Abstract
Objective
To examine relationships between prenatal perfluoroalkyl substance (PFAS) exposure and adiposity in children born to women who lived downstream from a fluoropolymer manufacturing plant.
Methods
Data are from a prospective cohort in Cincinnati, OH (HOME Study). We measured perfluorooctanoic (PFOA), perfluorooctane sulfonic (PFOS), perfluorononanoic (PFNA), and perfluorohexane sulfonic (PFHxS) acids in prenatal serum samples. We estimated differences in body mass index z-scores (BMI), waist circumference, and body fat at 8 years of age (n=204) and BMI between 2–8 years of age (n=285) according to PFAS concentrations.
Results
Children born to women in the top two PFOA terciles had greater adiposity at 8 years than children in the 1st tercile. For example, waist circumference (cm) was higher among children in the 2nd (4.3; 95% CI:1.7, 6.9) and 3rd tercile (2.2; 95% CI:−0.5, 4.9) compared to children in the 1st tercile. Children in the top two PFOA terciles also had greater BMI gains from 2–8 years compared to children in the 1st tercile (p<0.05). PFOS, PFNA and PFHxS were not associated with adiposity.
Conclusions
In this cohort, higher prenatal serum PFOA concentrations were associated with greater adiposity at 8 years and a more rapid increase in BMI between 2–8 years.
Keywords: Adiposity, children, endocrine disrupting chemicals, obesity, obesogens, perfluoroalkyl substances
Introduction
Childhood obesity is a major public health problem in the United States (US), where 17% of children are obese and another 15% are overweight.1 Excess adiposity is difficult to reverse once established and increases the risk of cardiometabolic, pulmonary, and musculoskeletal disorders.2 There is growing evidence that prenatal chemical exposures may play a role in childhood obesity risk by perturbing biological pathways involved in energy metabolism, appetite, or adipogenesis.3
Perfluoroalkyl substances (PFAS), which have been used in oil and water resistant textile coatings, non-stick cookware, food container coatings, floor polish, fire-fighting foam, and industrial surfactants, are a class of suspected obesogens that are persistent in the environment and humans.4–6 Biomonitoring studies demonstrate nearly ubiquitous exposure to four PFAS in the US population: perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS).7,8
Some experimental rodent and in vitro studies show that PFAS exposure may cause impaired glucose homeostasis, increased body weight, and altered adipocyte differentiation,9–12 while others do not.13 Four prospective epidemiological studies have examined the relationship between early life PFAS exposure and child or adult adiposity (Table S1).14–17 Two studies reported no associations between prenatal or early childhood PFAS exposure and child or adult adiposity.14,15 A third study reported that maternal serum PFOA concentrations during pregnancy were associated with increased adiposity, risk of being overweight or obese, and serum leptin concentrations in adults.16 A fourth study reported more rapid weight gain in girls born to women with higher serum PFOS concentrations during pregnancy.17
We used data from a cohort of pregnant women with median serum PFOA concentrations that were over two-times higher than pregnant women in the US to examine the relationship between prenatal PFAS exposures and longitudinal measures of adiposity in children between 2 and 8 years of age.
Methods
Study Participants
We used data from the HOME Study, a prospective cohort study designed to examine the impact of early life exposure to prevalent environmental chemicals.18,19 We recruited pregnant women from nine prenatal clinics associated with three hospitals in the Cincinnati, Ohio area from March 2003 to January 2006. The eligibility criteria at enrollment were: 1) 16±3 weeks gestation, 2) ≥18 years old, 3) living in a home built before 1978, 4) no history of HIV infection, and 5) not taking any medications for seizure or thyroid disorders. After research assistants explained study protocols, all women provided written informed consent for themselves and their children. The institutional review boards of Cincinnati Children’s Hospital Medical Center, the cooperating delivery hospitals, and the Centers for Disease Control and Prevention (CDC) approved this study.
Of the 468 women who initially enrolled in our study, 65 dropped out before delivery. We excluded ten women who delivered twins and three who delivered stillborn children. Among the remaining 390 singleton children, 222 (57%) completed a follow-up visit at an average of 8.1 years of age (range: 7.5–10). Our primary analysis of adiposity at 8 years of age included 204 (52%) children after excluding one with a genetic abnormality, three who were missing covariates, and 14 whose mothers were missing PFAS concentrations. We conducted additional analyses examining BMI, weight, and height z-score trajectories between 2 and 8 years of age. Of 316 (81%) children who returned to our study clinic at least once between 2 and 8 years of age, we excluded one with a genetic abnormality, four who were missing covariates, and 26 whose mothers were missing PFAS concentrations. These 285 (73%) children returned for 1,021 study visits at an average of 2.1 (n=243), 3.1 (n=219), 4.1 (n=167), 5.2 (n=188), or 8.1 (n=204) years of age.
Serum PFAS Concentrations
We collected serial blood samples from women at 16 and 26 weeks gestation and delivery and measured serum PFOA, PFOS, PFNA, and PFHxS concentrations using online solid phase extraction coupled to high performance liquid chromatography-isotope dilution tandem mass spectrometry.20 We analyzed 16-week samples in the majority of women (n=173, 87%) to reduce the potential impact of pregnancy-induced changes in serum volume. Because some women did not have a sufficient volume of serum from their 16 week sample to quantify PFAS concentrations, we used their 26-week (n=19, 9%) or birth sample (n=8, 4%). The limits of detection for this assay were 0.082 ng/mL (PFNA), 0.1 ng/mL (PFHxS, PFOA), and 0.2 ng/mL (PFOS). We detected the four PFAS in all samples. Each analytic batch included reagent blanks and quality control (QC) materials. The coefficients of variation of repeated measurements of the QC materials were ~6%.
