Abstract
Objective
At the cellular level, how excess adiposity promotes atherogenesis is not fully understood. One pathway involves secretion of adipokines that stimulate endothelial dysfunction through increased expression of the adhesion molecules. However, the relationship of adiposity to adhesion molecules that promote atherosclerosis is largely unknown.
Methods
Linear regression models were used to assess the sex-specific associations of soluble cellular adhesion molecules (sP- and sL-selectin, sICAM-1, sVCAM-1, and sHGF) and adiposity in 5,974 adults examined as part of the Multi-Ethnic Study of Atherosclerosis (MESA). Adiposity measures included body mass index (BMI), waist-to-hip-ratio (WHR), and, computed tomography measures of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT).
Results
The mean age was 64 years and 52% were female. In multivariable models adjusting for traditional cardiovascular risk factors, sHGF was positively associated with BMI, WHR, and VAT in both males and females, and sP-selectin with WHR and VAT in males. sVCAM-1 was inversely associated with VAT in females only.
Conclusions
Our results showed the relation of adiposity to soluble cellular adhesion proteins was similar across adiposity measures and for both sexes. However, the relationship between adiposity and sVCAM-1 and P-selectin may be modified by sex and the measure used to assess adiposity.
Keywords: adiposity, L-selectin, P-selectin, VCAM-1, ICAM-1, HGF
Introduction
Almost 35% of United States adults are obese, a condition associated with diabetes, atherosclerosis, and inflammation (1). How excess adiposity promotes atherogenesis is not fully understood. However, one mechanism involves secretion of the pro-inflammatory cytokines (i.e., adipokines)by adipose tissue and the macrophages in the interstitial tissue around adipocytes. Adipokines can stimulate endothelial dysfunction and subsequent atherogenesis by increasing endothelial permeability and leukocyte recruitment through increased expression of adhesion proteins including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and P--selectin (2).
Adhesion proteins are integral to fatty streak formation in the first stage of atherosclerosis, and are responsible for recruiting leukocytes, binding them to the endothelium, and aiding in transmigration across the endothelium (3). Therefore, these proteins are potentially early predictors of atherosclerosis, and have been linked to adiposity.. Soluble ICAM-1 (sICAM-1) levels are elevated in individuals with obesity, insulin resistance, and diabetes (4). The relationship of soluble VCAM-1 (sVCAM-1) with excess adiposity is less clear. That is, higher sVCAM-1 levels are associated with cardiovascular disease (CVD) and type 2 diabetes, but not body mass index (BMI) (5). Soluble L-selectin (sL-selectin) levels are negatively associated with obesity and weight loss surgery has been found to increase L-selectin expression (6). Soluble P-selectin (sP-selectin) has been associated with adiposity and both clinical and sub-clinical atherosclerosis(7), and has been shown to predict atherosclerosis independent of BMI and other CVD risk factors. In addition, soluble hepatocyte growth factor (sHGF) is positively associated with subclinical heart disease (8) and visceral adipose tissue (VAT) (9) with decreasing levels observed with weight loss (10). However, few studies have investigated the relation of various measures of adiposity to adhesion molecules.
Assessing the relationship between adiposity, adhesion proteins, and subsequent CVD risk is challenging due to the use of different measures to assess adiposity. In addition, it is rare for these studies to account for regional fat deposition. Large epidemiologic cohort studies show that higher BMI (11), waist circumference (WC) (12), and waist-to-hip-ratio (WHR) (13) are associated with increased risk of CVD. However, anthropometric measures such as BMI may result in misclassification with respect to adipose tissue types and do not accurately or adequately reflect the distribution, location, and complex biology of adipose tissue (14,15). This despite the fact that central adiposity confers significant metabolic risk but lower-body (gluteofemoral) fat (16) and larger hip circumference are associated with better metabolic and cardiovascular outcomes. Differences in regional fat deposition could be the distinguishing characteristics between metabolically healthy and unhealthy obesity (16). Therefore, regional measures of adiposity may provide a more refined representation of adiposity in chronic diseases and how adiposity contributes to CVD and diabetes risk profiles (14). Furthermore, there are recognized race/ethnicity differences in fat distribution that affects risk for metabolic diseases (17). Regional ectopic fat depots including subcutaneous adipose tissue (SAT) and VAT, measured via computed tomography (CT), appears to be better indicators of disease risk. In this regard, VAT contains a greater number of macrophages compared to SAT, is metabolically active, and associated with inflammation and adhesion proteins (18–21).
Our aim was to investigate how adiposity, measured by BMI, WHR, SAT, and VAT, are associated with soluble adhesion proteins (sP- and sL-selectin, sICAM-1, sVCAM-1, and sHGF) in a large, ethnically diverse population and assess effect modification by sex and race/ethnicity. We hypothesized that higher adiposity would be associated with higher adhesion protein levels in a dose-dependent manner independent of age, sex, and race/ethnicity.
