Abstract
NF-κB transcription factors regulate the expression of hundreds of genes primarily involved in immune responses. Signaling events leading to NF-κB activation constitute a major antiviral immune pathway. To replicate and persist within their hosts, viruses have evolved diverse strategies to evade and exploit cellular NF-κB immune signaling cascades for their benefit. We summarize recent studies concerning viral manipulation of the NF-κB signaling pathway downstream of pattern recognition receptors. Signal transduction mediated by pattern recognition receptors is a research frontier for both infectious disease and innate immunology.
1. Introduction
NF-κB proteins constitute a family of transcription factors (TFs) sharing Rel homology domain that regulate the transcription of genes downstream of promoters containing κB sequences [1–3]. The NF-κB-responsive genes encode a large array of effectors in the host antiviral defense system. Upon viral infection, cells initiate signaling events that activate NF-κB TFs, either via pattern recognition receptors (PRRs) or in response to entry processes per se. Thus, cellular NF-κB activation imposes selective pressure on viruses. Not surprisingly, numerous viruses modulate key components of the NF-κB pathway to evade antiviral responses or, even to promote viral infection. The classic examples of NF-κB activation by viral oncogenic proteins, such as Kaposi’s sarcoma-associated herpesvirus (KSHV) vFLIP [4], Epstein-Barr virus (EBV) LMP1 [5] and human T cell leukemia virus (HTLV) Tax proteins [6], have been extensively reviewed elsewhere [7]. In this review, we will summarize recent studies concerning NF-κB manipulation in the context of PRRs that represent a research frontier in infectious disease. To provide a view integrating viral modulation with host regulation of NF-κB activation, we review viral factors according to the targeted component of the NF-κB signaling pathway.
2. NF-κB activation by PRRs
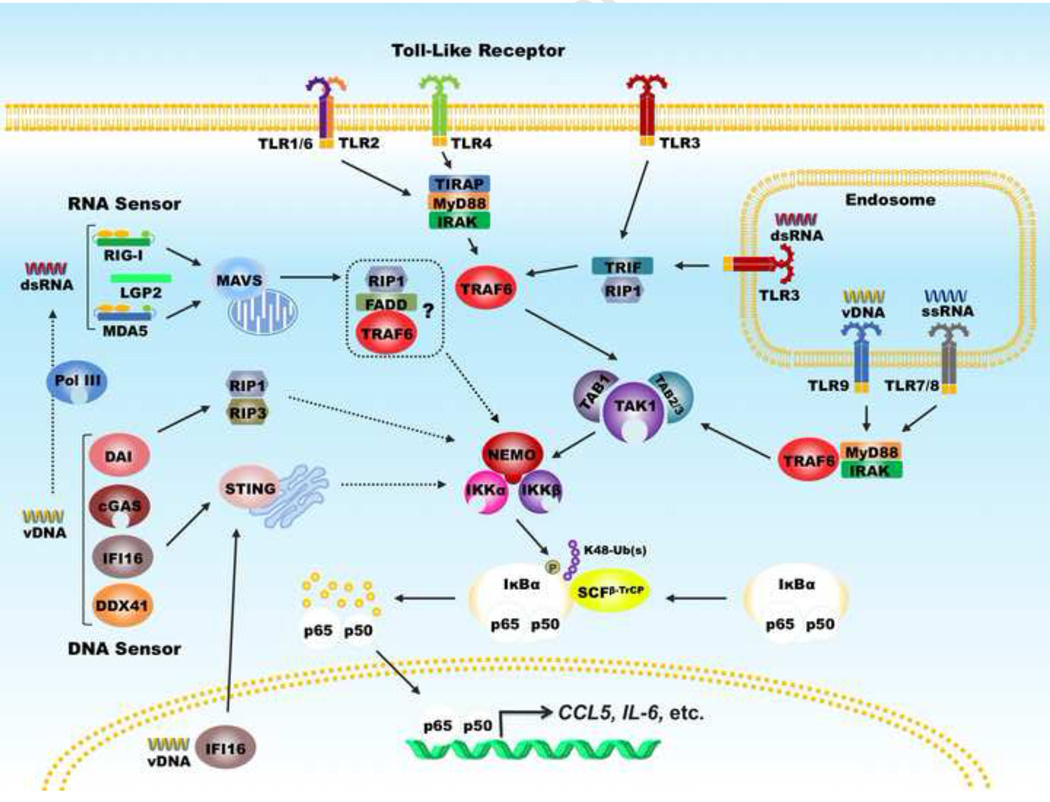
In resting cells, NF-κB TFs are kept as an inactive dimer by inhibitors of NF-κB (IκBs) in the cytoplasm [8]. Upon viral infection, PRRs, such as Toll-like receptors (TLRs), RIG-like receptors (RLRs) and DNA sensors, recognize pathogen-associated molecular pattern (PAMPs). When bound to cognate PAMPs, TLR3 can engage TRIF, while the other TLRs signal through myeloid differentiation primary response gene 88 (MyD88) [9]. RIG-I-like receptors, e.g., retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5), signal via mitochondria antiviral-signaling protein (MAVS) [10–13]. These adaptor molecules, in