Child Anthropometry
Children and their parents returned to our study clinic at 2, 3, 4, 5, and 8 years of age. At each study visit, we measured weight to the nearest 0.01 kg with the child dressed in undergarments or a dry diaper using a ScaleTronix scale (White Plains, NY). We measured height at these same study visits to the nearest 0.1 cm with the child standing straight without shoes or head coverings and heels positioned against the wall using an Ayrton Stadiometer Model S100 (Prior Lake, MN). At 8 years of age, waist circumference was measured by placing a plastic measuring tape around a horizontal plane defined by the left and right iliac crests. We also measured children’s body fat using a Tanita children’s body fat monitor at 8 years of age (Arlington Heights, IL). We calculated age (in months)- and sex-specific BMI z-scores using US references and considered children overweight or obese if their BMI z-score was ≥85th percentile according to NCHS standards.21
Confounding Variables
We selected potential confounders that might be associated with both PFAS concentrations and child adiposity using directed acyclic graphs (DAGs). Trained research assistants collected covariates using standardized computer-assisted interviews and medical chart reviews. Sociodemographic covariates included maternal race, age, education, marital status, employment, and household income. Perinatal variables included maternal depressive symptoms at 16 weeks gestation (Beck Depression Inventory-II),22 BMI at 16 weeks gestation, parity, and serum cotinine (a biomarker of tobacco smoke exposure).23 Dietary variables included the frequency of fresh fruit/vegetable and fish consumption during pregnancy, as well as prenatal vitamin use.
Statistical Analyses
First, we calculated univariate statistics of PFOA concentrations and BMI z-scores for each covariate. We also compared the distribution of PFAS concentrations among HOME Study women to the concentration distributions among pregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) during the 2003–2004 and 2005–2006 cycles.
Next, we examined the shape of the relationship between natural log-transformed PFAS concentrations and adiposity measurements at 8 years of age using 3-knot restricted cubic polynomial splines. We determined if there were non-linear associations by testing the significance of the non-linear spline term and examining graphs of the splines.24 We observed non-linear associations between PFOA and BMI (non-linearity p-value=0.012), waist circumference (non-linearity p-value=0.152), and body fat percent (non-linearity p-value=0.028). Thus, we examined PFOA using both splines and terciles. The associations between PFOS, PFNA, and PFHxS were all linear (non-linearity p-values≥0.199), and we categorized them as terciles. We quantified the risk of being overweight or obese at 8 years of age according to PFAS terciles using Poisson regression with robust standard errors.
We modeled children’s BMI z-scores between 2 and 8 years of age as a function of PFAS tercile, child age in months, an interaction term between age and PFAS tercile, and covariates using a linear mixed model with an unstructured covariance matrix to account for the correlated and repeated outcome measurements. This model allowed us to estimate the average linear BMI z-score slope between 2 and 8 years of age among children in each PFAS tercile and determine if these slopes statistically differed from one another.
Finally, because prior studies have observed sex-specific associations between PFAS and adiposity, we examined whether child sex modified the association between PFAS concentrations and child adiposity at 8 years of age using a product interaction term between child sex and PFAS terciles.16
Sensitivity Analyses
We also adjusted for prenatal urinary bisphenol A (BPA) concentrations because this chemical is a suspected obesogen.18 We measured BPA concentrations in urine samples collected from mothers at 16 and 26 weeks gestation using previously described methods.25 We also adjusted for duration of any breastfeeding and weekly gestational weight gain. Finally, we excluded infants who were born small for gestational age (<10th birth weight for sex and gestational age percentile) or low birth weight (<2,500 grams).
Results
Geometric mean PFOA (5.4 vs. 5.5 ng/mL), PFOS (13 vs. 13 ng/mL), PFNA (0.9 vs. 1.0 ng/mL), and PFHxS (1.4 vs. 1.6 ng/mL) concentrations were similar among women whose children did and did not complete follow-up at 8 years of age. On average, women included in the analysis were 29 years old at delivery (standard deviation [SD]: 5.9) and had an annual household income of $55,000 (SD: $42,000). Sixty percent were Caucasian, 45% were nulliparous, 62% were married, and 45% had a college degree or greater (Table 1).
Table 1.