Methods
Population
The Multi-Ethnic Study of Atherosclerosis (MESA) consists of 6,814 adults aged 45–84 years old. MESA participants underwent five examinations between 2000–2012 at six field centers located in Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and Saint Paul, MN and include 38% non-Hispanic white, 28% African, 22% Hispanic, and 12% Chinese Americans. The design of the cohort has been previously described(22).
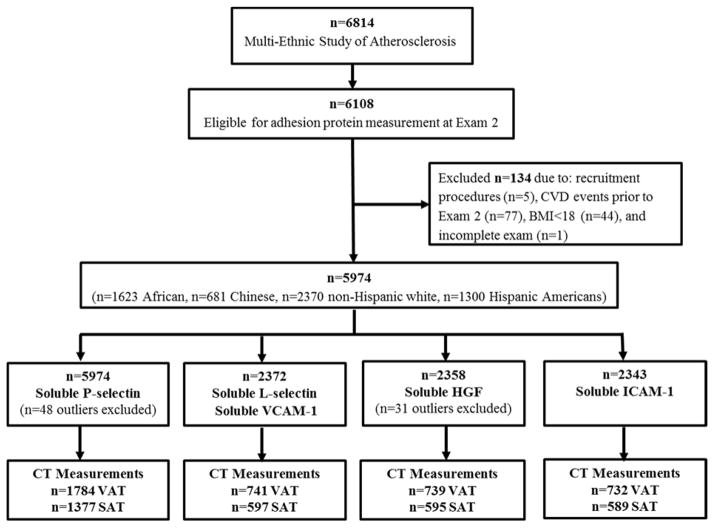
For this study, results from two MESA ancillary studies were used; Multiscale Biology of Atherosclerosis in the Cellular Adhesion Pathway (HL98077, PI: Bielinski) and Abdominal Body Composition, Inflammation, and Cardiovascular disease (HL088451, PI: Allison). Of those who participated in Exam 2 (2002–2004), 6,108 had plasma and serum samples eligible for adhesion protein measurement. We excluded a total of 134 samples, 2 Chinese and 3 Hispanic Americans from field centers that did not actively recruit these two ethnic groups, 77 participants with CVD events prior to Exam 2, 44 participants with BMI < 18, 7 sample failures, and 1 participant who did not complete Exam 2 or subsequent exams due to cognitive impairment. The final sample size included 5,974 participants including 1,623 African, 681 Chinese, 2,370 non-Hispanic white, and 1,300 Hispanic Americans. This study was conducted according to the principles of the Declaration of Helsinki, and informed consent was obtained from all participants. Institutional review boards at each research center approved the study.
Adiposity measurements
Anthropometric protocols were standard across sites. Participants wore light clothing and no shoes. Height was measured to the nearest 0.1 cm using an Accu-Hite stadiometer (Seca, Hanover, MD, USA), and weight was measured to the nearest pound using a Detecto Platform Balance Scale (Webb City, MO, USA). Waist and hip circumferences were measured to the nearest 0.1 cm using Gulick II 150-cm anthropometric tape measures (Country Technology Inc., Gays Mills, WI, USA), with the tape (standard 4 oz. tension) in the horizontal plane and participants standing. Waist circumference was measured at the umbilicus, and hip circumference around the widest circumference of the buttocks. Circumference measurements were either taken around either light tight-fitting clothing or no clothing. BMI was calculated as weight (kg)/height2 (m2). WHR was calculated from waist and hip circumferences.
A random subset of MESA subjects underwent CT scanning of the abdomen at Exam 2 or 3 (23,24). These scans were used to measure areas of visceral and subcutaneous adiposity at three different vertebral levels. Data collection methods are described elsewhere(23). In brief, 6 mm thick slices were used, with two each at the L2–L3, L3–L4 and L4–L5 disc spaces. Fat tissue was classified as −190 ≤ fat pixel ≤ −30 Hounsfield Units. Two analysts from a centralized reading center (UCSD, La Jolla, CA) independently assessed each scan; intraclass correlations were 0.92–0.99 for different body composition measures. Due to its high correlation with total abdominal volume and lower standard error of estimates, VAT and SAT were defined (in cm2) as the average of two slices from L2–L3, and adjusted for height.
Adhesion protein measurements
sP-selectin levels were measured on 5,974 participants and the other adhesion proteins on a subset of 2,372. Figure 1 shows the sample sizes and exclusions for each protein. sP-selectin was measured in plasma by a quantitative sandwich enzyme-linked immunosorbent assay using the Human Soluble P-selectin/CD62P Immunoassay Kit (R&D Systems, Minneapolis, MN). Two independent groups of samples were assayed. A different lot of ELISA reagent and different human control pool was used for each group. The inter-assay CV for group 1 was 6.7% at a control concentration of 182 mg/dL; the inter-assay CV for group 2 was 9.7% at a control concentration of 79 mg/dL. The minimum detectable level was 0.5 ng/mL for both assay groups.