turn, recruit TNF receptor-associated factor (TRAF) and transforming growth factor β-activated kinase 1 (TAK1) [14]. TRAF, e.g., TRAF6, catalyzes the synthesis of K63-linked polyubiquitin chains that recruit and activate the IKK kinase complex, comprising IKKα, IKKβ and IKKγ (also known as NEMO) [15]. Activated IKK phosphorylates IκBs, e.g., IκBα, which primes IκBs for ubiquitination by SCFβ-TrCP complex and subsequent degradation by the 26S proteasome [16,17]. Destruction of IκBs unleashes the NF-κB dimer (primarily consisting of p65 [also known as RelA] and p50) which then translocates into the nucleus to up-regulate the expression of hundreds of genes including an array of inflammatory cytokines that constitute a robust antiviral innate immune response (Figure 1). Recent studies have implicated several DNA sensors, including cyclic GMP-AMP synthase (cGAS) [18], interferon-γ inducible protein 16 (IFI16) [19], DDX41 [20] and DAI [21], in sensing viral DNA and activating downstream NF-κB and interferon regulatory factor (IRF) signaling via the STING adaptor.
Figure 1. Signaling of NF-κB activation downstream of pattern recognition receptors.
Pattern recognition receptors, including Toll-like receptors, RIG-I-like receptors and DNA receptors, interact with their cognate adaptors and activate the canonical IKKαβγ complex. The TRAF molecules and TAB-TAK1 complex link distinct adaptors to the IKKαβ kinase. IKKα or IKKβ phosphorylates inhibitors of NF-κB that is subsequently degraded by the proteasome, releasing the p65-p50 dimer to translocate into the nucleus and up-regulate the expression of a large set of inflammatory cytokines. Dashed lines indicate that the signaling events are not well defined.
As intracellular obligate “parasites”, viruses have evolved diverse strategies to modulate NF-κB signaling. For example, human KSHV and EBV, two oncogenic gamma herpesviruses, activate NF-κB during latency to exploit its pro-survival roles. This contributes to oncogenic transformation [22]. Human immune-deficient virus 1 (HIV-1) and herpes simplex virus 1 (HSV-1) usurp NF-κB to activate viral gene expression [23,24]. Murine gamma herpesvirus 68 (γHV68), a model herpesvirus for human KSHV and EBV, activates RIG-I and IKKβ to promote viral lytic gene expression, while dampening host inflammatory immune gene expression [25–28]. Other viruses inhibit NF-κB activation primarily to evade host inflammatory and immune responses (Table 1). These viruses deploy diverse factors that target nearly every key step of NF-κB signaling pathway, from PRRs to NF-κB-dependent transcription [29].
Table 1.
