., 2013; Rosenbloom et al., 2011) and cerebrospinal fluid (de Souza et al., 2011; Seguin et al., 2011) biomarkers of PCA subjects follow patterns similar to those in AD. Although the type of neuropathology in PCA is the same as that in typical AD, the distribution of the neuropathology is distinct. Both PET-amyloid imaging (Rosenbloom et al., 2011) and neuropathology studies suggest similarities in the regional distribution of the amyloid pathology between PCA and typical AD, but a strikingly greater burden of neurofibrillary tangle pathology in posterior brain regions in PCA subjects as compared to typical AD (Crutch et al., 2012). Thus, PCA is considered an atypical form of AD, although other pathologies, including Creutzfeldt-Jakob disease, corticobasal degeneration or Lewy body disease can also rarely present as PCA (Renner et al., 2004; Tang-Wai et al., 2004).
Given its distinct clinical presentation and limited heterogeneity in etiology with AD as the main underlying neuropathology, uncovering the genetics of PCA is important as it can identify genetic factors that are both common with and distinct from those in typical AD, hence providing insight about pathophysiology of both conditions. Further, given that the onset age for PCA is generally younger, thus often prompting clinical genetic screens, discovery of the full pathogenic mutation spectrum that can lead to PCA can aid the clinician in the choice of genetic tests. To date, there are only case reports of PCA subjects with missense variants in genes previously implicated in early-onset AD (EOAD) (Saint-Aubert et al., 2013; Sitek et al., 2013; Tremolizzo et al., 2014), frontotemporal dementia (FTD) (Caroppo et al., 2015; Rossi et al., 2014), and Creutzfeldt-Jakob disease (CJD) (Depaz et al., 2012).
In this first systematic mutation screen of 124 subjects with clinical PCA or posterior AD pathology, we utilized a customized Illumina exome genotyping array, NeuroX, to evaluate genes known to harbor pathogenic variants in EOAD [APP (Goate et al., 1991), PSEN1 (Sherrington et al., 1995) and PSEN2 (Rogaev et al., 1995)], LOAD [APOE (Yu et al., 2014) and TREM2 (Guerreiro and Hardy, 2013; Kleinberger et al., 2014)], FTD [GRN (Perry et al., 2013; Wojtas et al., 2012) and MAPT (Carney et al., 2014; Wojtas et al., 2012), and CJD [PRNP (Guerreiro et al., 2014)], for their role in PCA. NeuroX is enriched for risk variants involved in neurological disorders, thus providing an efficient platform for rapid screening of genes of interest (Ghani et al., 2015). Our findings have implications for the mutation spectrum in PCA as well as the utility of NeuroX.
2. MATERIALS AND METHODS
2.1. Subjects
All subjects were recruited at Mayo Clinic Jacksonville in Florida or Mayo Clinic Rochester in Minnesota. Sixty seven subjects had clinical diagnosis of PCA, five of whom also had pathologic diagnosis; and 57 had neuropathological diagnosis as posterior AD, but not a clinical diagnosis (Table 1). Clinical diagnosis of PCA was made according to published core criteria (Tang-Wai et al., 2004) as follows: presentation of visual complaints in the absence of significant primary ocular disease; relative preservation of anterograde memory and insight early in the disorder; insidious onset and gradual progression; disabling visual impairment throughout the disorder; absence of stroke or tumor; absence of early parkinsonism and hallucinations. All subjects with pathologic diagnoses were evaluated by one neuropathologist (DWD). For the neuropathologic diagnosis, senile plaques (per 10 × field) and neurofibrillary tangles (NFT) (per 40 × field) are counted in the mid-frontal, superior temporal, inferior parietal, motor, visual (Brodmann’s area 17=BA 17) and entorhinal cortices, in addition to two sectors of the amygdala. NFTs are also counted in the nucleus basalis of Meynert. Visual association cortex (BA 18) is also scanned in all cases and counted in those with severe NFT pathology. All subjects with neuropathologic diagnosis of posterior AD have disproportionate severity of NFT in BA17 and BA18 compared to typical AD cases, but could have additional pathologies including Lewy bodies, vascular disease, hippocampal sclerosis or progressive supranuclear palsy. The overall pattern of NFT severity in BA17 and BA18 is greater than that in the frontal cortex in posterior AD, such that this diagnosis is not simply a function of the overall disease severity, but reflects disproportionate, focal involvement of the visual cortices. Thus, subjects with posterior AD had neuropathology characteristics of PCA (Crutch et al., 2012). The disproportionate involvement of the posterior cortices was not determined by objective criteria, but subjectively. All neuropathologically diagnosed cases had Braak stage of 5.5 or 6, except for one patient with a Braak stage of 4. Our rationale for including posterior AD cases in our cohort stems from prior neuropathologic studies of PCA, which consistently identified higher tangle counts in posterior cortical regions (Crutch et al., 2012). Only subjects with complete age, sex, ethnicity and APOE genotype information are included in the study.
Table 1.