Figure 1.
Study design.
sL-selectin was measured in serum on using the Human soluble L-selectin/CD62L Immunoassay Kit, a quantitative sandwich enzyme-linked immunosorbent assay (R&D Systems); minimum detectable level was 0.3 ng/mL, inter-assay CV was 6.7% at a mean control concentration of 943 ng/mL. sICAM-1 was measured in serum using the Human sICAM-1 Instant ELISA (Bender MedSystems GmbH, Vienna, Austria) with a minimum detectable level of 2.17 ng/mL; the inter-assay CV was 9.1% at a mean concentration of 261 ng/mL. Of importance, this assay is not affected by variant rs5491 in the ICAM1 gene, which is common in African Americans and blocks the detection of soluble ICAM-1 by antibodies used in some ELISA assays. sVCAM-1 was measured using a human Quantikine ELISA Kit (R&D Systems); the minimum detectable level was 0.6 ng/mL, and the inter-assay CV was 3.6% at a mean concentration of 564 ng/mL. sHGF was measured in serum using the Quantikine Human HGF Immunoassay kit (R&D Systems), with a lower limit of detection of 40 pg/mL and inter-assay CV of 6.2% at a mean concentration of 946 pg/mL.
Other measurements
A combination of self-administered and interview-administered questionnaires was used to collect data such as smoking history, alcohol intake, and medication use. Resting seated blood pressure was measured three times using an automated oscillometric method (Dinamap), and the average of the second and third readings are used in analyses. Hypertension was defined as systolic blood pressure (SBP) of ≥140 mm Hg, diastolic blood pressure (DBP) of ≥90 mm Hg, or taking antihypertensive medication. Diabetes was defined as any participant who self-reported a physician diagnosis, used diabetic medication, or had a fasting glucose ≥126 mg/dL (i.e., 7 mmol/L). Serum glucose was assayed by a hexokinase/glucose-6-phosphate dehydrogenase method, and triglycerides and cholesterol were assayed by enzymatic methods. HDL cholesterol was measured after precipitation of non-HDL-cholesterol with magnesium/dextran and LDL was calculated via the Friedewald equation. Hemoglobin A1c was assayed using an automated high performance liquid chromatography methods (Tosoh HPLC Glycohemoglobin Analyzer, Tosoh Bioscience, Inc., San Francisco, CA, USA). Measurements from Exam 2 for all of these variables were used.
Statistical analysis
Outliers were excluded as appropriate (n = 14 for sHGF n = 48 for sP-selectin). Participant characteristics were compared across groups using analysis of variance (Kruskal-Wallis) and chi-square (exact) tests as appropriate. Regression models were used to compare demographic and traditional cardiovascular risk factors by sex adjusting for race. Primary endpoints include soluble adhesion protein levels: sL-selectin, sP-selectin, sICAM-1, sVCAM-1, and sHGF. To address differences in sP-selectin levels across groups, we obtained residuals from a linear regression model using log-transformed raw sP-selectin values as a response and group status as a predictor. All references of sP-selectin levels heretofore refer to these residual values. Linear regression was performed to quantify the relationship between BMI, WHR, VAT, and SAT and adhesion proteins controlling for age and race/ethnicity. In a subsequent model, total cholesterol, HDL cholesterol, systolic blood pressure, hypertension treatment, current smoking, and diabetes status were added as covariates. We assessed effect modification by sex and race/ethnicity. Assumptions of linearity for each were evaluated using generalized additive models with cubic B-splines. From these, there were no indications of major departures from linearity. We used a Bonferroni correction to account for multiple comparisons (0.05/5 proteins × 2 sexes = 0.005). Given that adiposity measurements are highly correlated (some as high as 80% in our sample), additional Bonferroni correction by measurement type would produce overly conservative results due to dependence on the test statistics, reducing the effective number of tests.
Results
Characteristics of the MESA participants are listed in Table 1. Pairwise plots of adhesion molecules verses obesity measures by sex are included in Figures S1–S5. Significant differences by sex were observed for all clinical and CT adiposity measures as well as for sP- and sL-selectin and sHGF (Table 2). Compared to men, women had lower mean sP-selectin levels (P < 0.001), but higher mean sL-selectin (P < 0.001) and HGF (P = 0.001) levels. These differences were largely consistent within race/ethnic strata (Table S1); further, no interaction between race/ethnicity and the association between adiposity and adhesion proteins was found. Therefore we present results stratified by sex, but not race/ethnicity.
TABLE 1.