Maternal serum PFOA concentrations (ng/mL) and child BMI z-scores at 8 years of age according to maternal sociodemographic, perinatal, dietary, and environmental factors during pregnancy (HOME Study)
| Variable | N (%) | PFOA Med (25th, 75th) |
BMI Z-Score Mean (SD) |
|---|---|---|---|
| Overall | 204 | 5.3 (3.7, 7.7) | 0.35 (1.0) |
| Maternal Age | |||
| 18–25 years | 55 (27) | 6.1 (4.4, 8.4) | 0.41 (1.06) |
| >25–35 years | 120 (59) | 4.7 (3.6, 7.0) | 0.37 (0.98) |
| >35 years | 29 (14) | 5.5 (4.0, 9.4) | 0.19 (0.97) |
| p-value | 0.144 | 0.628 | |
| Maternal Race | |||
| White | 122 (60) | 5.6 (3.8, 9.0) | 0.18 (0.94) |
| Black | 72 (35) | 4.9 (3.7, 6.2) | 0.67 (0.96) |
| Other | 10 (5) | 6.4 (3.7, 10) | 0.25 (1.44) |
| p-value | 0.007 | 0.003 | |
| Maternal Education | |||
| Bachelor's/Grad/Professional | 92 (45) | 5.4 (3.9, 8.9) | 0.32 (0.92) |
| Tech school/Some College | 59 (29) | 5.4 (3.6, 7.3) | 0.01 (1.05) |
| High School or Less | 53 (26) | 5.0 (3.8, 7.2) | 0.80 (0.93) |
| p-value | 0.612 | <0.0001 | |
| Marital Status | |||
| Married | 126 (62) | 5.3 (3.7, 8.9) | 0.25 (0.97) |
| Not Married | 78 (38) | 5.4 (4.0, 7.2) | 0.52 (1.02) |
| p-value | 0.330 | 0.067 | |
| Income | |||
| >$80,000 | 52 (25) | 5.7 (3.8, 9.9) | 0.26 (0.87) |
| $40–80,000 | 65 (32) | 5.3 (4.2, 7.2) | 0.24 (0.95) |
| $20–40,000 | 33 (16) | 5.5 (4.0, 6.6) | 0.36 (1.09) |
| <$20,000 | 54 (26) | 4.8 (3.6, 6.6) | 0.58 (1.10) |
| p-value | 0.096 | 0.256 | |
| Parity | |||
| 0 | 92 (45) | 6.5 (5.1, 9.6) | 0.27 (1.03) |
| 1 | 59 (29) | 4.6 (3.5, 5.7) | 0.37 (0.95) |
| 2+ | 53 (26) | 4.2 (3.4, 7.1) | 0.48 (1.00) |
| p-value | <0.0001 | 0.473 | |
| Depressive Symptoms | |||
| Minimal: <14 | 160 (78) | 5.4 (3.7, 8.1) | 0.28 (0.97) |
| Mild: 14–19 | 25 (12) | 6.0 (4.1, 8.8) | 0.45 (1.01) |
| Moderate/Severe | 19 (9) | 4.3 (3.5, 5.3) | 0.82 (1.17) |
| p-value | 0.012 | 0.072 | |
| Serum Cotinine | |||
| <LOD | 66 (32) | 4.7 (3.5, 6.2) | 0.26 (0.91) |
| LOD - 3 ng/mL | 115 (56) | 6.0 (4.2, 9.0) | 0.38 (1.03) |
| >3 ng/mL | 23 (11) | 4.3 (3.6, 5.5) | 0.52 (1.11) |
| p-value | 0.052 | 0.517 | |
| Employment | |||
| Unemployed | 40 (20) | 5.0 (3.3, 5.9) | 0.51 (1.08) |
| Employed | 164 (80) | 5.5 (4, 8.3) | 0.32 (0.98) |
| p-value | 0.040 | 0.263 | |
| Fresh Fruit and Veg | |||
| Monthly | 25 (12) | 5.8 (3.3, 6.6) | 0.37 (0.72) |
| Weekly | 109 (53) | 5.5 (4.2, 7.9) | 0.36 (0.99) |
| Daily or More | 70 (34) | 4.7 (3.5, 8.8) | 0.34 (1.10) |
| p-value | 0.066 | 0.983 | |
| BMI | |||
| <25 | 85 (42) | 5.4 (3.9, 8.9) | −0.01 (0.96) |
| 25–30 | 67 (33) | 5.0 (3.7, 7.7) | 0.39 (0.86) |
| >30 | 52 (25) | 5.2 (3.7, 7.3) | 0.90 (0.98) |
| p-value | 0.469 | <0.001 | |
| Prenatal Vitamins | |||
| Rarely/Never | 29 (14) | 5.0 (3.8, 6.0) | 0.46 (1.18) |
| Weekly/Daily | 175 (86) | 5.4 (3.7, 8.1) | 0.34 (0.97) |
| p-value | 0.170 | 0.517 | |
| Fish Intake | |||
| None | 29 (14) | 5.3 (3.7, 7.4) | 0.60 (0.09) |
| Any | 175 (86) | 5.4 (3.7, 7.7) | 0.31 (1.01) |
| p-value | 0.780 | 0.145 |
The p-values are from linear regression models regressing BMI z-scores or natural log-transformed PFOA concentrations on each covariate.