Cohort Description
| Group | No. | Female, n (%) | APOE ε4 copies 0/1/2 (%)a | Mean age +/− SD (range)b |
|---|---|---|---|---|
| Clinical PCA | 67 | 43 (64%) | 35/24/8 (52.2/35.8/11.9) | 61.6 ± 8.5 (42–83) |
| Posterior AD pathology | 57 | 42 (74%) | 16/30/11 (28.1/52.6/19.3) | 80.0 ± 10.4 (58–99) |
| Combined cases | 124 | 85 (69%) | 51/54/19 (41.1/43.5/15.3) | 70.0 ± 13.2 (42–99) |
| pc | 0.26d | 0.01d | < 0.0001e |
Demographics for cases clinically diagnosed as PCA, pathologically diagnosed as Posterior AD, and combined cases are shown.
The number of subjects with no, one or two copies of APOE ε4 allele (percentage of subjects);
Age at onset is used for subjects recruited by clinical diagnosis and age at death is used for autopsied samples;
Comparisons are between PCA and Posterior AD cases.
P values for chi-square test;
P value for two-sided, unpaired t-test.
2.2. Analysis of NeuroX genotypes
One hundred and twenty four PCA samples were genotyped using the NeuroX, which is a customized Human Exome BeadChip (Illumina, Inc., San Diego, CA) containing 242,901 variants from the standard Illumina exome content, and an additional 24,706 custom content variants focusing on neurologic diseases (Nalls et al., 2015). It should be noted that since NeuroX is a commercially available array with predetermined content, not all known dementia gene variants that meet the inclusion criteria used in our study are present on this array, as described below in more detailed and as shown in Table 2. Variant clustering and genotyping was achieved using Illumina’s GenomeStudio software v2011.1 and the genotyping module v1.9.4. The CHARGE cluster file v1.0 (Grove et al., 2013) was used to cluster the standard content, and the remaining variants were clustered using GenomeStudio’s clustering algorithm. All variants with a GenTrain Score < 0.7 had their clusters visually assessed and manually adjusted where appropriate. Any variants that were impossible to cluster or showing irregularities were zeroed. All genotype calls were exported to PLINK (Purcell et al., 2007) format in the forward orientation, and the resulting dataset was subjected to quality control using PLINK v1.07. Variants with greater than 10% missing data were excluded, resulting in 265,525 remaining variants with an average call rate of 99%. All samples had call rates above 99%.
Table 2.
Number of variants per gene of interest in NeuroX.
| Pathogenic Nature Unclear | Pathogenic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Gene | Database | Designed on NeuroX | Worked on NeuroX | Database | Designed on NeuroX | Worked on NeuroX | Database | Designed on NeuroX | Worked on NeuroX |
| From the AD&FTD Mutation Database | APP | 31 | 9 | 9 | 1 | 1 | 1 | 24 | 6 | 6 |
| PSEN1 | 197 | 77 | 76 | 8 | 4 | 4 | 185 | 70 | 69 | |
| PSEN2 | 25 | 10 | 10 | 7 | 3 | 3 | 13 | 6 | 6 | |
| GRN | 149 | 32 | 32 | 35 | 10 | 10 | 69 | 12 | 12 | |
| MAPT | 73 | 11 | 11 | 2 | 0 | 0 | 44 | 1 | 1 | |
| HPPa | PRNP | 42 | 5 | 5 | 0 | 0 | 0 | 24 | 1 | 1 |
| AD riskb | APOE | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | 1 |
| TREM2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | |
| Total | 8 | 520 | 147 | 145 | 53 | 18 | 18 | 362 | 99 | 97 |
Variants listed are SNPs or small indels (duplication not included). Pathogenicity annotation per the AD&FTDMDB (molgen.vib-ua.be/Public/MutationDatabase/Default.cfm) or the ahuman prion point mutations listed in http://www.madcow.org/prion_point_mutations.html).
AD risk variants with confirmed pathogenicity (Guerreiro and Hardy, 2013; Kleinberger et al., 2014; Yu et al., 2014).
As the goal of this study was to evaluate the contribution to PCA of known disease-associated variants in known dementia genes, we focused on variants that were previously reported in dementia patients, and for which there was evidence in the literature of pathogenicity either through their segregation with disease in families and/or through evaluation of their biological consequences at the molecular level. Therefore, we evaluated in this study only those variants that were annotated in the AD and FTD mutation database (AD&FTDMDB) (Cruts et al., 2012) as “pathogenic” or “pathogenic nature unclear”, in addition to variants in the Human Prion Protein (HPP) Mutation Database (http://www.mad-cow.org/prion_point_mutations.html, both accessed on 12/01/2014) which were annotated as “causative”, as well as the well-established AD risk missense variants in APOE (Cys112Arg, a.k.a APOE ε4) and TREM2 (Arg47His). Of the 415 variants (SNPs/insertions/deletions) in APP, PSEN1, PSEN2, APOE, TREM2, GRN, MAPT and PRNP that met these criteria, NeuroX contains assays for 117, of which 115 passed the genotype QC described above (Table 2). Although we used the annotation provided by the AD&FTDMDB (“pathogenic” or “pathogenic nature unclear”) for our variant selection criteria, in this study we make a distinction between pathogenic variants that are “deterministic” (sufficient to cause disease), versus variants that were demonstrated to be “risk factors” (variants that increase the risk of disease, but which are neither necessary nor sufficient to cause the disease) based on published data.