Viral inhibitors of the NF-κB signaling pathway
| Cellular target | Viral protein | Virus | Mechanism | Refs | |
|---|---|---|---|---|---|
| PRR Receptor and Adaptor | RIG-I | V protein | Measles virus | Blocks PP1 phosphatase activities | [41] |
| G protein | Human metapneumovirus virus | Binds to CARD domain of RIG-I and blocks its dimerization with MAVS | [42] | ||
| Human respiratory syncytial virus | [43] | ||||
| MDA5 | V protein | Measles virus | Blocks PP1 phosphatase activities | [40] | |
| TLR2 | RTA | Gamma-herpesvirus 68, Kaposi’s sarcoma-associated virus (KSHV) | Down-regulates TLR2 expression | [37] | |
| TLR4 | Down-regulates TLR4 expression | [37] | |||
| TLR3 | NS1 | West Nile Virus (WNV) | Unknown | [78] | |
| MAVS | NS3/4a | Hepatitis C Virus (HCV) | Cleaves MAVS into non-functional fragments | [30] | |
| Protease 3C | Coxsackievirus B | [32] | |||
| TRIF | Protease 3CD | Hepatitis A Virus (HAV) | Cleaves TRIF into non-functional fragments | [34] | |
| Protease 3C | Enteroviruses | [33] | |||
| MyD88 | ICP0 | Human Simplex Virus 1 (HSV-1) | Targets MyD88 for proteasomal degradation | [36] | |
| TIRAP | Targets TIRAP for proteasomal degradation | [36] | |||
| TRAF6 | BPLF1 | Epstein-Barr Virus (EBV) | Interacts with and deubiquitinates TRAF6 | [79] | |
| IKK kinases | IKKγ (NEMO) | Protease 3C | HAV | Cleaves NEMO into non-functional fragments | [44] |
| NSP4 | Porcine reproductive and respiratory syncytia virus | Sequesters IKKγ from IKKα and IKKβ | [45] | ||
| IKKα IKKβ |
PB1-F2 | Influenza A virus | Interacts with and blocks IKK kinases activation | [47] | |
| NS1 | [46] | ||||
| Polymerase | Hepatitis B Virus (HBV) | [48] | |||
| IκBs | IκBs | Ankyrin-repeat proteins | Poxvirus | Competes for SCF ubiquitin ligase complex | [51] |
| A49 | Poxvirus | Mimics IκBs to target β-TrCP | [52] | ||
| NSP1 | Human Rotavirus | Targets β-TrCP for proteasomal degradation | [53] | ||
| NFκB | RelA | US3 | HSV-1 | Hyperphosphorylates RelA to prevent its nuclear translocation | [54] |
| EMV150 | Ectromelia | Interacts with RelA and blocks its nuclear translocation | [57] | ||
| ICP0 | HSV-1 | [55] | |||
| ORFV002 | Poxvirus | [56] | |||
| ORF002 | Vaccinia virus | Competes RelA for CBP/p300 | [59] | ||
| VP16 | HSV-1 | [60] | |||
| ORF61 | Varicella-zoster virus | Blocks RelA nuclear translocation, mechanism unknown | [80] | ||
| P50 | ICP0 | HSV-1 | Targets p50 for proteasomal degradation | [55] | |
| UXT | BGLF4 | EBV | Phosphorylates co-activator UXT to dampen NFκB activity | [61] | |
3. Inactivation of NF-κB signaling cascades
3.1 TLRs, RLRs and their adaptors
PRRs detect PAMPs integral to pathogen infection, including pathogen structural components and replication intermediates. In viral infection, TLRs and RLRs often recognize viral nucleic acid. One common mechanism to disrupt innate immune signaling downstream of these receptors is an inactivating cleavage of the crucial adaptor molecules, namely MAVS for RLRs and TRIF or MyD88 for TLRs by virally encoded proteases. Following the discovery of MAVS cleavage by HCV NS3/4a protease [12,30], studies revealed that a number of RNA viruses deploy similar strategies to proteolytically destroy MAVS and TRIF molecules, which halts innate immune signaling at a very early step [31]. Recent work showed that hepatitis A virus, coxsackievirus B and enteroviruses encode cysteine proteases that target TRIF to disable TLR3 signaling [32–34]. A TLR2-mediated immune response is implicated in the pathogenesis of HSV-1 [35] and the ICP0 E3 ligase function targets MyD88 and Mal (TIRAP) for degradation to suppress the inflammatory response downstream of TLR2 [36]. Murine γHV68 and human KSHV express multiple proteins interfering with TLR signaling, including ORF21, ORF31, ORF50 and K4.2 (KSHV-specific). The ORF50 (RTA) TF initiates ubiquitous gene expression for murine γHV68 and human KSHV, and serves as an E3 ligase that downregulates TLR2 and TLR4 expression [37]. The mechanistic action of the other three proteins of KSHV and γHV68 in evading TLR signaling remains unclear.