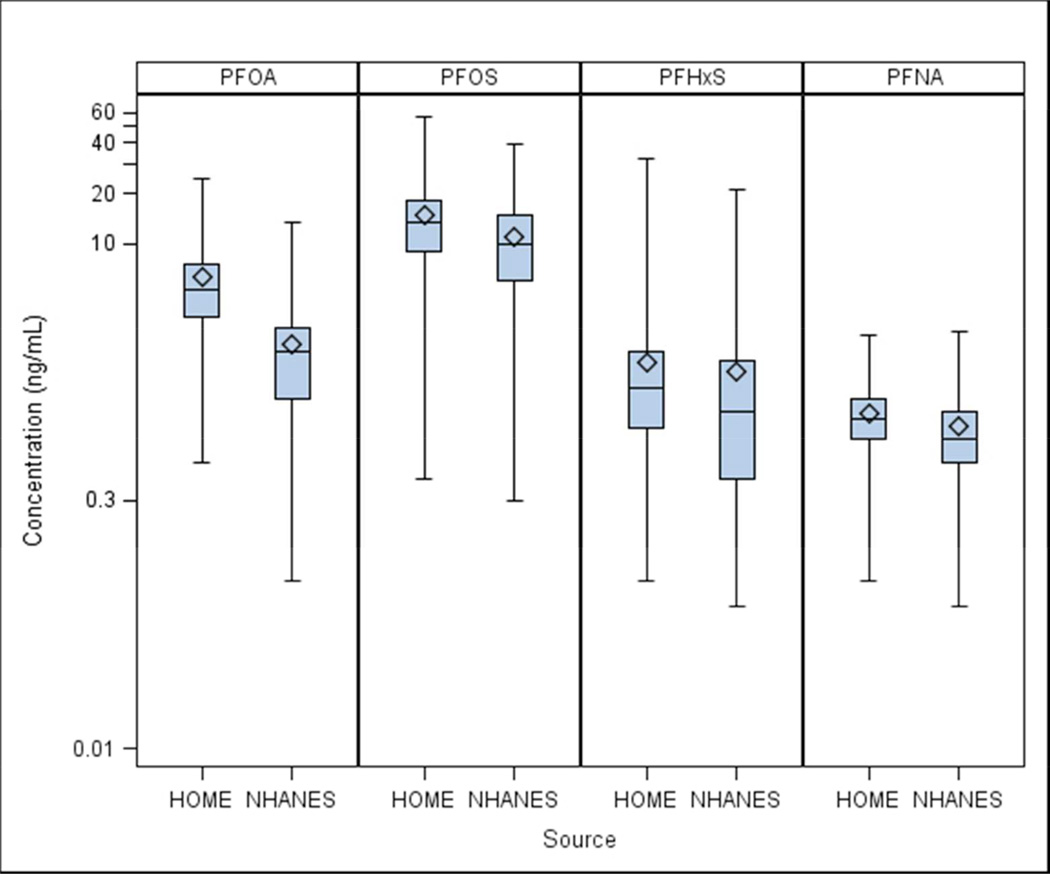
Median PFOA concentrations were over 2-times as high among HOME Study women compared to pregnant women in the NHANES 2003–2004 and 2005–2006 cycles (5.3 vs. 2.3 ng/mL) (Figure 1, Table S2). Notably, the 5th percentile of PFOA concentrations (2.4 ng/mL) among HOME Study women was approximately the median among NHANES women. Median PFOS concentrations were slightly higher in the HOME Study women compared with NHANES women (13 vs. 11 ng/mL); PFHxS and PFNA concentrations were similar in both groups of women.
Figure 1.
Serum PFAS concentrations in pregnant women from the NHANES in the US (n=174) and pregnant women from the HOME Study in Cincinnati, OH (n=204) (2003–2006)
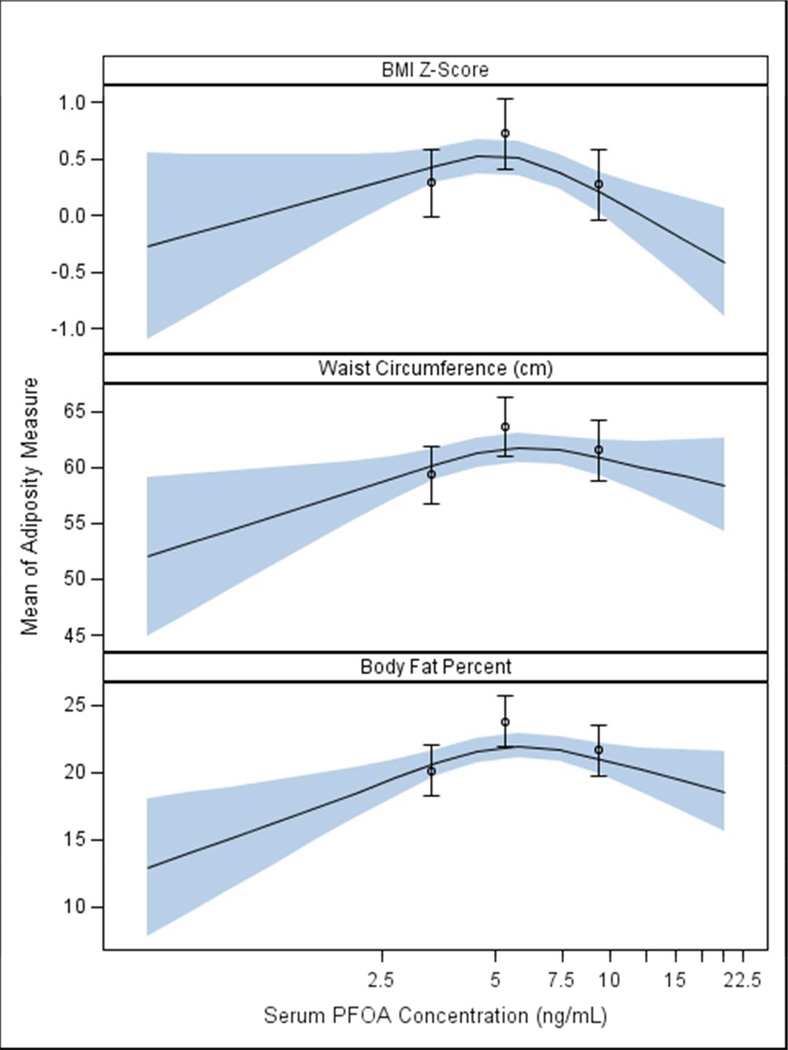
After adjusting for covariates, the association between PFOA concentrations and all three adiposity measurements was positive up to the 50th percentile of PFOA concentrations and then declined at concentrations above the 50th percentile (Figure 2 and Table S3). We observed a similar dose-response relationship when we examined PFOA terciles and child adiposity (Table 2). For example, compared to children in the 1st PFOA tercile, body fat was elevated among children born to women in the 2nd tercile (3.6%; 95% CI: 1.8, 5.5), but less elevated among children in the 3rd tercile (1.5 %; 95% CI: −0.4, 3.4). Children born to women in the 2nd and 3rd terciles of PFOA concentrations had an 84% (RR: 1.84; 95% CI: 0.97, 3.50) and 54% (RR: 1.54; 95% CI: 0.77, 3.07) increased risk of being overweight or obese at 8 years of age compared to children born to women in the 1st tercile, respectively.