To date, there are six case reports of PCA patients with mutations in known dementia genes (Caroppo et al., 2015; Depaz et al., 2012; Rossi et al., 2014; Saint-Aubert et al., 2013; Sitek et al., 2013; Tremolizzo et al., 2014). Of these six variants, four (PSEN1 G223R, PSEN2 M239I, MAPT V363I, and GRN R110X) are listed in the AD&FTDMUTDB, as “pathogenic”, but only PSEN2 M239I is included on the NeuroX array. PSEN1 I211M is not on the AD&FTDMUTDB or in NeuroX. While the PRNP 120bp insertion from the published PCA case report is listed in the HPP mutation database, annotation of causality is not provided, and it was not present on NeuroX.
Sanger sequencing was used to validate the NeuroX genotypes that were indicative of the presence of a variant of interest. Validated variants were formally tested for association with the risk of PCA with a Fisher’s exact in RStudio v0.98.1091 using counts from the Exome Variant Server’s European-American cohort as controls (http://evs.gs.washington.edu/EVS/, accessed on 12/01/2014). Using simulation-based power calculations we have an estimated 80% power to detect a minimum odds ratio (OR)=1.45 from a variant with MAF=30%, similar to the effect size and MAF of AD risk variants detected in our previous study (Carrasquillo et al., 2014). We are also powered to detect variants with an OR≤3.70 and MAF≥1%, and rarer variants with MAF≥0.1% and OR≤15. Linkage disequilibrium between APOE ε4 and its NeuroX proxy (rs769449) was evaluated in Haploview 4.0 (Barrett, 2009) using the NeuroX rs769449 data and pre-existing APOE ε4 genotypes in this cohort (Carrasquillo et al., 2014).
3. RESULTS
3.1. Variant screen in PCA
We assessed 124 subjects in our cohort, 67 of whom were diagnosed as PCA clinically and 57 with posterior AD neuropathology (Table 1). There was no difference in the sex composition between the clinically and pathologically diagnosed subjects although APOE ε4 frequency was higher in posterior AD (0.46) compared with clinical PCA (0.30), as we previously reported in a slightly larger cohort including these 124 subjects (Carrasquillo et al., 2014). Notably, the APOE ε4 frequency in both subgroups is enriched compared to control frequencies (0.12). The significant difference in mean age is explained by the use of age-at-onset for the clinical PCA subjects and age-at-death for posterior AD cases.
Our NeuroX variant screen of 124 PCA subjects identified one rare pathogenic variant (TREM2 Arg47His) and one rare potentially pathogenic variant (PSEN2 Ser130Leu) (Table 3). Both of these rare variants are in genes known to be involved in AD, and were confirmed by Sanger sequencing (Figure 1). Neither variant has been previously reported in a PCA patient.
Table 3.
Rare NeuroX pathogenic variants or of pathogenic nature unclear that are present in at least one case subject.
| Fisher’s Exact Test a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Amino acid change | dbSNP variant ID | NeuroX variant name | Minor allele count - Cases | Major allele count - Cases | Minor allele count - Controls (EVS) | Major allele count - Controls (EVS) | P | OR | LCI | UCI |
| PSEN2 | Ser130Leu | rs63750197 | exm153953 | 1 | 247 | 9 | 8591 | 0.248 | 3.86 | 0.09 | 28.04 |
| TREM2 | Arg47His | rs75932628 | exm545529 | 1 | 247 | 22 | 8578 | 0.480 | 1.58 | 0.04 | 9.84 |
Pathogenic variants or variants of “pathogenic nature unclear” (according to the AD&FTDMDB) that were present in at least one case subject are listed along with the results from a aFisher’s exact test comparing allele frequencies in the 124 PCA cases versus the frequency in 4,300 European Americans, according to the Exome Variant Server (EVS). P, Fisher’s exact p-value; OR, odds ratio; LCI, lower 95% confidence interval; UCI, upper 95% confidence interval.
Figure 1. Sequence chromatograms from the carriers of the (a) PSEN2 Ser130Leu and (b) TREM2 Arg47His variants.
In both panel (a) and (b), the variant site is indicated by the black arrow in the upper chromatogram, while the sequence from a control sample lacking the variant is shown in the lower chromatogram.
Although NeuroX genotypes also identified a rare pathogenic GRN frameshift mutation, Gln130fs, Sanger sequencing did not confirm this variant and instead revealed a non-pathogenic, synonymous variant, rs25646 (Asp128), indicating an error in the annotation and/or design of this NeuroX assay. This error has also been previously noted by others (Ghani et al., 2015).
The common APOE ε4 (rs429358) pathogenic variant could not be genotyped on the NeuroX given that it failed genotype cluster QC, as also previously noted (Ghani et al., 2015). Nevertheless, the genotypes for a strong APOE ε4 proxy (rs769449; D’=1.0, r2=0.80) on the NeuroX, demonstrated highly significant association with risk of PCA (124 PCA vs. 4,300 European-American controls, Fisher’s exact OR= 4.24, p=1.5×10−19), as expected (Carrasquillo et al., 2014). No other pathogenic or potentially pathogenic variants were found in this screen.
