Abstract
Purpose
Examine the relationship between changes in cardiometabolic risk profiles and subsequent cardiovascular disease (CVD).
Methods
The study sample included 5,557 Multi-Ethnic Study of Atherosclerosis participants, recruited in 2000 from six U.S. counties. Standardized scores were calculated for metabolic and cardiovascular components relative to accepted clinical cutpoints, and summed to create an index of cardiometabolic risk. CVD events/deaths were assessed after exam 3 (years 2004–2005) through December 2011. Cox proportional hazards models were used to examine the association between change in the cardiometabolic index (exam 3 minus exam 1) and subsequent cardiovascular outcomes, adjusted for demographics, socioeconomic status, medication, and stratified by tertiles of baseline cardiometabolic risk.
Results
We found a 31% relative increase in the CVD event rate per standard deviation change in the cardiometabolic index among those in the highest tertile of baseline cardiometabolic risk (HR: 1.31, 95% CI: 1.14, 1.50); associations were not statistically significant in the lower tertiles of baseline risk.
Conclusions
We found that larger increases in the cardiometabolic index over time were significantly associated with higher risk for subsequent CVD events among those with elevated cardiometabolic risk at baseline. These findings highlight the importance of monitoring temporal changes in risk factor profiles for predicting cardiovascular outcomes.
Keywords: Cardiometabolic index, cardiovascular disease
Introduction
Allostatic load (AL) is a multi-system, biological risk score that incorporates measures from multiple biological regulatory systems to assess overall health across these systems [1]. Each of the components usually considered in most measures of AL have been shown independently associated with cardiovascular disease (CVD), including high risk lipid levels [2], high relative weight [3], high glucose [4], high blood pressure [5], high heart rate [6] as well as inflammatory markers [7]. Research has suggested, however that there is additional predictive value to assessing these health factors in one index [1,8]. Studies have indeed shown AL to be a significant predictor of subsequent risks for overall mortality, cognitive and physical functional decline and self-reported cardiovascular events [9,10].
We propose to extend this research by examining the association between change in AL over time and risk for subsequent CVD events. In this study, we consider a restricted version of AL that is focused on cardiometabolic risk factors due to data availability. We recently published one of the first studies to examine changes in this cardiometabolic index over time, and found a significant association between low socioeconomic status (SES) and increasing cardiometabolic index over time, among those starting out with lower levels of the cardiometabolic index at baseline (<median) [11]. This research raised basic questions regarding the progression of cardiometabolic risk profiles over time, and highlighted the overall dearth of research on changes in cardiometabolic risk, as well as the relationship between this process and subsequent “hard” outcomes, including frank CVD and CVD-related mortality.
Data from the Multi-Ethnic Study of Atherosclerosis (MESA) provide the opportunity to investigate longitudinal patterns of change in a multi-system index of biological risk and the relationship between such changes and subsequent CVD morbidity and mortality in a multi-ethnic cohort.
We propose to assess relationships between changes in cardiometabolic risk profiles during the first 4 years of follow-up (exams 1–3) and subsequent cardiovascular events and mortality during the remaining 6–7 years of follow-up.
Material and Methods
The MESA study is a prospective cohort study of the determinants of subclinical CVD with a multiethnic, population-based sample of 6814 men and women aged 45–84 years, including White, African American, Chinese, and Hispanic participants [12]. Participants were recruited from 6 US communities: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. The baseline examination took place July 2000 through August 2002, a second follow-up examination between September 2002 and February 2004, a third between March 2004 and September 2005, a fourth between September 2005 and May 2007, and a fifth between June 2010 and March 2012. Among those screened and eligible for the baseline examination, the participation rate was 59.8%, and the retention rates were 92.0%, 89.0%, 87.0%, and 76% of the original cohort through examinations 2–5, respectively. Details of the study design and recruitment for MESA have been published [12].