Figure 2.
Adjusted restricted cubic polynomial spline of maternal serum PFOA concentrations during pregnancy and child BMI z-score, waist circumference, and body fat percent at 8 years of age among HOME Study women and their children (n=204)a
a-Adjusted for maternal age, race, education, income, parity, employment, marital status, depressive symptoms, BMI at 16 weeks gestation, fruit/vegetable consumption, fish consumption, prenatal vitamin use, and maternal serum cotinine concentrations. The waist circumference model is also adjusted for child age in months.
The solid line represents the mean BMI, waist circumference, or body fat percent from the adjusted restricted cubic polynomial spline. The shaded area is the 95% confidence interval. The non-linearity p-values were 0.002, 0.019, and 0.002 for BMI z-scores, waist circumference, and body fat % models, respectively. Estimated mean differences in adiposity at 8 years of age according to various maternal PFOA concentrations are presented in Table S3.
The point estimates represent the mean BMI, waist circumference, or body fat percent for the 1st (0.5–4.2 ng/mL), 2nd (4.3–6.4 ng/mL), and 3rd (6.6–25 ng/mL) maternal PFOA terciles. The errors bars represent the 95% CI of these means.
Table 2.
Adjusted association between maternal serum PFOA concentrations during pregnancy and child adiposity measurements at 8 years of age (n=204)a
| PFAS Tercile | N Overweight or Obese/N Total |
Median (Range) of Concentrations (ng/mL) |
BMI z-score Difference (95% CI) |
Waist Circumference Difference (95% CI) |
Body Fat Percent Difference (95% CI) |
RR for overweight/ obesity (95% CI)b |
|---|---|---|---|---|---|---|
| PFOA Tercile 1 | 13/67 | 3.3 (0.5–4.2) | Ref | Ref | Ref | Ref |
| PFOA Tercile 2 | 21/68 | 5.3 (4.3–6.4) | 0.44 (0.13, 0.74) | 4.3 (1.7, 6.9) | 3.6 (1.8, 5.5) | 1.84 (0.97, 3.50) |
| PFOA Tercile 3 | 17/69 | 9.4 (6.6–25) | −0.01 (−0.33, 0.30) | 2.2 (−0.5, 4.9) | 1.5 (−0.4, 3.4) | 1.54 (0.77, 3.07) |
-Adjusted for maternal age, race, education, income, parity, employment, marital status, depressive symptoms, BMI at 16 weeks gestation, fruit/vegetable consumption, fish consumption, prenatal vitamin use, and maternal serum cotinine concentrations. The waist circumference model is also adjusted for child age in months.
-Defined as having a BMI z-score ≥ 1.0364 at 8 years of age.