Exam 2 characteristics by race/ethnicity, mean (standard deviation) or percent
| Characteristics | European American | Chinese American | African American | Hispanic American |
|---|---|---|---|---|
| N | 2,622 | 803 | 1,893 | 1,496 |
| Age, years | 63 (10) | 62 (10) | 62 (10) | 61 (10) |
| Sex, % female | 52 | 51 | 56 | 52 |
| Body mass index, kg/m2 | 28 (5.1) | 24 (3.3) | 30 (5.9) | 29 (5.1) |
| Waist to hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) |
| Systolic blood pressure, mmHg | 124 (20) | 125 (22) | 132 (22) | 127 (22) |
| Diastolic blood pressure, mmHg | 70 (10) | 72 (10) | 75 (10) | 72 (10) |
| Hypertension, % yes | 39 | 38 | 60 | 42 |
| Antihypertensive therapy, % yes | 33 | 29 | 50 | 33 |
| Hypertension categories, % | ||||
| Normotensive, SBP < 140 and DBP < 90 mm Hg | 62 | 63 | 41 | 59 |
| Controlled hypertensive, SBP < 140 and DBP < 90 mm Hg | 28 | 26 | 48 | 30 |
| Uncontrolled hypertensive, SBP ≥ 140 or DBP ≥ 90 mm Hg | 11 | 12 | 12 | 12 |
| Diabetes mellitus, % yes | 6 | 13 | 18 | 18 |
| Hemoglobin A1c | 5.4 (0.6) | 5.8 (0.9) | 5.9 (1.1) | 5.9 (1.2) |
| Total cholesterol, mg/dL | 196 (35) | 193 (32) | 190 (36) | 198 (38) |
| HDL cholesterol, mg/dL | 52 (16) | 50 (13) | 52 (15) | 48 (13) |
| LDL cholesterol, mg/dL | 117 (30) | 115 (29) | 117 (33) | 120 (33) |
| Triglycerides, mg/dL | 133 (90) | 143 (85) | 105 (69) | 157 (101) |
| Any antilipidemic therapy, % yes | 18 | 14 | 16 | 13 |
| Statin use, % yes | 17 | 13 | 15 | 12 |
| Current smoker, % yes | 12 | 5.6 | 18 | 14 |
| Smoking status, % | ||||
| Never | 44 | 75 | 45 | 54 |
| Former | 44 | 19 | 37 | 33 |
| Current | 12 | 5.6 | 18 | 14 |
| Current use of alcohol, % yes | 72 | 31 | 49 | 47 |
SBP, systolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
TABLE 2.
Measures of adiposity and levels of soluble adhesion proteins by sex-adjusted for race/ethnicity measured at Exam 2 unless otherwise specified, mean (standard deviation) or percentage
| Characteristics | Females | Males | P-valuea |
|---|---|---|---|
| Age, years | 64 (10) | 64 (10) | 0.74 |
| Body mass index, kg/m2 | 29 (6) | 28 (4) | <0.001 |
| Waist circumference, cm | 97 (16) | 100 (12) | <0.001 |
| Waist to hip ratio, % | <0.001 | ||
| Men ≤ 0.95, women ≤ 0.80 | 12 | 41 | |
| Men 0.96–1.0, women 0.81–0.85 | 16 | 34 | |
| Men > 1.0, women > 0.85 | 71 | 25 | |
| CT measures of fat, Exam 2/3 | |||
| VAT, cm2 | 0.54 (0.16) | 0.64 (0.14) | <0.001 |
| SAT, cm2 | 0.95 (0.03) | 0.91 (0.04) | <0.001 |
| Soluble Adhesion Proteins | |||
| sP-selectin, ng/mL (n = 5,974) | 35 (12) | 39 (13) | <0.001 |
| sL-selectin, ng/mL (n = 2,372) | 931 (200) | 847 (190) | <0.001 |
| sVCAM-1, ng/mL (n = 2,372) | 730 (225) | 746 (235) | 0.2 |
| sICAM-1, ng/mL (n = 2,343) | 255 (87) | 251 (86) | 0.56 |
| sHGF, ng/mL (n = 2,358) | 1,002 (234) | 965 (226) | 0.001 |
Sex comparisons from regression models adjusting for race/ethnicity.
CT, computed tomography; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule; HGF, hepatocyte growth factor.
BMI, WHR, and VAT were associated with higher levels of HGF in both males and females (Table 3, Tables S2, S3, and S4). The magnitude of association was consistently higher in males compared to females for every measure of adiposity investigated (sex interaction for BMI, P = 0.022; WHR, P = 0.001. and VAT P = 0.29). For example, each 1-unit increase in WHR was associated with an increase of 1,094 pg/ml in HGF levels for males and 658 pg/ml for females. Likewise, increased adiposity was associated with higher levels of sP-selectin in both males and females (Table 3). The associations were attenuated with adjustment for additional risk factors but remained significant in males for WHR and VAT (sex-interaction P = 0.0063 and 0.13, respectively). Higher VAT was inversely associated with sVCAM-1 in females only (sex interaction P = 0.09). For each 1-unit increase in VAT, sVCAM-1 levels decreased by 203 ng/ml in females, but only 40 ng/ml (not significant) in males. Neither sL-selectin nor sICAM-1 were significantly associated with any of the measures of adiposity after accounting for CVD risk factors. None of the adhesion proteins were significantly associated with levels of SAT.