Study Sample
Our study sample included MESA participants who had no CVD events before MESA exam 3 (when follow-up for incidence of CVD begins in this analysis), attended MESA examinations 1 and 3, had available data for measuring cardiometabolic risk factors at exams 1 and 3, and had no missing follow-up data for CVD after exam 3. Of the original 6814, 89 were excluded due to experiencing a CVD event before exam 3 and missing exam 3, and an additional 119 were excluded due to non-CVD related death before exam 3. Another 142 participants were excluded due to experiencing a CVD event before exam 3 (although they attended that MESA exam). An additional 659 were excluded due to missing exam 3, and 248 participants were excluded due to missing follow-up surveillance for cardiovascular events after exam 3 (n=35), missing cardiometabolic risk factor data at exam 1 or 3 (n=33), missing education, home ownership or medication use (n=36), or missing car/ land ownership or investment data (n=144). Our final study sample was n=5557. As described in Table 1, those excluded from the analyses were significantly older, had higher cardiometabolic risk at each examination, included more African American, Hispanic and immigrant participants, had lower levels of SES, and higher levels of medication use at baseline.
Table 1.
Frequency distributions for select characteristics
| % or Mean, Median, STD | Study Sample (n=5557) |
Excluded MESA Sample (n=1257)a |
|---|---|---|
| Age [range: 44–84]* | 61.50, 61.0, 10.04 | 65.03, 66.0, 10.57 |
| Male | 46.73 | 49.01 |
| Race/Ethnicity* | ||
| African American | 26.63 | 32.70 |
| Chinese | 12.02 | 10.82 |
| Hispanic | 21.07 | 25.86 |
| White | 40.27 | 30.63 |
| Born outside the US* | 30.72 | 35.06 |
| Income* | ||
| Income <=$19,999 | 20.41 | 33.89 |
| Income $20–39,999 | 25.72 | 25.46 |
| Income $40–74,999 | 26.97 | 20.29 |
| Income >=$75,000 | 23.97 | 11.61 |
| Income Missing | 2.93 | 8.75 |
| Education* | ||
| Education: <=high school | 33.63 | 47.97 |
| Education: some college | 28.79 | 27.31 |
| Education: >=college | 37.57 | 24.72 |
| Wealth | ||
| Home ownership (yes vs. no)* | 69.01 | 58.66 |
| Land/property ownership (yes vs. no) | 30.45 | 27.40 |
| Car ownership (yes vs. no)* | 83.10 | 79.94 |
| Investments/stocks/bonds (yes vs. no)* | 64.12 | 50.63 |
| Medication use at baseline | ||
| Hypertensive medication* | 35.61 | 44.42 |
| Statins | 14.61 | 15.94 |
| Insulin* | 1.39 | 3.62 |
| Any new medication use at visit 2 or 3* | 23.39 | 16.87 |
| Cardiometabolic Index at baseline (continuous)* | −7.87, −7.89, 3.83 | −6.46, −6.60, 4.01 |
| Difference in Cardiometabolic Index (exam 3-exam 1)* | 0.06, 0.18, 2.64 | −0.61, −0.36, 3.09 |
| Incidence of any CVD event after exam 3* | 6.57 | 7.07 |
| Follow-up timeb (years) [range: 0.01–7.8]* | 6.68, 7.15, 1.52 | 6.29, 6.89, 1.83 |
Sample sizes were reduced when data missing: Nativity status(n=1235), Education(n=1234), home ownership(n=1224), land(n=635), car(n=648), investment(n=634), hypertension medication(n=1254), statin/insulin(n=1242), cardiometabolic index (n=1209), difference in index (n=354), study follow-up time(n=213) and incidence of CVD event (n=608 including those who attended visit 3 and those who did not and still have passive MESA surveillance).
Follow-up time included time between exam 3 (approximately 3 years after baseline MESA exam) and end of surveillance period at year 11 of the MESA study.
Difference between included and excluded sample is statistically significant, p<0.05.
Outcome
Cardiovascular disease and mortality events were ascertained based on MESA participant surveillance after exam 3 (calendar period March 2004-September 2005), through December 31, 2011. Participants were contacted by telephone every 9–12 months and asked about hospital admission, CVD outpatient diagnoses, procedures and deaths. Occasional medical visits were ascertained through regular MESA study exams, participant call-ins, medical record abstractions or obituaries. Self-reported diagnoses were all verified with copies of hospital/physician medical records or death certificates, and next-of-kin interviews were obtained for out of hospital CVD deaths. Two physicians independently reviewed and classified all CVD events and assigned incidence dates. In this study, we considered all CVD events according to the MESA definition, including myocardial infarction, resuscitated cardiac arrest, definite/probable angina, stroke, or deaths related to stroke, coronary heart disease or other CVD.