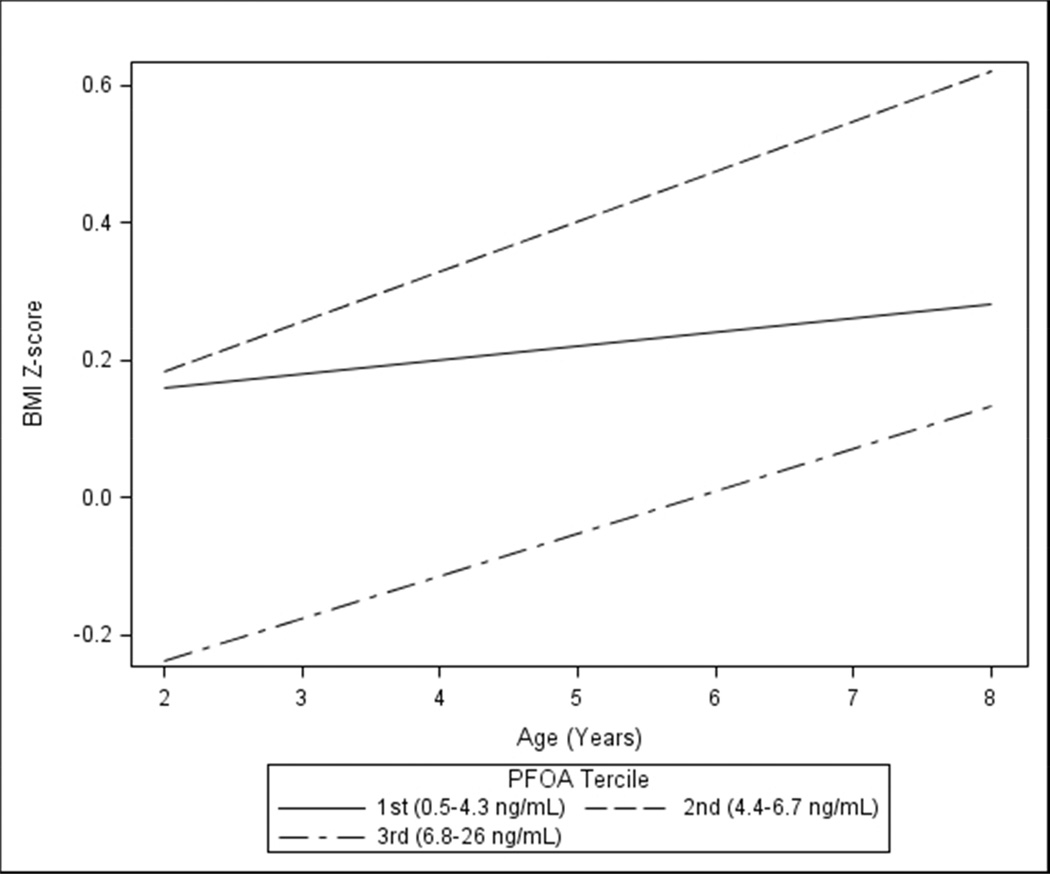
Between 2 and 8 years of age, BMI z-scores increased at a greater rate among children in the 2nd (0.44; 95% CI: 0.23, 0.64; 2nd PFOA tercile×age p-value=0.033) and 3rd (0.37; 95% CI: 0.14, 0.60; 3rd PFOA tercile×age p-value=0.110) PFOA tercile compared to children in the 1st tercile (0.12; 95% CI: −0.08, 0.32) (Figure 3). The BMI z-score slope of the top two terciles was significantly greater than the slope of the 1st tercile (PFOA×age p-value=0.03). These increases in BMI z-score were driven by greater increases in weight relative to height between 2 and 8 years of age among children born to women in the top two serum PFOA terciles (Supplemental Figure S1).
Figure 3.
Adjusted BMI z-scores slopes between 2 and 8 years of age by maternal PFOA concentration tercile among HOME Study women and their children (n=285, 1,012 repeated observations)a
a-Adjusted for maternal age, race, education, income, parity, employment, marital status, depressive symptoms, BMI at 16 weeks gestation, fruit/vegetable consumption, fish consumption, prenatal vitamin use, maternal serum cotinine concentrations, and child age in months.
PFOS, PFHxS or PFNA concentrations were not associated with child adiposity at 8 years of age (Table S4) or changes in BMI z-scores between 2 and 8 years of age (all effect measure modification p-value between PFAS tercile and age>0.23).
Child sex did not modify the associations between maternal PFOA, PFOS, or PFNA concentrations and adiposity at 8 years of age (effect measure modification p-values>0.20). Our results were similar when we excluded children who were born low birth weight or small for gestational age, and when we adjusted for prenatal urinary BPA concentrations, breastfeeding duration, or gestational weight gain.
Discussion
In this prospective cohort of women with serum PFOA concentrations above the national average, we observed that higher PFOA concentrations during pregnancy were associated with greater BMI, waist circumference, and body fat in their children at 8 years of age. The association between PFOA concentrations and child adiposity was non-linear; adiposity did not increase further among children born to women with PFOA concentrations above the 50th percentile. The increase in body fat we observed among children born to women in the 2nd and 3rd PFOA terciles was equivalent to a 1.1 and 0.4 kg increase in body fat for a child of average weight in the cohort at 8 years of age (29 kg), respectively. In addition, higher PFOA concentrations during pregnancy were associated with greater gains in child BMI between 2 and 8 years of age. In contrast, maternal serum PFOS, PFNA and PFHxS concentrations were not associated with child adiposity at 8 years of age.