TABLE 3.
Adjusted associations of soluble adhesion protein levels and adiposity, stratified by sex
| sP-selectin | sL-selectin ng/mL (SD = 200) | sICAM-1 ng/mL (SD = 87) | sVCAM-1 ng/mL (SD = 232) | sHGF pg/mL (SD = 234) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | |||||||||||
| Beta (S.E.) |
P-value | Beta (S.E.) |
P-value | Beta (S.E.) |
P- value |
Beta (S.E.) |
P- value |
Beta (S.E.) |
P-value | Beta (S.E.) |
P-value | Beta (S.E.) |
P-value | Beta (S.E.) |
P- value |
Beta (S.E.) |
P-value | Beta (S.E.) |
P-value | |
| Clinical Measures of Adiposity | ||||||||||||||||||||
| Body mass index, kg/m2 (Exam 2) | n=3,129 | n=2,845 | n=1,253 | n=1,119 | n=1,239 | n=1,104 | n=1,253 | n=1,119 | n=1,245 | n=1,113 | ||||||||||
| Model 1 | 0.005 (0.001) | <0.0001 | 0.007 (0.001) | <0.0001 | 2.1 (1.0) | 0.039 | 1.3 (1.4) | 0.35 | 1.8 (0.44) | <0.0001 | 1.2 (0.63) | 0.053 | 1.2 (1.1) | 0.26 | 0.68 (1.6) | 0.68 | 11 (1.1) | <0.0001 | 16 (1.5) | <0.0001 |
| Model 2 | 0.002 (0.001) | 0.027 | 0.004 (0.002) | 0.009 | 1.9 (1.1) | 0.09 | −0.29 (1.5) | 0.85 | 0.61 (0.47) | 0.20 | −0.04 (0.69) | 0.95 | −0.45 (1.2) | 0.71 | −1.6 (1.8) | 0.38 | 8.9 (1.2) | <0.0001 | 14 (1.6) | <0.0001 |
| Waist-to-hip ratio (Exam 2) | n=3,126 | n=2,845 | n=1,252 | n=1,119 | n=1,238 | n=1,104 | n=1,252 | n=1,119 | n=1,244 | n=1,113 | ||||||||||
| Model 1 | 0.42 (0.07) | <0.001 | 0.87 (0.10) | <0.0001 | 55 (75) | 0.46 | −2.2 (100) | 0.98 | 99 (34) | 0.003 | 182 (46) | <0.0001 | −18 (83) | 0.83 | 273 (119) | 0.022 | 658 (81) | <0.0001 | 1,094 | <0.0001 |
| Model 2 | 0.24 (0.08) | 0.002 | 0.65 (0.11) | <0.0001 | 72 (82) | 0.38 | 6.3 (108) | 0.95 | 14 (35) | 0.69 | 113 (49) | 0.021 | −142 (90) | 0.11 | 263 (127) | 0.038 | 424 (86) | <0.0001 | 926 (114) | <0.0001 |
| CT Measures of Adiposity | ||||||||||||||||||||
| Visceral adipose tissue (Exam 2/3)t, cm2 | n=911 | n=873 | n=378 | n=363 | n=373 | n=359 | n=378 | n=363 | n=376 | n=363 | ||||||||||
| Model 1 | 0.27 (0.06) | <0.0001 | 0.37 (0.08) | <0.0001 | −119 (62) | 0.056 | 78 (70) | 0.27 | 23 (30) | 0.45 | 3.6 (38) | 0.92 | −203 (64) | 0.002 | −40 (79) | 0.61 | 277 (68) | <0.0001 | 332 (84) | <0.0001 |
| Model 2 | 0.17 (0.07) | 0.016 | 0.32 (0.09) | 0.0002 | −171 (67) | 0.011 | −13.4 (76) | 0.86 | −31 (30) | 0.31 | 43 (39) | 0.27 | −253 (70) | 0.0003 | −79 (85) | 0.35 | 200 (72) | 0.006 | 340 (90) | 0.0002 |
| Subcutaneous adipose tissue (Exam 2/3)t cm2 | n=720 | n=656 | n=310 | n=286 | n=306 | n=282 | n=310 | n=286 | n=308 | n=286 | ||||||||||
| Model 1 | 0.36 (0.34) | 0.28 | 0.40 (0.31) | 0.2 | 281 (331) | 0.40 | 346 (237) | 0.14 | −136 (160) | 0.40 | 52 (129) | 0.69 | 248 (334) | 0.46 | 329 (267) | 0.22 | 145 (347) | 0.68 | 589 (290) | 0.043 |
| Model 2 | 0.60 (0.35) | 0.082 | 0.44 (0.33) | 0.18 | 264 (340) | 0.44 | 437 (248) | 0.08 | 75 (154) | 0.63 | 12 (133) | 0.93 | 307 (351) | 0.38 | 382 (282) | 0.18 | 535 (347) | 0.12 | 622 (308) | 0.045 |
Model 1 = adjusted for age and race/ethnicity.