Exposure
We use a restricted measure of AL based on the metabolic and cardiovascular markers measured in MESA [11]. We refer to this restricted measure as a cardiometabolic index to distinguish it from other more broadly based measures of AL that reflect a wider array of biological systems, including inflammatory markers and stress hormones (that were not available longitudinally in MESA) [11]. Metabolic indicators included waist-to-hip ratio (WHR), triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and glucose (triglycerides, LDL cholesterol and glucose values were only included for those who fasted >=10 hours; glucose was log-transformed due to skewed values). Cardiovascular measures included systolic blood pressure, resting heart rate and pulse pressure. To reduce the undue influence of outliers, each of the biomarker values were Winsorised at the 1st and 99th percentiles [13]. Standardized scores were then calculated to indicate where the individual’s values place them (in standard deviation (SD) units) relative to accepted clinical thresholds. These thresholds are 0.90 WHR for men and 0.85 for women [14], 200 mg/dL for triglycerides [15], 160 mg/dL for LDL cholesterol [15], 40 mg/dL HDL cholesterol [15], 126 mg/dL of glucose [14], 140 mmHg for systolic blood pressure [5], 60 mmHg for pulse pressure [16], and 90 beat/min for heart rate [17]. Standardized scores for the individual parameters were summed across all systems to create a total cardiometabolic index. The index was set to missing if values were missing for more than half (>=5) of the 8 components in the score (n=0 had a missing risk score at baseline, n=4 at exam 2, n=0 at exam 3). For those missing <5 components, we imputed values based on the mean of each component across all available exams per participant (each participant had data values available for at least 2 other exams). Imputed values were used for n=1102 participants who were missing heart rate data at exam 2 due to a delay in initiation of heart rate data collection at that exam; only n=261 other values were imputed. We chose to use a cardiometabolic index constructed from continuous values of biology, unlike the more traditional measures of CVD risk, such as the Framingham risk score and the metabolic syndrome, which use categorized versions of biology, because the latter are not sensitive to changes in biology that do not cross category thresholds.
Change in the cardiometabolic index over time was measured as the cardiometabolic index at exam 3 minus the index at exam 1.
Covariates
We considered standard risk factors associated with CVD [18,19] as potential confounders in these analyses, including age (at MESA exam 1), gender, race/ethnicity (African American, Hispanic, Chinese and white), participant nativity (born abroad vs. born in the U.S.), individual-level SES and medication use. Individual SES measures included income and education reported at MESA exam 1 (categorized into approximate quartiles: ≤$19,999, $20–39,999, $40–74,999 and ≥$75,000 for income and ≤complete high school, some college, and ≥college for education), as well as wealth measures (home ownership, car ownership, land/other property ownership, investments; each of these considered as binary “yes/no” measures). Most of the wealth measures (aside from home ownership that was obtained at exam 1) were only available at MESA exam 2. Medication use included use of hypertension medication, statins, and insulin at MESA exam 1 (“yes/no”) and “new” use of any of these medications at exams 2–3 (“yes/no”).
Analysis
Initial descriptive analyses included examining the cardiometabolic score and the changes in the score between exam 1 and 3. The Kaplan-Meier method was used to construct descriptive survival curves for the time to first cardiovascular event (subsequent to exam 3) stratified by both distribution tertiles of change in cardiometabolic score (exam 3-exam 1) and exam 1 (hereafter referred to as baseline) tertiles of cardiometabolic score. Cox proportional hazards models were fit to examine the association between change in cardiometabolic score (continuous measure) and the time to subsequent cardiovascular morbidity/mortality events (between exam 3 and end of follow-up at year 11). We tested the Cox proportional hazards assumption by including time dependent covariates in the model and determining that none were statistically significant. The models were adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (all 4 binary measures), and medication use (3 terms considering the use of 3 types of medication at exam 1, and one term indicating new medication use at exams 2–3). Considering the MESA study design including a multi-ethnic cohort, we explored the possibility of interaction between race/ethnicity and change in cardiometabolic risk by including those interaction terms in the models.