Women in our cohort had above average PFOA exposures, with approximately 50% having serum PFOA concentrations greater than or equal to the 95th percentile of pregnant women in the US at the same time (2003–2006). This allowed us to examine the dose-response relationship at these higher levels that were still within the range of interest for the general population. The higher serum PFOA concentrations among women in our cohort could be due to ingestion of drinking water contaminated with PFOA released by the DuPont Washington Works plant in Parkersburg, West Virginia. This plant, located ~250 miles upstream of Cincinnati along the Ohio River, has manufactured fluoropolymers since the 1950s, and as recently as 2000, was emitting over 80,000 lbs of PFOA into the environment per year.26,27 Serum PFOA concentrations among pregnant women from the communities surrounding this plant are elevated (median: 14.3 ng/mL) due to consumption of PFOA contaminated drinking water.26,28 This contamination may affect other communities along the Ohio River, including the city of Cincinnati, because they draw their water supplies from the river. Indeed, a study of school-age Cincinnati girls observed elevated serum PFOA concentrations (median: 7.3 ng/mL) and reported that serum PFOA concentrations were positively associated with ingestion of drinking water that may have been contaminated with PFOA.29
Four previous cohort studies have examined the associations between PFAS exposures and child or adult adiposity (Table S1). A study of 665 adults from Denmark reported that prenatal PFOA concentrations were associated with increased BMI and waist circumference at 20 years of age, with stronger associations among females.16 Another Danish study of 811 children did not observe any associations between prenatal PFOA or PFOS concentrations and child BMI or waist circumference at 7 years of age.14 A third study of 447 girls from the United Kingdom found that prenatal PFOS concentrations were associated with more rapid growth between birth and 20 months of age.17 Finally, a study of 8,764 adults who lived near the DuPont Washington Works Plant and had exceptionally high PFOA exposure did not find an association between estimated early life PFOA exposure and BMI or risk of overweight or obesity.15
Misclassified child or adult adiposity could cause discrepant results across studies. The Andersen et al. and Barry et al. studies, which reported no association between PFAS exposure and adult or child BMI relied on self- or parent-reported weight and height.14,15 There are welldocumented errors in self- and parent-reported anthropometry that could misclassify BMI and attenuate associations towards the null.30,31 In contrast, the present study and the two prior studies reporting associations between prenatal PFAS exposure and increased adiposity or accelerated growth used standardized anthropometric measurements or clinical records for the majority of participants. These more accurate measures of adiposity might have resulted in less misclassification and enhanced statistical precision. However, the present study and prior ones are limited by reliance on less-refined measures of adiposity. Future studies would benefit from using more detailed adiposity measures, including densitometry, bioelectric impedance, or dual energy X-ray absorptiometry.
Repeated assessments of weight and height from 2–8 years of age are a strength of our study. These longitudinal data provide additional insight that might not be apparent in studies measuring adiposity at only one time point in childhood. An additional strength of this study is that we were able to estimate child adiposity using bioelectric impedance at 8 years of age. However, the relatively modest sample size for analyses of our 8-year outcomes limits the statistical precision of these estimates. In addition, while it is possible that selection bias could have arisen due to loss to follow-up, this seems unlikely given that maternal serum PFAS concentrations were similar between parent/child pairs who did and did not complete follow-up at 8 years of age.
We speculate that the non-linear associations we observed between PFOA and adiposity at 8 years of age may be due to the growth restrictive effect that PFOA has on the fetus and more rapid gains in adiposity during childhood.32 This is similar to the phenomenon observed among tobacco smoke exposed children who are smaller at birth, grow more rapidly than their unexposed peers, and are at increased risk of obesity later in life.33,34 Additional follow-up of this cohort will enable us to determine if children with higher prenatal PFOA exposure continue to gain adiposity more rapidly and have excess adiposity at later ages.
Our dose-response relationship is consistent with a threshold effect. In vitro studies suggest that PFOA exposures above a certain level activate biological mechanisms, like the constitutive androstane receptor, that may dampen the obesogenic effects observed at lower exposures.35,36 This may be one reason for discrepant findings in prior studies, particularly in the study examining exceptionally high PFOA exposure in Ohio and West Virginia. Regardless of the biological or non-causal mechanism(s) responsible for our findings, these results highlight the need to consider longitudinal patterns of adiposity and the shape of dose-response relationships when studying chemical obesogens.
We found that maternal serum PFOA concentrations, and not the other PFAS, were associated with both child adiposity at 8 years of age and accelerated BMI z-score trajectories between 2 and 8 years of age. Rodent studies suggest that the effect of prenatal PFOA exposure on neonatal death, growth, and development is dependent on the peroxisome proliferator activated receptor-α, while prenatal PFOS exposure is not.37 Thus, it is possible that individual PFAS act via different mechanisms. Future animal and epidemiological studies should continue to investigate this class of chemicals and identify potential mechanisms of action that might explain their associations with growth and adiposity. In addition to identifying individual chemical obesogens, future studies should consider the cumulative or synergistic impact of multiple chemical exposures.
While our unadjusted and covariate-adjusted results were similar (results not shown), residual confounding from factors associated with both PFAS exposure and child adiposity may have biased our results. This bias may arise if certain maternal dietary patterns or behaviors during pregnancy, such as packaged food consumption, were associated with both higher PFAS exposure and child dietary patterns that are associated with child adiposity.38 Water consumption may be another confounder since water contamination is a likely source of PFOA exposure in our study participants and it may be associated with lifestyle factors that predict subsequent child adiposity. Unfortunately, we did not collect information about children’s diet or the water consumption habits of mothers. Finally, children were at different stages of pubertal development when they returned for the 8-year visit (range: 7.5–10 years), which may have influenced their adiposity.39 While we did not measure Tanner Stage, future studies should carefully consider whether this adjustment is appropriate since PFAS exposure may be associated with later pubertal development.40
Conclusions
In this cohort of pregnant women who had above average serum PFOA concentrations, increasing serum PFOA levels during pregnancy were associated with greater BMI, waist circumference, and body fat in children at 8 years of age in a non-monotonic fashion. Higher serum PFOA concentrations during pregnancy were also associated with more rapid increases in BMI in children between 2 and 8 years of age. Other prospective cohort studies with longitudinal and detailed measures of child adiposity should confirm these findings.