In resting cells, RIG-I is kept in a latent state by an intramolecular interaction that is secured by phosphorylation [38]. Thus, dephosphorylation is a prerequisite step for RIG-I activation by RNA [39]. Measles virus V protein blocks this action of the PP1 phosphatase to suppress RIG-I- and MDA-5-dependent innate immune responses [40,41]. Human metapneumovirus and respiratory syncitial virus express secreted glycosylated G proteins, in addition to their membrane-anchored longer isoform. The secreted G proteins bind to the CARD domain of RIG-I, but not that of MDA-5, blocking RIG-I dimerization with MAVS and nullifying RIG-I-mediated signal transduction [42,43].
3.2. IKK kinases
The core event of NF-κB signaling cascade is the activation of the trimeric IκB kinase (IKK) comprising catalytic IKKα or IKKβ and the scaffold protein IKKγ. While IKKα and IKKβ show some redundancy in their phosphorylation of downstream substrates, the scaffolding IKKγ is absolutely required for the recruitment and activation of IKKs [3]. Hepatitis A virus 3c protease cleaves IKKγ to block activation of the IKK kinase complex, whereas the NSP4 protein of porcine reproductive and respiratory syncytial virus binds to IKKγ and effectively sequesters it from IKKα and IKKβ [44,45]. Additionally, IKK kinases are targeted by the polymerase of hepatitis B virus, the NS1 protein and the PB1-F2 polypeptide of influenza A virus [46–48]. These viral factors physically associate with IKK kinases and prevent their activation in response to viral infection, thereby terminating upstream signaling. Collectively, viruses target the assembly and phosphorylation of the IKK complex to prevent proper activation of IKK kinases.
3.3. Degradation of IκBs
IκBs keep NF-κB TFs in an inactive state that is readily activated upon acute stimulation by viral infection or TNF-α treatment. The degradation of IκBs is governed by serine phosphorylation within the N-terminal domain, subsequent ubiquitination by the β-TrCP E3 ligase and ultimately proteolytic cleavage by the proteasome [49,50]. Poxviruses express a family of multiple proteins containing ankyrin-repeat sequences, the signature structural element of IκBs and some of these viral proteins interfere with the degradation of IκBs [51]. For example, the vaccinia virus A49 protein harbors a sequence mimicking IκBα to block the action of the β-TrCP E3 ligase responsible for recognizing and ubiquitinating phosphorylated IκBs, thus stabilizing IκBs and inhibiting NF-κB activation [52]. The non-structural protein 1 (NSP1) of human rotavirus instead blocks the degradation of IκBs by inducing the degradation of β-TrCP [53]. In essence, viral proteins display structural mimicry of IκBs and β-TrCP to stabilize IκBs and avoid NF-κB activation.
3.4 NF-κB TFs
Degradation of IκB subunits unleashes the p50–p65 dimer that translocates into the nucleus and, in collaboration with other TFs and activators, up-regulates anti-viral gene expression. Recent studies have identified viral proteins that inhibit NF-κB activation by targeting its nuclear translocation and transcriptional activation.