Considering that when cardiometabolic risk is low, change in the risk score is unlikely to have the same association with subsequent CVD events as is change at levels closer to clinically defined higher risk of disease, we explored the potentially different implications of change in cardiometabolic scores for those starting with low (≤−9.53), medium (−9.54 to −6.31) or high (>−6.31) scores at baseline by including interaction terms for these levels (distribution tertiles) and change in cardiometabolic score. When considered separately, the interaction term for the middle tertile and highest tertiles were each marginally significant compared to the other levels (p=0.097 and p=0.06, respectively). In addition, we considered the potential bias that methodologists have highlighted concerning the inclusion of baseline values of the exposure as a control when estimating the association between change in the exposure of interest and an outcome [20]. We addressed both these issues by examining relationships between change in the cardiometabolic index and our outcome both with and without adjustment for baseline cardiometabolic risk (continuous), recognizing that the true effect size falls somewhere between those two estimates [20]. We also examined the models stratified by tertiles of the baseline cardiometabolic score as an alternative method of controlling for baseline while reducing (albeit not eliminating) potential bias from inclusion of the baseline as a covariate in regression, and to isolate potentially different implications of change in score for those starting with different levels of cardiometabolic risk at baseline. Finally, to assess if change in cardiometabolic risk substantively improved the models in addition to baseline measures of risk, we utilized log-likelihood ratio tests to compare the model fit between models included baseline cardiometabolic score, with and without change in the cardiometabolic score.
Results
The analytic sample is described in Table 1, with an average cardiometabolic score of −7.87 (standard deviation (SD): 3.83) at baseline, a modest average increase of 0.06 in cardiometabolic score between exam 1 and exam 3, and substantial variability in change across the sample (SD: 2.64). Average follow-up time after exam 3 (for CVD event monitoring) was 6.68 years (SD: 1.52, range: 0.01–7.80), and 6.57% of the sample experienced a CVD event over the follow-up period. The average change in cardiometabolic score between exams 1 and 3, SD, and the number of CVD events per tertile of baseline cardiometabolic risk was as follows: 1.11 (SD:2.08), n=54 (2.94%) CVD events for tertile 1; 0.23 (SD:2.42), n=115 (6.27%) events for tertile 2; −1.12 (SD: 2.84), n=196 (10.37%) events for tertile 3 (data not shown).
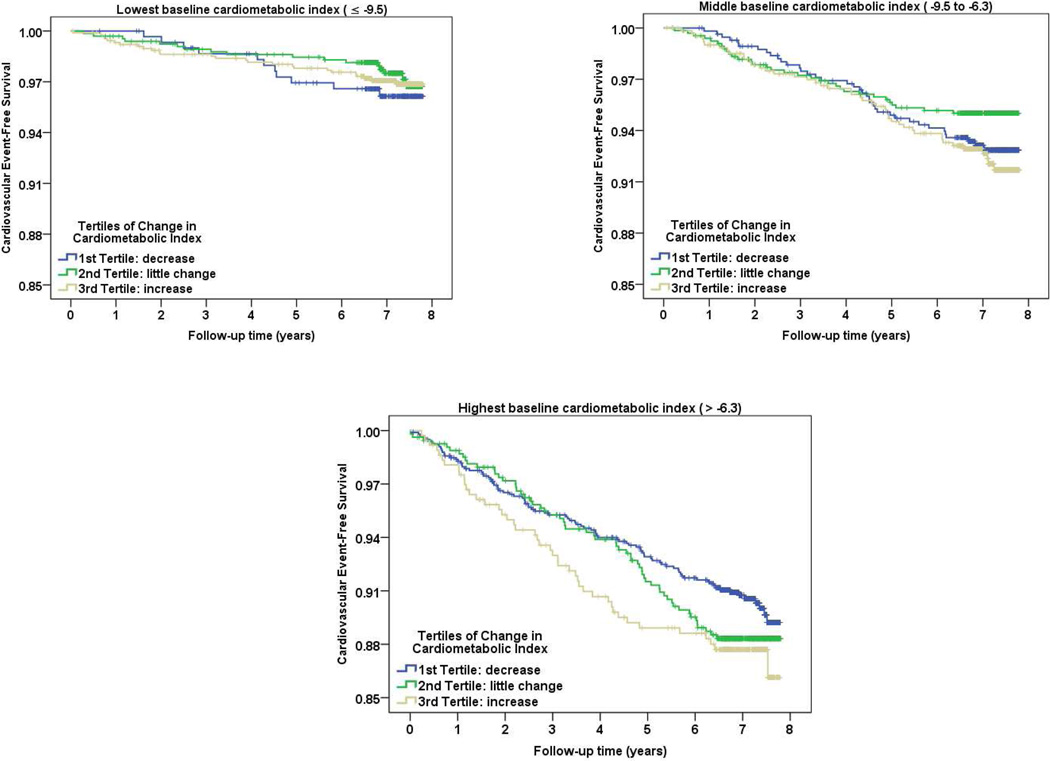
In Figures 1a–c, Kaplan-Meir plots illustrate the unadjusted trends for CVD event-free survival for those with increasing cardiometabolic risk over time (change > +1.14), compared to those with minimally changing (change −0.91 to + 1.14) or decreasing cardiometabolic risk (change ≤ −0.91), stratified by tertiles of baseline cardiometabolic risk. In the lower two tertiles of baseline cardiometabolic risk, CVD event-free survival did not appear to differ by change in cardiometabolic score (p-value=0.7 for Figure 1a, p=0.2 for Figure 1b). Among those with the highest tertile of baseline cardiometabolic risk, the survival curves started diverging early, with lowest survival among those who experienced an increase in cardiometabolic risk, however, those differences were also not statistically significant (p=0.2 for Figure 2c).