Model 2 = adjusted for model 1 + total cholesterol, HDL cholesterol, systolic blood pressure, hypertension treatment, current smoking, diabetes status.
CT Adiposity Measures also adjusted for height.
CT, computed tomography; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule; HGF, hepatocyte growth factor.
Discussion
Extending previous findings, we assessed the relationship between measures of adiposity and soluble cellular adhesion proteins in a large multi-ethnic population. We observed that increased levels of sHGF and sP-selectin were associated with greater adiposity. sHGF was positively associated with all the clinical measures of adiposity whereas, for CT measures, the association was exclusive to VAT. Furthermore, we found evidence of a sex-interaction between sP-selectin levels and adiposity. We propose that the effect of adiposity on adhesion proteins and even endothelial dysfunction and subsequent atherosclerosis may be somewhat modified by the measure used to assess adiposity, and perhaps by sex for some proteins.
The relationship between sHGF and adiposity has been previously documented. In a sample of 65 lean and subjects with obesity, Rehman et al. (8) showed that serum HGF was, on average, more than three times higher in subjects with obesity versus their lean counterparts. Corroborating these results in larger populations, Faber et al. reported that serum HGF significantly increased with greater adiposity as assessed by both BMI and VAT (9). However, neither of these studies reported sex-specific analyses. Sex-specific associations of adiposity and HGF were investigated in 1,806 Framingham Heart Study participants, where it was observed that the association of VAT and serum HGF was stronger in females (25). In contrast, we report that the association was greater in magnitude for males. These conflicting results could be due to differences in the population composition as MESA is more ethnically diverse and older (mean age of 64 vs. 45 years old) with higher average BMI. Similar to previous studies, the association in MESA was highly significant and consistent by sex and independent of other risk factors, as well as the measure used to assess adiposity. These results suggest that sHGF could serve as an important marker of chronic disease risk due to its strong correlation with excess adiposity and putative participation in atherosclerotic lesions (26) and type 2 diabetes (27).
sP-selectin was associated with WHR and VAT but not BMI. This pattern may indicate that the relationship of sP-selectin to adiposity is dependent on measures that account for central adiposity and regional fat deposition. Prior reports have reported mixed results. P-selectin was significantly related to VAT and SAT independent of CVD risk factors, but not independent of BMI/waist circumference (28) and P-selectin significantly correlated with pericardial and intrathoracic fat after adjusting for age and sex, but not in multivariable models (29), in the Framingham Heart Study population. In contrast, P-selectin and BMI or WHR were neither correlated after adjusting for just age, sex, and smoking, nor in multivariable models accounting for CVD risk factors (30). These studies, with ours, suggest that P-selectin may not be related to BMI after accounting for other factors, but P-selectin is likely related to VAT, although this may not be independent of BMI, and may be stronger for males than for females. That there was a sex-specific interaction is not particularly surprising given that males and females often differ in fat distribution, and sex-specific interactions have been reported in some of the very adipokines associated with adiposity that promote atherosclerosis, including leptin (31) and adiponectin (32).
In contrast to Pou et al. (28), we did not include BMI, WC, and VAT in the same model as they were highly correlated in our sample. Our results indicate that WHR may be more strongly related to certain fat depots and adhesion protein levels than BMI. Thus, sP-selectin may serve as one instance where simply measuring adiposity via BMI may be insufficient. As noted previously, BMI may lead to the mischaracterization of health since it cannot adequately account for the variability in fat deposition or muscle that is related to metabolic risk or subclinical indicators of CVD (18–21). The mechanism behind this likely deals with the different characteristics of each fat depot; while abdominal adipose tissues exhibit higher lipid turnover and more rapid storage of diet-derived fat, gluteofemoral tissue has lower triglyceride turnover, less sensitivity to stress-stimulated lipolysis, and stores a higher proportion of fatty acids that have been recirculated (16). Since sP-selectin levels have been associated with both clinical and sub-clinical atherosclerosis (7), future studies should further investigate how P-selectin may be related to fat distribution, particularly noting sex-specific differences.
sVCAM-1 was negatively associated with VAT in females in the MESA sample. Previous investigations on adiposity and sVCAM-1 are limited in number with mixed results. Two larger studies have shown that VCAM-1 was not associated with BMI (5) or WHR (30). In contrast, Bosanska et al. (33) reported increased VCAM-1 mRNA expression with higher VAT (33). Likewise, increased sVCAM-1 correlated with BMI (34) (35). It is important to note that neither of these latter two studies accounted for CVD risk factors. Our results suggest that sex may modify the relationship between adiposity and sVCAM-1, and that only using measures that account for fat deposition and composition may truly elucidate this relationship.