3.2. Description of the TREM2 Arg47His carrier
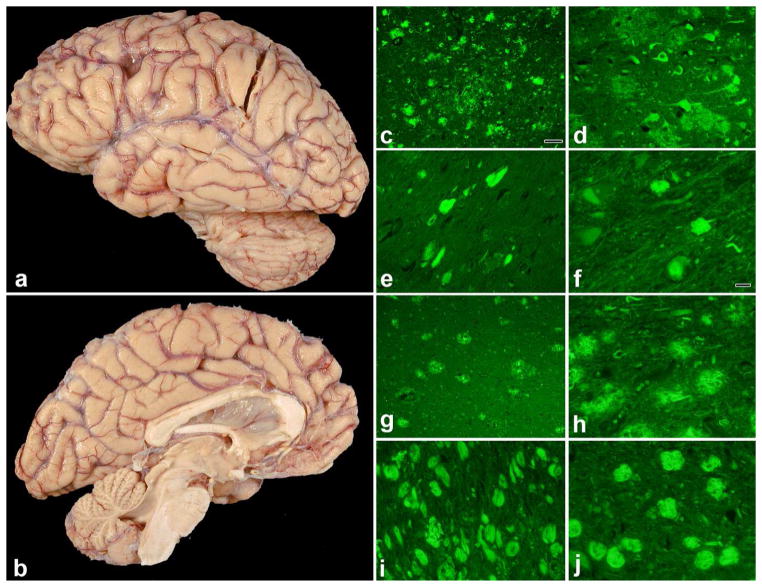
The TREM2 Arg47His variant was found in a subject with posterior AD pathology. There was no detailed clinical data available on this female patient, who had onset of symptoms at age 71, a rather long disease duration (14 years) and APOE ε3/ε4 genotype. Brain weight was 1,020 grams with cortical atrophy demonstrating occipital predominance on gross anatomy. Figures 2a and b depict the lateral and medial views, respectively, of gross anatomy of the brain from this subject where the occipital lobe atrophy is evident. The gap between the dorsal cerebellar surface and the ventral occipital lobe surface is especially notable in Figure 2b, which further demonstrates the occipital atrophy.
Figure 2. Neuropathologic features of the TREM2 Arg47His carrier and typical AD neuropathology for comparison.
(a) cortical atrophy on lateral view (slight occipital predominance), (b) medial view demonstrating the occipital atrophy, (c) thioflavin-S fluorescent microscopic images of Brodmann area 18, (d) middle frontal gyrus, (e) hippocampal CA1 sector and (f) basal nucleus of Meynert. Figures 2 (g–j) correspond to the same regions as in (c–f), respectively, but are from an autopsied subject with typical AD neuropathology. Scale bar in (c) is 50 microns; scale bar in (f) is 20 microns (applies to d, e and f). The same magnifications are utilized for the corresponding figures (g–j).
There was advanced AD pathology in the posterior brain regions with Thal phase = 5 and Braak stage = 6. Thioflavin-S staining of senile plaques and neurofibrillary tangles shows severe neurofibrillary tangle pathology in the visual association cortex (BA18) (Figure 2c), but less dense pathology in the frontal cortex, hippocampus and basal nucleus of Meynert (Figures 2d–f). There was also TDP-43, Type 1 pathology and secondary pathologic diagnoses of hippocampal sclerosis and vascular disease in this patient.
For comparison, the same regions of a patient with typical AD neuropathology are shown in Figures 2g–j. The typical AD subject was matched to the posterior AD patient for Thal phase, Braak stage and gender; was also Caucasian and had age at death of 82. Brain weight of the typical AD subject was 920 grams, with less severe neurofibrillary tangle pathology in BA18 (Figure 2g), but more severe pathology in the frontal cortex, hippocampus and basal nucleus of Meynert (Figures 2h–j). Hence, the disproportionate involvement of the posterior cortical regions is evident in the posterior AD subject.