Figure 1.
(a–c). Survival function of cardiovascular event by levels of change in cardiometabolic risk, stratified by tertiles of baseline cardiometabolic risk.
Legend: Tertiles of change in Cardiometabolic Index
Blue: 1st Tertile: decrease
Green: 2nd Tertile: little change
Grey: 3rd Tertile: increase
The Cox proportional hazards model for the overall sample indicated no association between change in cardiometabolic score and CVD events when baseline cardiometabolic score was not included in the model (hazard ratio (HR): 1.04 per SD1 of change in cardiometabolic score, 95% confidence interval (CI): 0.94–1.15; see Table 2). When baseline cardiometabolic score was added to the model, both baseline and change scores were statistically significant. Analyses stratified by baseline cardiometabolic score further revealed that the strongest and only statistically significant association between increase in cardiometabolic score over time and risk of subsequent CVD events was among those starting off with the highest levels of cardiometabolic risk (HR: 1.22 per SD of change in cardiometabolic score, 95% CI: 1.06–1.40; see Table 2). Stratified models were re-run with additional adjustment for baseline cardiometabolic score within strata, yielding similar trends and an even greater hazard ratio for those starting out with the highest levels of cardiometabolic risk at baseline (HR: 1.31 per SD of change in cardiometabolic score, 95% CI: 1.14–1.50; see Table 2).
Table 2.
Adjusted hazard ratios (from Cox proportional hazards models) for subsequent CVD events per standard deviation increase in cardiometabolic index (between exams 1 and 3)a
| Model without baseline cardiometabolic risk |
Model with baseline cardiometabolic risk |
|
|---|---|---|
| Hazard Ratios (95% CI)b | Hazard Ratios (95% CI) | |
| Overall sample n=5557, #events=365 |
1.04 (0.94–1.15) | 1.24 (1.11–1.38)*** |
| Sample with baseline cardiomet score≤−9.5 n=1834 (tertile=1) #events=54 |
1.19 (0.85, 1.66) | 1.26 (0.91, 1.76) |
| Sample with −9.5<baseline cardiomet score≤−6.3 n=1833 (tertile=2) #events=115 |
1.04 (0.85, 1.27) | 1.06 (0.87, 1.30) |
| Sample with baseline cardiomet score>−6.3 n=1890 (tertile=3) #events=196 |
1.22 (1.06, 1.40)** | 1.31 (1.14, 1.50)*** |
CI: Confidence Interval
Main predictor: change in cardiometabolic risk score (exam 3 minus exam 1), adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (4 binary measures) and medication use (3 terms indicating 3 different types of medication at baseline and one term for any new medication use during follow-up).
The standard deviation of the cardiometabolic index change score (exam 3 minus exam 1) was 2.6.
p<0.05
p<0.01
p<0.001
Log likelihood ratio tests (LRT) comparing models that included baseline cardiometabolic score with and without change in cardiometabolic score, indicated statistically significant improvements with the addition of the change score, both in the overall sample (LRT p=0.0001), and in the top tertile of baseline score (LRT p=0.0002). LRT yielded no statistically significant improvements with the addition of change for the lowest and second tertiles of baseline score.
Interactions terms for race/ethnicity and change in cardiometabolic risk did not indicate any statistically significant interactions in the overall or stratified models.