We found that sICAM-1 was associated with greater adiposity; however, this relationship was not independent of traditional CVD risk factors. Our results concur with those reported by Miller and Cappuccio, who found that although WHR correlated with sICAM-1, the relationship was not independent of traditional CVD risk factors (30). In contrast, increased levels of sICAM-1 correlated with VAT and SAT independent of CVD risk factors (28). The discrepancy could be due to differences in modeling; whereas Pou et al. (28) adjusted for age, sex, menopausal status and hormone replacement therapy, and behavioral characteristics including smoking, aspirin, alcohol intake, and physical activity index, we adjusted for traditional CVD risk factors. Although sICAM-1 appears to be related to adiposity, the conflicting studies make it difficult to definitively report whether this result is independent of other CVD risk factors.
No measures of adiposity were associated with sL-selectin in MESA. Two prior studies reported conflicting results; one found lower levels of sL-selectin in patients with severe obesity compared to normal weight individuals (6). In contrast, Ponthiuex et al. (36) reported that sL-selectin levels were uncorrelated with BMI after adjustment for age and sex. The relationship of sL-selectin as a risk factor for CVD is questionable as prior investigations in MESA did not find sL-selection levels to increase risk of atherosclerosis (37).
Our study had several advantages, first we assessed adiposity using 4 different measures, which have varying levels of precision and association with atherosclerosis risk, in contrast to prior studies that used a single clinical measure of adiposity (5,30,34). While the impact of VAT on cardiometabolic risk is well-established (38,39), few have investigated how central adiposity is related to soluble adhesion protein levels. Contrary to other studies, we reported sex-specific results due to significant interactions between both sex and adhesion protein levels and adiposity measures. Limitations of our study include the cross-sectional design precluding observing adiposity and adhesion protein levels over time, and the relatively small sample size for the CT measures of adiposity may have precluded our ability to detect effect modification by sex and race/ethnicity. Furthermore, only frozen samples were available for measurement of soluble adhesion proteins, therefore, we have no measures the cellular expression of the membrane bound proteins (e.g., ICAM-1, VCAM-1, and L-selectin) as this type of sample is not suitable for flow cytometry.
Conclusions
Our results showed the relation of adiposity to adhesion proteins may be modified by both sex and the measure used to assess adiposity for some proteins. When assessing cardiovascular risk in overweight and patients with obesity, instead of simply relying on BMI and traditional cardiovascular risk factors such as cholesterol levels and blood pressure, it may be more reflective of risk to measure adiposity with WHR or CT scans that account for regional fat deposition. Further, because adhesion proteins are directly related to the development and instability of atherosclerotic plaques, adding these measures may improve patient outcomes by providing more targeted therapy. Future studies examining the relationship between adiposity and adhesion proteins should ideally use multiple measures of adiposity, and especially measures that account for regional fat deposition; furthermore, studies must examine these relationships in light of possible sex-specific effects.
Supplementary Material
Study Importance Questions.
What is already known about this subject? Please remember to also include this between the title page and structure abstract in your paper
Soluble cellular adhesion pathway proteins are associated with traditional measures of adiposity such as body mass index in predominantly white populations.
Regional measures of adiposity may provide a more refined representation of adiposity in chronic diseases as opposed to a single clinical measure of adiposity.
What does this study add? Please remember to also include between the title page and structured abstract in your paper
In a large multi-ethnic cohort, we observed that increased levels of hepatocyte growth factor and P-selectin were associated with greater adiposity and found evidence of a sex-interaction between P-selectin levels and adiposity.
The effect of adiposity on adhesion pathway proteins may be somewhat modified by the measure used to assess adiposity.
Acknowledgments
Funding agencies: MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support is provided by grants and contracts N01 HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169. Funding for adhesion protein levels was provided by NHLBI by grant R01HL98077. Funding for body composition measures was provided by NHLBI by grant R01HL088451.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators.
Footnotes
Disclosures: The authors declare no conflict of interest.