3.3. Description of the PSEN2 Ser130Leu carrier
The PSEN2 Ser130Leu variant was found in a clinically diagnosed female PCA patient, who at age 41 presented with a three year history of progressive visuospatial impairment with no memory difficulties at the time. Her symptoms started as prosopagnosia and difficulty reading her computer screen despite progressively increasing the font size. She was prescribed reading glasses with no benefit. She then developed difficulties understanding spatial relationships as manifested by problems with dressing, driving and putting a complex visual scene together. Her neurological examination showed Kokmen short test of mental status (STMS) score of 35/38 with impairment in drawing a clock face, copying a cube and calculation. Simultanagnosia and prosopagnosia were also noted on the exam with no ocular apraxia or optic ataxia. Visual acuity was intact (20/30) bilaterally. She had saccadic eye pursuits. There was no evidence of parkinsonism and the rest of her neurological examination was unremarkable. Neuropsychological assessment revealed severe visual agnosia, intact attention and verbal memory with relative sparing of language abilities. Follow-up examination after a year of her initial evaluation revealed a decline in her STMS (25/38), elements of Gerstmann syndrome with mild finger agnosia, right-left confusion, agraphia and acalculia; left visual field neglect, left apraxia, left greater than right diffuse increase in deep tendon reflexes, and bilateral cortical sensory loss with astereognosis bilaterally. Interim decline in visuospatial impairment along with new-onset word finding difficulties were reported with no decline in episodic memory. On repeat neuropsychometric evaluation, she was unable to perform any of the visual tasks involving Benton Facial Recognition, Famous Faces, Visual Form Discrimination, and Boston Naming. There was mild decline in verbal fluency while logical memory skills remained relatively stable. Comprehensive laboratory workup including but not limited to serum paraneoplastic panel, thyroperoxidase antibody, ceruloplasmin, autoimmune markers (c-ANCA, p-ANCA, ANA, ENA, rheumatoid factor), vitamin B12, TSH, complete blood count; cerebrospinal fluid (CSF) studies including Whipple, Lyme, Borrelia burgdorferi PCR, glucose, protein, cell count, 14-3-3 protein level were unremarkable. Magnetic Resonance Imaging (MRI) of the brain at initial presentation showed diffuse mild cortical atrophy, more prominent in the right temporo-parieto-occipital region. Single voxel spectroscopy demonstrated decrease of N-acetylaspartate (NAA) in the right parieto-occipital white matter. MRI re-imaging a year later showed similar findings. 18F-fluorodexyglucose positron emission tomography (FDG-PET) showed decreased metabolism in the corresponding areas, along with left temporoparietal hypometabolism to a lesser extent. Her clinical diagnosis was Probable Posterior Cortical Atrophy. This subject also had APOE ε3/ε4 genotype.
4. DISCUSSION
In this systematic mutation screen of known dementia genes in 124 subjects with clinical PCA or posterior AD pathology, we investigated 115 variants that have been categorized in AD&FTD (Cruts et al., 2012) or HPP mutation databases as “pathogenic”, “pathogenic nature unclear” or “causative”. These variants were selected because they resided in one of the eight genes that have variants that lead to or unequivocally increase risk for AD (APP, PSEN1, PSEN2, APOE, TREM2), FTD (MAPT, GRN) or CJD (PRNP); and also because they reside on the NeuroX chip and passed QC. We identified one rare pathogenic variant (TREM2 Arg47His) and one rare potentially pathogenic variant (PSEN2 Ser130Leu). Although the APOE ε4 allele could not be successfully genotyped with the NeuroX, we were able to detect highly significant association with a different APOE variant on the NeuroX (rs769449, p=1.5×10−19) that is in near perfect linkage disequilibrium with APOE ε4 (D’=1.0, r2=0.80), thus serving as an excellent proxy for APOE ε4, and confirming the highly significant APOE ε4 association with PCA that we had previously reported (Carrasquillo et al., 2014).
Until recently, mutation screens in PCA have been limited to case reports which identified PSEN1 I211M (Sitek et al., 2013), PSEN1 G223R (Saint-Aubert et al., 2013), PSEN2 M239I (Tremolizzo et al., 2014), MAPT V363I (Rossi et al., 2014), GRN R110X (Caroppo et al., 2015), in addition to a family with PCA phenotype in which a 120 base pair octapeptide repeat insertion mutation was identified within PRNP (Depaz et al., 2012). A mutation screen of known dementia genes in a cohort of 227 early-onset probable AD subjects revealed PSEN1 P218L and PSEN1 I238M mutations in two subjects with memory and vision loss, although this was not a specific screen for PCA (Wojtas et al., 2012). These studies highlight the mutation spectrum in AD, FTD and CJD genes which can lead to the clinical presentation of PCA. Amongst these eight reports, five pertain to AD genes, although it is difficult to make inferences regarding mutation frequency based on case reports.
Our study is useful in that it provides estimations of the type and frequency of such mutations in PCA given that it is a sizable cohort rather than a case report. Both rare variants identified in our study are in AD genes (PSEN2 and TREM2), which is consistent with the finding that much of PCA etiopathology is attributed to AD. Since two out of 124 screened subjects had a coding variant, we can estimate that 1.6% of PCA subjects harbor such a variant, likely in an AD gene. However, it must be noted that this is likely an underestimate, considering that our screen was limited to variants present in the NeuroX. In addition, as we have not imposed any age restrictions in our cohort, this estimate is likely to be higher amongst the younger PCA subjects.
This is the first identification of the TREM2 Arg47His variant in a patient with posterior AD pathology. Although we did not have further clinical information on this subject, posterior AD is the pathology characteristic of PCA patients based on prior reports and reported subjects with both clinical and pathologic data (Carrasquillo et al., 2014; Crutch et al., 2012). The pathogenicity of the TREM2 Arg47His variant has been previously validated by replication of its association in numerous AD case-controls series (Guerreiro and Hardy, 2013; Jonsson et al., 2013) and by functional molecular studies (Kleinberger et al., 2014). One of the initial studies on TREM2 association with AD risk (Guerreiro and Hardy, 2013) included pathology descriptions for the TREM2 Arg47His variant and reported typical AD neuropathology. Given this and the role of this variant in AD risk, it is unlikely that TREM2 Arg47His is driving the posterior distribution of AD pathology. Rather, it is likely that this variant is involved in pathways common to both typical AD and PCA, with other genetic and/or environmental factors influencing vulnerability of posterior regions in some subjects.