Sensitivity analyses
We re-fit the models additionally adjusting for new medication use reported at MESA exams during the CVD event follow-up period (exams 4–5) since the medication indicators in the main models relate to exams 1–3, when changes in cardiometabolic score were assessed. However, the results of those models indicated similar trends to our main findings (Appendix, Table A1.). We also repeated the original models including indicators for stopping medication use over the course of MESA exams 1–3 (one term for each type of medication) and our results were virtually unchanged (Appendix Table A2). In addition, we re-ran the models excluding all those who required imputation of missing risk factors. The results were similar to our main models (Appendix Table A3).
Finally, considering reduced power for the models in strata with lower levels of baseline cardiometabolic score due to fewer CVD events, we re-ran the models with equal numbers of events in each of the three groups rather than by tertiles of baseline score (Appendix Table A4). These findings (additionally adjusted for baseline score within strata) showed statistically significant associations limited to the two higher levels of baseline score (HR: 1.26, 95%CI: 1.06–1.50 for highest level, HR: 1.27, 95%CI: 1.06–1.53 for middle level). The model for the lowest level of baseline score, similar to our main findings, indicated no statistically significant relationship between change of score and CVD events (HR: 1.12, 95%CI: 0.91–1.39).
Discussion
Our results indicate increased risk of CVD events associated with increases over time in the cardiometabolic index, among those who start out with higher levels of cardiometabolic risk. In this group with elevated cardiometabolic risk at baseline, we estimated relative increase in the CVD event rate to be between 22%–31% per standard deviation increase in the cardiometabolic index, adjusted for age, gender, race/ethnicity, nativity, SES and medication use (Table 2). This finding highlights the importance of monitoring changes in risk scores over time, especially for those with adverse cardiometabolic risk profiles, to improve the prediction of subsequent CVD morbidity and mortality.
Other studies have found increased risk of CVD events related to increases over time in individual cardiovascular risk factors, and noted that the impact of an increase differed by baseline values of the risk factors [21,22]. Our study suggests that baseline values are indeed crucial in determining the effects of further change in CVD risk factors and in the cumulative cardiometabolic score. In previous analyses, we had noted that the rate of increase in the cardiometabolic score over time also varies by starting levels. In particular, we found lower rates of increased cardiometabolic score over time associated with those starting with elevated levels of baseline cardiometabolic risk [11]. Our current findings show that despite these generally lower rates of increase, nonetheless, additional elevation at those levels may have graver consequences for CVD risk.
The strengths of this analysis include its large and racially/ethnically diverse population with multiple biomarkers available over time, allowing the unique opportunity to examine longitudinal changes in cardiometabolic scores. Moreover, MESA data provide the unique ability to follow participants for incidence of CVD events. There are also, however, several limitations that should be acknowledged. Our index of cardiometabolic risk does not take into account other potentially important biological systems (e.g., inflammation, neuroendocrine) that are related to the incidence of CVD. Moreover, we did not distinguish between pharmaceutically controlled and native values of risk factors in the creation of the cardiometabolic score; however, we did adjust for medication use in the regression models. Finally, we considered that our inability to detect a significant effect of change in the index on subsequent incident events in the bottom two tertiles of baseline risk may be the result of the significantly fewer events in the lower tertiles. We addressed this issue by considering models with equal numbers of events in each of the three groups rather than by tertiles of baseline cardiometabolic score (Appendix Table A4). Our findings indicated that, even with this new stratification, we still did not see a statistically significant relationship between change of score and CVD events in the lowest group. While the newly defined middle group showed similar significant effects to the highest group, the cutpoint also shifted so that it included levels of baseline risk that fell into the top tertile of the distribution (i.e., our original cutpoint of >−6.3). The similarity of effects in the newly defined middle and top groups is thus consistent with our main results indicating that those with higher baseline risk are at greater risk of CVD events related to subsequent changes in their cardiometabolic risk scores.
Conclusion
These findings demonstrate the significant impact of temporal change in cardiometabolic profiles on subsequent event risk among those who have elevated risk profiles, as well as the value of using continuous measures of risk factors to assess and monitor change in risk. These results further suggest that different stages in the natural progression of cardiometabolic dysregulation may have different implications for future CVD events. Identifying the differential contributions to disease risk associated with patterns of change in cardiometabolic risk profiles from different starting levels of cardiometabolic risk can better inform policy interventions aimed at mitigating and preventing disease.