Supplementary Material
What is Known.
Perfluoroalkyl substances are man-made persistent chemicals and suspected obesogens.
Some studies report that prenatal exposure to perfluoroalkyl substances may increase the risk of childhood obesity, but few have examined longitudinal patterns of child adiposity.
What this Study Adds
This study examined longitudinal measures of adiposity from two to eight years of age in a prospective cohort of children born to women who lived downstream of a fluoropolymer manufacturing plant and had elevated prenatal serum perfluorooctanoic acid concentrations.
Higher prenatal perfluorooctanoic acid concentrations were associated with increased adiposity at eight years of age in a non-monotonic fashion, as well as more rapid gains in BMI from two to eight years of age.
Acknowledgements
We would like to acknowledge the Centers for Disease Control and Prevention (CDC) laboratory staff who performed the analyses. We thank Dr. David Savitz for his helpful feedback on a draft of this manuscript. We are grateful to our participants for the time they have given to our study.
Funding: This work was supported by NIEHS grants R00 ES020346, PO1 ES11261, R01 ES014575, and R01 ES020349.
Dr. Lanphear has served as an expert witness and a consultant to the California Attorney General’s Office for the plaintiffs in a public nuisance case related to childhood lead poisoning, but he has not personally received any compensation for these services. Dr. Lanphear has also served as a paid consultant on a US Environmental Protection Agency research study related to childhood lead poisoning. Dr. Braun was financially compensated for conducting a re-analysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. None of these activities are directly related to the present study.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 3.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. International journal of andrology. 2012;35:437–448. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EFSA. Perfluoroctane sulfonate, perfluorooctanoic acid and their salts: Scientific opinion of the panel on contaminants in the food chain. EFSA Journal. 2008;653:1–131. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated environmental assessment and management. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. The Journal of steroid biochemistry and molecular biology. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. Journal of toxicology and environmental health. 2013;76:409–421. doi: 10.1080/15287394.2013.771547. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environmental health perspectives. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicological sciences : an official journal of the Society of Toxicology. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 10.Taxvig C, Dreisig K, Boberg J, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Molecular and cellular endocrinology. 2012;361:106–115. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicology in vitro : an international journal published in association with BIBRA. 2013;27:1634–1643. doi: 10.1016/j.tiv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Molecular and cellular endocrinology. 2009;304:97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Ngo HT, Hetland RB, Sabaredzovic A, Haug LS, Steffensen IL. In utero exposure to perfluorooctanoate (PFOA) or perfluorooctane sulfonate (PFOS) did not increase body weight or intestinal tumorigenesis in multiple intestinal neoplasia (Min/+) mice. Environmental research. 2014;132C:251–263. doi: 10.1016/j.envres.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. American journal of epidemiology. 2013;178:921–927. doi: 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- 15.Barry V, Darrow LA, Klein M, Winquist A, Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environmental research. 2014;132C:62–69. doi: 10.1016/j.envres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environmental health perspectives. 2012;120:668–673. doi: 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environmental health perspectives. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun JM, Lanphear BP, Calafat AM, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environmental health perspectives. 2014;122:1239–1245. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty SR, Khoury JC, Morrow AL, Lanphear BP. Reporting individual test results of environmental chemicals in breastmilk: potential for premature weaning. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2008;3:207–213. doi: 10.1089/bfm.2008.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218:2133–2137. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Hyattsville, Maryland: National Center for Health Statistics; 2000. [2001/02/24]. [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Beck Depression Inventory - 2nd Edition (BDI-II) San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 23.Braun JM, Daniels JL, Poole C, et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health. 2010;9:53. doi: 10.1186/1476-069X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine. 2010 doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 25.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622:150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 26.Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisbee SJ, Brooks AP, Jr, Maher A, et al. The C8 health project: design, methods, and participants. Environmental health perspectives. 2009;117:1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environmental health perspectives. 2013;121:1207–1213. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinney SM, Biro FM, Windham GC, et al. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut. 2014;184:327–334. doi: 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinbami LJ, Ogden CL. Childhood Overweight Prevalence in the United States: The Impact of Parent-reported Height and Weight. Obesity. 2009;17:1574–1580. doi: 10.1038/oby.2009.1. [DOI] [PubMed] [Google Scholar]
- 31.Hattori A, Sturm R. The obesity epidemic and changes in self-report biases in BMI. Obesity (Silver Spring, Md. 2013;21:856–860. doi: 10.1002/oby.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson PI, Sutton P, Atchley DS, et al. The Navigation Guide-Evidence-Based Medicine Meets Environmental Health: Systematic Review of Human Evidence for PFOA Effects on Fetal Growth. Environmental health perspectives. 2014 doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. International journal of epidemiology. 2006;35:121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 34.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. International journal of obesity (2005) 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Molecular pharmaceutics. 2008;5:35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- 36.Peters JM, Gonzalez FJ. Why toxic equivalency factors are not suitable for perfluoroalkyl chemicals. Chemical research in toxicology. 2011;24:1601–1609. doi: 10.1021/tx200316x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reproductive toxicology. 2009;27:246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. International journal of hygiene and environmental health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Espinosa MJ, Fletcher T, Armstrong B, et al. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environmental science & technology. 2011;45:8160–8166. doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.