The PSEN2 Ser130Leu identified in our NeuroX screen has likewise not been previously reported in a PCA patient. However, a different PSEN2 variant (Met239Ile) was previously reported in a PCA case report (Tremolizzo et al., 2014). Others have argued that PSEN2 Ser130Leu is a benign variant (Sassi et al., 2014; Wojtas et al., 2012), based on its presence in 1 control out of 179 (MAF=0.005) and the fact that this amino acid change is localized in the hydrophilic loop 1 in which deterministic AD mutations have not been reported. However, this variant is predicted to be possibly damaging by Polyphen2 (Adzhubei et al., 2013), and was only observed in 9 out of 8591 European-American population controls from the ESP (PSEN2 Ser130Leu, ESP control MAF=0.001), which is even lower than that reported in ESP controls for the TREM2 Arg47His variant (MAF=0.0026). It is possible that like TREM2 Arg47His, and unlike the Mendelian PSEN2 mutations, PSEN2 Ser130Leu is a risk, and not a deterministic variant, for both typical AD and PCA. Molecular studies will be needed to test the effect of the PSEN2 Ser130Leu variant on functional outcomes including APP processing.
Although the association of the TREM2 Arg47His and PSEN2 Ser130Leu variants with PCA risk does not reach significance given their rarity, they appear to be enriched in the PCA cohort vs. population controls from ESP. It should be noted that both subjects with these variants have APOE ε3/ε4 genotype. The potential interaction of APOE ε4 with either rare variant in leading to PCA remains to be established, however at least for the subject with PSEN2 Ser130Leu variant, the presence of two pathogenic variants likely led to the very early age at onset at 38.
We acknowledge that analyzing jointly the clinical PCA and posterior AD cases may introduce heterogeneity into our analyses because many of the posterior AD cases lack clinical information thus raising possibility that some of the posterior AD subjects might not have fit a clinical diagnosis of PCA. However, the correlation between a clinical diagnosis of PCA and a neuropathology classification of posterior AD has been published by several other groups, noting a very high (80–100%) concordance rate (Ala et al., 1996; Alladi et al., 2007; Renner et al., 2004; Tang-Wai et al., 2004; Victoroff et al., 1994). Also, in a previous publication (Carrasquillo et al., 2014), we demonstrated that there are no significant differences between clinical PCA and posterior AD cases, in terms of percentage of affected females or the direction of risk association with APOE ε4, APOE ε2, CLU or BIN alleles. Therefore, we have analyzed clinical PCA and posterior AD cases jointly, as this study design provides more power to detect PCA risk factors, by increasing the sample size.
In addition to the results on this first pathogenic dementia variant screen in PCA, our study also provides another application for the customized array NeuroX, which includes exome content on variants selected from 12,000 individual exome and whole genomes (Grove et al., 2013), as well as custom content based on mutations or variants associated with neurological diseases, including AD and FTD GWAS hits, rare variants with large effect sizes, and rare variants identified through exome sequencing studies (Nalls et al., 2015). Although NeuroX could potentially lack rare, novel PCA susceptibility variants, it is an efficient platform for evaluating the effect of known putative functional variation, as its content represents ~95% of the known exome variants. That said, caution needs to be exercised in interpreting the results of NeuroX, as we and others (Ghani et al., 2015) determined an error in assay design/annotation for the GRN Gln130fs variant, which in fact denotes a non-pathogenic synonymous variant (rs25646). Further, assays can be prone to failure, such as the APOE ε4 (rs429358) pathogenic variant, which could lead to inability to assess all possible coding mutations in genes of interest.
In summary, in the present study we demonstrate that some of the coding variability in TREM2 and PSEN2 that has been previously implicated in AD is also present in posterior AD and PCA cases, respectively, hence expanding the mutation spectrum for this condition. We also validate the APOE ε4 association that we reported previously. Taken together with our previous finding of the association of AD GWAS loci with PCA risk (Carrasquillo et al., 2014), our studies provide novel information towards elucidating the genetic underpinnings of this disease by identifying specific genetic variants that are common to AD and PCA. Further investigation is needed to identify genetic factors that preferentially influence the vulnerability of posterior regions in PCA. Studies that investigate the newer AD risk loci (Lambert et al., 2013) as well as novel genes and variants should prove invaluable towards the refinement of our understanding of PCA pathophysiology and future treatments.
Highlights.