Acknowledgements
Financial Support
This work was supported by the following grants from the National Institutes of Health: 5R01HI101161-03, N01-HC-95160:25, and 1R21AG046589. Funding for statistical analyses were supported in part by the NIH/National Center for Advancing Translational Science (NCTAS) UCLA CTSI, grant UL1TR000124, and by the Analysis and Cost Effectiveness Core of the UCLA Older Americans Independence Center, NIH 5P30-AG028748-09. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
List of Abbreviations and Acronyms
- CVD
cardiovascular disease
- MESA
Multi-Ethnic Study of Atherosclerosis
- US
United States
- AL
allostatic load
- WHR
waist to hip ratio
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- SD
standard deviation
- HR
hazard ratio
- CI
confidence interval
Appendix
Table A1.
Final models additionally adjusting for medication use reported during the CVD follow-up period (between exams 4–5)a
| Model without baseline cardiometabolic risk |
Model with baseline cardiometabolic risk |
|
|---|---|---|
| Hazard Ratios (95% CI)b | Hazard Ratios (95% CI) | |
| Overall sample n=5315, #events=325 |
1.02 (0.92–1.14) | 1.25 (1.11–1.40)*** |
| Sample with baseline cardiomet score ≤−9.5 n=1778 (tertile=1) #events=48 |
1.27 (0.89, 1.81) | 1.37 (0.96, 1.95) |
| Sample with −9.5<baseline cardiomet score ≤−6.3 n=1755 (tertile=2) #events=105 |
1.03 (0.83, 1.27) | 1.05 (0.85, 1.31) |
| Sample with baseline cardiomet score >−6.3 n=1782 (tertile=3) #events=182 |
1.23 (1.06, 1.43)** | 1.32 (1.13, 1.53)*** |
CI: Confidence Interval
Main predictor: change in cardiometabolic risk score (exam 3 minus exam 1), adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (4 binary measures) and medication use (3 terms indicating 3 different types of medication at baseline and one term for any new medication use during follow-up), and new medication use during CVD follow-up period (exams 4–5). Sample size is reduced due to missing values for medication use at exams 4 and 5.
The standard deviation of the cardiometabolic index change score (exam 3 minus exam 1) was 2.6.
p<0.05
p<0.01
p<0.001
Table A2.
Final models additionally adjusting for indicator of stopped medication use across MESA exams 1–3a
| Model without baseline cardiometabolic risk |
Model with baseline cardiometabolic risk |
|
|---|---|---|
| Hazard Ratios (95% CI)b | Hazard Ratios (95% CI) | |
| Overall sample n=5557, #events=365 |
1.04 (0.94–1.16) | 1.24 (1.11–1.38)*** |
| Sample with baseline cardiomet score ≤−9.5 n=1834 (tertile=1) #events=54 |
1.18 (0.84, 1.66) | 1.26 (0.90, 1.77) |
| Sample with −9.5<baseline cardiomet score ≤−6.3 n=1833 (tertile=2) #events=115 |
1.05 (0.86, 1.29) | 1.07 (0.87, 1.32) |
| Sample with baseline cardiomet score >−6.3 n=1890 (tertile=3) #events=196 |
1.22 (1.07, 1.40)** | 1.32 (1.14, 1.52)*** |
CI: Confidence Interval
Main predictor: change in cardiometabolic risk score (exam 3 minus exam 1), adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (4 binary measures) and medication use (3 terms indicating 3 different types of medication at baseline and one term for any new medication use during follow-up), and indication of stopped medication usage (3 terms for 3 medication types: hypertensive, statin and insulin) over the course of MESA exams 1–3.
The standard deviation of the cardiometabolic index change score (exam 3 minus exam 1) was 2.6.
p<0.05
p<0.01
p<0.001
Table A3.
Final models excluding all values requiring imputationa
| Model without baseline cardiometabolic risk |
Model with baseline cardiometabolic risk |
|
|---|---|---|
| Hazard Ratios (95% CI)b | Hazard Ratios (95% CI) | |
| Overall sample n=5514, #events=361 |
1.03 (0.93–1.15) | 1.22 (1.10–1.36)*** |
| Sample with baseline cardiomet score ≤−9.5 n=1829 (tertile=1) #events=53 |
1.19 (0.85–1.66) | 1.26 (0.90–1.77) |
| Sample with −9.5<baseline cardiomet score ≤−6.3 n=1826 (tertile=2) #events=115 |
1.04 (0.85–1.28) | 1.06 (0.87–1.30) |
| Sample with baseline cardiomet score >−6.3 n=1859 (tertile=3) #events=193 |
1.20 (1.05–1.38)** | 1.28 (1.12–1.48)*** |
CI: Confidence Interval
Main predictor: change in cardiometabolic risk score (exam 3 minus exam 1), adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (4 binary measures) and medication use (3 terms indicating 3 different types of medication at baseline and one term for any new medication use during follow-up).