Author contributions: MJC, CLW, NBL and SJB designed the research; MJC and SJB conducted the research; MJC, PAD and PSK analyzed data and performed the statistical analyses; MJC, JSP, PAD, MMS, MdA, HS, WT, CB, CLW and NBL contributed in the conception, design, data analysis, drafting and revising of the manuscript and MAA, MYT, NQH and SJB contributed in data collection, interpretation of results, writing and reviewing of the manuscript.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 3.Galkina E, Ley K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 4.Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387–394. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 5.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, et al. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes. 2000;49:485–491. doi: 10.2337/diabetes.49.3.485. [DOI] [PubMed] [Google Scholar]
- 6.Cottam DR, Schaefer PA, Shaftan GW, Angus LDG. Dysfunctional immune-privilege in morbid obesity: implications and effect of gastric bypass surgery. Obes Surg. 2003;13:49–57. doi: 10.1381/096089203321136584. [DOI] [PubMed] [Google Scholar]
- 7.Bielinski SJ, Berardi C, Decker PA, Kirsch PS, Larson NB, Pankow JS, et al. P-selectin and subclinical and clinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;240:3–9. doi: 10.1016/j.atherosclerosis.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/s0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 9.Faber DR, van der Graaf Y, Westerink J, Kanhai DA, Monajemi H, Visseren FLJ, et al. Hepatocyte growth factor and interferon-γ inducible protein-10 are related to visceral adiposity. Eur J Clin Invest. 2013;43:369–378. doi: 10.1111/eci.12054. [DOI] [PubMed] [Google Scholar]
- 10.Swierczynski J, Korczynska J, Goyke E, Adrych K, Raczynska S, Sledzinski Z. Serum hepatocyte growth factor concentration in obese women decreases after vertical banded gastroplasty. Obes Surg. 2005;15:803–808. doi: 10.1381/0960892054222678. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 12.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes-Austin JM, Wassel CL, Jiménez J, Criqui MH, Ix JH, Rasmussen-Torvik LJ, et al. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: The Multi-Ethnic Study of Atherosclerosis (MESA) Vasc Med. 2014 doi: 10.1177/1358863X14537545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 15.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 16.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 17.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 18.Adrielle Lima Vieira R, Nascimento de Freitas R, Volp AC. Adhesion molecules and chemokines; relation to anthropometric, body composition, biochemical and dietary variables. Nutr Hosp. 2014;30:223–236. doi: 10.3305/nh.2014.30.2.7416. [DOI] [PubMed] [Google Scholar]
- 19.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 20.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Eitzman DT. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol. 2009;7:169–179. doi: 10.2174/157016109787455680. [DOI] [PubMed] [Google Scholar]
- 21.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC: Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE, et al. Visceral adiposity and left ventricular remodeling: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25:667–676. doi: 10.1016/j.numecd.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaess BM, Pedley A, Massaro JM, Larson MG, Corsini E, Hoffmann U, et al. Relation of vascular growth factors with CT-derived measures of body fat distribution: the Framingham Heart Study. J Clin Endocrinol Metab. 2011;97:987–994. doi: 10.1210/jc.2011-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Calderon TM, Fallon JT, Berman JW. Hepatocyte growth factor is a survival factor for endothelial cells and is expressed in human atherosclerotic plaques. Atherosclerosis. 164:79–87. doi: 10.1016/s0021-9150(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 27.Rajpathak SN, Wassertheil-Smoller S, Crandall J, Liu S, Ho GYF. Hepatocyte growth factor and clinical diabetes in postmenopausal women. Diabetes Care. 2010;33:2013–2015. doi: 10.2337/dc10-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 29.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity. 2010;18:1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MA, Cappuccio FP. Cellular adhesion molecules and their relationship with measures of obesity and metabolic syndrome in a multiethnic population. Int J Obes. 2006;30:1176–1182. doi: 10.1038/sj.ijo.0803264. [DOI] [PubMed] [Google Scholar]
- 31.Chai S-B, Sun F, Nie X-L, Wang J. Leptin and coronary heart disease: A systematic review and meta-analysis. Atherosclerosis. 2014;233:3–10. doi: 10.1016/j.atherosclerosis.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin GA, Barrett-Connor E, May S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 2006;65:506–513. doi: 10.1111/j.1365-2265.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 33.Bosanska L, Michalsky D, Lacinova Z, Dostalova I, Bartlova M, Haluzikova D, et al. The influence of obesity and different fat depots on adipose tissue gene expression and protein levels of cell adhesion molecules. Physiol Res. 2010;59:79–88. doi: 10.33549/physiolres.931705. [DOI] [PubMed] [Google Scholar]
- 34.Yamakado M, Ishizaka Y, Takahashi E, Nakadate T. P-536: Role of soluble adhesion molecules in the pathogenesis of hypertensive vascular lesions in obesity. Am J Hypertens. 2003;16:231A–232A. [Google Scholar]
- 35.Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard J-M, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: The PRIME Study. Atherosclerosis. 2003;170:169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 36.Ponthieux A, Herbeth B, Droesch S, Haddy N, Lambert D, Visvikis S. Biological determinants of serum ICAM-1, E-selectin, P-selectin and L-selectin levels in healthy subjects: the Stanislas study. Atherosclerosis. 2004;172:299–308. doi: 10.1016/j.atherosclerosis.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Berardi C, Decker PA, Kirsch PS, de Andrade M, Tsai MY, Pankow JS, et al. Plasma and serum L-selectin and clinical and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Transl Res. 2014;163:585–592. doi: 10.1016/j.trsl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Despres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 39.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.