First systematic mutation screen of known dementia genes in a sizable PCA cohort
NeuroX used to detect the mutation frequency and spectrum in PCA
Variants compared against controls from the NHLBI Exome Sequencing Project
Two rare variants not previously reported in PCA, TREM2 Arg47His and PSEN2 Ser130Leu
Acknowledgments
Support for this research was provided by the National Institutes of Health grants: National Institute on Aging (R01 AG032990 to NET; U01 AG046139 to NET and SGY; and R01 AG018023 to NRG-R and SGY; AG025711, AG017216, AG003949 to DWD); National Institutes on Neurologic Diseases and Stroke (R01 NS080820 to NET), Mayo Alzheimer’s Disease Research Center: (P50 AG016574 to DWD, NRG-R, SGY, and NET); MMC is supported in part by an MNIRGD Alzheimer’s Association grant. IB is supported by a Joint Alzheimer’s Research UK/University of Nottingham Faculty of Medicine PhD studentship. The work in the KM lab is supported by funding from ARUK and KM is a member of the Alzheimer’s Society Grant Advisory Board. We thank the patients and their families for their participation, without whom these studies would not have been possible.
Footnotes
DISCLOSURE STATEMENT
B. Boeve, M.D. has served as an investigator for clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals and GE Healthcare, and serves on the Scientific Advisory Board of the Tau Consortium. N. Graff-Radford, M.D. has served as a consultant to Codman and received grant support from Elan Pharmaceutical Research, Pfizer Pharmaceuticals, Medivation, and Forrest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7(Unit 7):20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala TA, Frey WH, 2nd, Clark HB. Posterior cortical atrophy: neuropathological correlations. Arch Neurol. 1996;53(10):958. doi: 10.1001/archneur.1996.00550100020006. [DOI] [PubMed] [Google Scholar]
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130(Pt 10):2636–45. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009;2009(10) doi: 10.1101/pdb.ip71. pdb ip71. [DOI] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Carney RM, Kohli MA, Kunkle BW, Naj AC, Gilbert JR, Zuchner S, Pericak-Vance MA. Parkinsonism and distinct dementia patterns in a family with the MAPT R406W mutation. Alzheimers Dement. 2014;10(3):360–5. doi: 10.1016/j.jalz.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Belin C, Grabli D, Maillet D, De Septenville A, Migliaccio R, Clot F, Lamari F, Camuzat A, Brice A, Dubois B, Le Ber I. Posterior Cortical Atrophy as an Extreme Phenotype of GRN Mutations. JAMA Neurol. 2015;72(2):224–8. doi: 10.1001/jamaneurol.2014.3308. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, Khan Q, Murray ME, Krishnan S, Aakre J, Pankratz VS, Nguyen T, Ma L, Bisceglio G, Petersen RC, Younkin SG, Dickson DW, Boeve BF, Graff-Radford NR, Ertekin-Taner N. Late-onset Alzheimer disease genetic variants in posterior cortical atrophy and posterior AD. Neurology. 2014;82(16):1455–62. doi: 10.1212/WNL.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170–8. doi: 10.1016/S1474-4422(11)70289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Theuns J, Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat. 2012;33(9):1340–4. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza LC, Corlier F, Habert MO, Uspenskaya O, Maroy R, Lamari F, Chupin M, Lehericy S, Colliot O, Hahn-Barma V, Samri D, Dubois B, Bottlaender M, Sarazin M. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer’s disease. Brain. 2011;134(Pt 7):2036–43. doi: 10.1093/brain/awr130. [DOI] [PubMed] [Google Scholar]
- Depaz R, Haik S, Peoc’h K, Seilhean D, Grabli D, Vicart S, Sarazin M, DeToffol B, Remy C, Fallet-Bianco C, Laplanche JL, Fontaine B, Brandel JP. Long-standing prion dementia manifesting as posterior cortical atrophy. Alzheimer Dis Assoc Disord. 2012;26(3):289–92. doi: 10.1097/WAD.0b013e318231e449. [DOI] [PubMed] [Google Scholar]
- Ghani M, Lang AE, Zinman L, Nacmias B, Sorbi S, Bessi V, Tedde A, Tartaglia MC, Surace EI, Sato C, Moreno D, Xi Z, Hung R, Nalls MA, Singleton A, St George-Hyslop P, Rogaeva E. Mutation analysis of patients with neurodegenerative disorders using NeuroX array. Neurobiol Aging. 2015;36(1):545, e9–14. doi: 10.1016/j.neurobiolaging.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rosser M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Grove ML, Yu B, Cochran BJ, Haritunians T, Bis JC, Taylor KD, Hansen M, Borecki IB, Cupples LA, Fornage M, Gudnason V, Harris TB, Kathiresan S, Kraaij R, Launer LJ, Levy D, Liu Y, Mosley T, Peloso GM, Psaty BM, Rich SS, Rivadeneira F, Siscovick DS, Smith AV, Uitterlinden A, van Duijn CM, Wilson JG, O’Donnell CJ, Rotter JI, Boerwinkle E. Best practices and joint calling of the HumanExome BeadChip: the CHARGE Consortium. PLoS One. 2013;8(7):e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Bras J, Wojtas A, Rademakers R, Hardy J, Graff-Radford N. A nonsense mutation in PRNP associated with clinical Alzheimer’s disease. Neurobiol Aging. 2014;35(11):2656, e13–6. doi: 10.1016/j.neurobiolaging.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Hardy J. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369(16):1569–70. doi: 10.1056/NEJMc1306509. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleo A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sanchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, van der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M, Haass C. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6(243):243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, Destefano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thornton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Hollingworth P, Ramirez A, Hanon O, Fitzpatrick AL, Buxbaum JD, Campion D, Crane PK, Baldwin C, Becker T, Gudnason V, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MJ, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuinness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannfelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltunen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–8. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136(Pt 3):844–58. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Bras J, Hernandez DG, Keller MF, Majounie E, Renton AE, Saad M, Jansen I, Guerreiro R, Lubbe S, Plagnol V, Gibbs JR, Schulte C, Pankratz N, Sutherland M, Bertram L, Lill CM, DeStefano AL, Faroud T, Eriksson N, Tung JY, Edsall C, Nichols N, Brooks J, Arepalli S, Pliner H, Letson C, Heutink P, Martinez M, Gasser T, Traynor BJ, Wood N, Hardy J, Singleton AB. NeuroX, a fast and efficient genotyping platform for investigation of neurodegenerative diseases. Neurobiol Aging. 2015;36(3):1605, e7–e12. doi: 10.1016/j.neurobiolaging.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Lehmann M, Yokoyama JS, Karydas A, Lee JJ, Coppola G, Grinberg LT, Geschwind D, Seeley WW, Miller BL, Rosen H, Rabinovici G. Progranulin mutations as risk factors for Alzheimer disease. JAMA Neurol. 2013;70(6):774–8. doi: 10.1001/2013.jamaneurol.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175–80. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St George-Hyslop PH. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376(6543):775–8. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76(21):1789–96. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Bastone A, Piccoli E, Morbin M, Mazzoleni G, Fugnanesi V, Beeg M, Del Favero E, Cantu L, Motta S, Salsano E, Pareyson D, Erbetta A, Elia AE, Del Sorbo F, Silani V, Morelli C, Salmona M, Tagliavini F. Different mutations at V363 MAPT codon are associated with atypical clinical phenotypes and show unusual structural and functional features. Neurobiol Aging. 2014;35(2):408–17. doi: 10.1016/j.neurobiolaging.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Saint-Aubert L, Payoux P, Hannequin D, Barbeau EJ, Campion D, Delisle MB, Tafani M, Viallard G, Peran P, Puel M, Chollet F, Demonet JF, Pariente J. MR, (18)F-FDG, and (18)F-AV45 PET correlate with AD PSEN1 original phenotype. Alzheimer Dis Assoc Disord. 2013;27(1):91–4. doi: 10.1097/WAD.0b013e318251d87c. [DOI] [PubMed] [Google Scholar]
- Sassi C, Guerreiro R, Gibbs R, Ding J, Lupton MK, Troakes C, Al-Sarraj S, Niblock M, Gallo JM, Adnan J, Killick R, Brown KS, Medway C, Lord J, Turton J, Bras J, Morgan K, Powell JF, Singleton A, Hardy J. Investigating the role of rare coding variability in Mendelian dementia genes (APP, PSEN1, PSEN2, GRN, MAPT, and PRNP) in late-onset Alzheimer’s disease. Neurobiol Aging. 2014;35(12):2881, e1–6. doi: 10.1016/j.neurobiolaging.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin J, Formaglio M, Perret-Liaudet A, Quadrio I, Tholance Y, Rouaud O, Thomas-Anterion C, Croisile B, Mollion H, Moreaud O, Salzmann M, Dorey A, Bataillard M, Coste MH, Vighetto A, Krolak-Salmon P. CSF biomarkers in posterior cortical atrophy. Neurology. 2011;76(21):1782–8. doi: 10.1212/WNL.0b013e31821ccc98. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Sitek EJ, Narozanska E, Peplonska B, Filipek S, Barczak A, Styczynska M, Mlynarczyk K, Brockhuis B, Portelius E, Religa D, Barcikowska M, Slawek J, Zekanowski C. A patient with posterior cortical atrophy possesses a novel mutation in the presenilin 1 gene. PLoS One. 2013;8(4):e61074. doi: 10.1371/journal.pone.0061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Susani E, Mapelli C, Isella V, Bertola F, Ferrarese C, Appollonio I. First Report of PSEN2 Mutation Presenting as Posterior Cortical Atrophy. Alzheimer Dis Assoc Disord. 2014 doi: 10.1097/WAD.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Victoroff J, Ross GW, Benson DF, Verity MA, Vinters HV. Posterior cortical atrophy. Neuropathologic correlations. Arch Neurol. 1994;51(3):269–74. doi: 10.1001/archneur.1994.00540150063018. [DOI] [PubMed] [Google Scholar]
- Wojtas A, Heggeli KA, Finch N, Baker M, Dejesus-Hernandez M, Younkin SG, Dickson DW, Graff-Radford NR, Rademakers R. C9ORF72 repeat expansions and other FTD gene mutations in a clinical AD patient series from Mayo Clinic. Am J Neurodegener Dis. 2012;1(1):107–18. [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]