The standard deviation of the cardiometabolic index change score (exam 3 minus exam 1) was 2.6.
p<0.05
p<0.01
p<0.001
Table A4.
Models stratified by equal number of CVD eventsa
| Model without baseline cardiometabolic risk |
Model with baseline cardiometabolic risk |
|
|---|---|---|
| Hazard Ratios (95% CI)b | Hazard Ratios (95% CI) | |
|
Levels of Baseline cardiomet score by equal # CVD events |
||
| Sample with baseline cardiomet score by equal≤−7.5 n=3034 (tertile=1) #events=121 |
1.04 (0.84, 1.29) | 1.12 (0.91, 1.39) |
| Sample with −7.5<baseline cardiomet score ≤−4.7 n=1428 (tertile=2) #events=120 |
1.27 (1.06, 1.53)* | 1.27 (1.06, 1.53)* |
| Sample with baseline cardiomet score >−4.7 n=1095 (tertile=3) #events=124 |
1.16 (0.98, 1.37) | 1.26 (1.06, 1.50)* |
CI: Confidence Interval
Main predictor: change in cardiometabolic risk score (exam 3 minus exam 1), adjusted for age, gender, race/ethnicity, nativity, income, education, wealth (4 binary measures) and medication use (3 terms indicating 3 different types of medication at baseline and one term for any new medication use during follow-up), and new medication use during CVD follow-up period (exams 4–5). Sample size is reduced due to missing values for medication use at exams 4 and 5.
The standard deviation of the cardiometabolic index change score (exam 3 minus exam 1) was 2.6.
p<0.05
p<0.01
p<0.001
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This refers to the standard deviation of the difference between cardiometabolic index at exam 3-exam 1. The SD is 2.6 (see Table 1).
References
- 1.Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program (NCEP) Expert Panel (2001) Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006 Feb 14;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. Epub 2005 Dec 27. [DOI] [PubMed] [Google Scholar]
- 4.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011 Nov 2;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007 Aug 28;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. Epub 2007 Aug 13. Review. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005 Jun 28;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 8.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci. 2001 Apr 10;98(8):4770–4775. doi: 10.1073/pnas.081072698. Epub 2001 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006 May-Jun;68(3):500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- 10.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002 Jul;55(7):696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- 11.Merkin SS, Karlamangla A, Roux AV, Shrager S, Seeman TE. Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: multi-ethnic study of atherosclerosis. Am J Public Health. 2014 Apr;104(4):e48–e55. doi: 10.2105/AJPH.2013.301841. Epub 2014 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Pace LA. Contemporary Statistical Methods. R Recipes: A Problem Solution Approach. Apress. (1 Edition) 2014 Dec 19;:157–166. [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program (NCEP) Expert Panel (2001) Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Haider AW, Larson MG, Franklin SS, Levy D Framingham Heart Study. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003 Jan 7;138(1):10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 17.Seccareccia F, Pannozzo F, Dima F, Minoprio A, Menditto A, Lo Noce C, et al. Heart rate as a predictor of mortality: the MATISS project. American Journal of Public Health. 2001;91(8):1258–1263. doi: 10.2105/ajph.91.8.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford ES, Giles WH, Mokdad AH. The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004 May 19;43(10):1791–1796. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005 Mar 15;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 20.Cain KC, Kronmal RA, Kosinski AS. Analysing the relationship between change in a risk factor and risk of disease statistics in medicine. Stat Med. 1992 Apr;11(6):783–797. doi: 10.1002/sim.4780110609. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005 Feb;59(2):134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda S, Shimada K, Fujita M, Yoshiyama M, Yoshikawa J, Kohro T, Hayashi D, Yamazaki T, Nagai R. Changes in serum cholesterol levels determine future risk of cardiovascular events in patients with acute coronary syndrome in the Japanese Coronary Artery Disease (JCAD) Study. J Cardiol. 2013 Jun;61(6):387–392. doi: 10.1016/j.jjcc.2013.02.006. [DOI] [PubMed] [Google Scholar]