HSV-1 expresses a serine/threonine kinase, US3, which potently phosphorylates p65 at serine 75 [54]. Mutations that abolished the kinase activity of US3 also impaired its ability to block NF-κB activation. With kinase-active US3, hyperphosphorylated p65 was retained in the cytoplasm, even when cells were stimulated with TNF-α, a cytokine that induces acute NF-κB activation. It is not clear whether the US3 homologues of other herpesviruses possess a similar function. Additionally, the ICP0 E3 ligase of HSV-1 antagonizes NF-κB activation via both p50 and p65. While ICP0 degraded p50 via the RING finger domain that interacts with the Rel homology domain of p50, ICP0 bound p65 and blocked its nuclear translocation [55]. A similar strategy is deployed by the mouse poxvirus [56], ectromelia virus, in blocking p65 nuclear translocation. In cells expressing the ectromelia virus EMV150 protein, one member of the EMV ankyrin-repeat-containing family, IκB degradation was observed while p65 resided in the cytoplasm of cells stimulated with TNF-α. Mutational analysis showed the BTB/Kelch domain of EMV150 was sufficient to interact with and retain p65 in the cytoplasm, implicating the BTB/Kelch domains which are shared by various cellular proteins in regulating p65 nuclear translocation [57]. These studies emphasized that the nuclear translocation of NF-κB subunits is a crucial step in mounting a cellular anti-viral response.
The transcriptional activity of NF-κB is modulated by post-translational modifications and transcription co-activators/co-repressors. For example, the phosphorylation of p65 at ser529 is necessary for its interaction with and subsequent acetylation by CBP/p300. Further enhanced by ser536 phosphorylation, acetylation of p65 at lys310 increases its transcription activity [58]. The p65-CBP/p300 interaction is targeted by vaccinia virus and HSV-1. These viruses encode ORF002 and VP16 that compete with CBP/p300 for binding to p65 [59,60]. P65 acetylation and transcription are then severely attenuated, thereby suppressing inflammatory cytokine expression. UXT is a transcriptional co-activator that enhances NF-κB-mediated gene expression. The EBV BGLF4 kinase phosphorylates UXT and dampens the transcription activity of NF-κB [61].
4. Activation of the NF-κB signaling pathway by viral proteins
Some viruses activate the NF-κB signaling pathway to promote viral infection and/or cell proliferation [62]. These viruses have evolved distinct mechanisms to target key signaling components of the NF-κB pathway, such as PRRs and IKKs. We highlight viral factors of NF-κB activation in relation to their cellular targets (Table 2).
Table 2.
Viral activators of the NF-κB signaling pathway
| Cellular target | Viral protein | Virus | Mechanism | Refs | |
|---|---|---|---|---|---|
| Receptor | RIG-I | vGAT | Gamma-herpesvirus 68, Kaposi’s sarcoma-associated virus (KSHV) | vGAT interacts with and activates RIG-I via deamidation | [28] |
| TLR2 | gB | Herpes simplex virus-1 (HSV-1) | gB interacts with and activates TLR2 in a MyD88/TRAF6 dependent manner | [64] | |
| gH/gL | HSV-1 | gH/gL interacts with and activates TLR2 | [63] | ||
| Kinase | IKKα/β | Vpr | Human immunodeficiency virus type 1 (HIV-1) | Vpr interacts with and activates IKKα/β through enhancing their phosphorylation | [68] |
| IKKε | vGPCR | KSHV | vGPCR interacts with and activates IKKε, which in turn promotes p65 phosphorylation | [66] | |
| TAK1 | gp41CD | HIV-1 | gp41CD interacts with TAK1 and TAK1 activity is required for gp41CD-induced NF-κB activation | [67] | |
| vGPCR | KSHV | vGPCR interacts with and activates TAK1 through inducing its phosphorylation and ubiquitination | [65] | ||
| Vpr | HIV-1 | Vpr interacts with and activates TAK1 through inducing its phosphorylation | [69] | ||
| Others | IκBα | Tat | HIV-1 | Tat interacts with IκBα and prevents its binding to the NF-κB complex | [70] |
| NEMO | HBx | Hepatitis B virus | HBx interacts with p22-FLIP and NEMO to form a ternary complex | [71] | |
| NFX1-91 | E6 | Human papillomavirus | E6 targets NFX1-91 for degradation | [72] | |
| RelA | Tat | HIV-1 | Tat interacts with p65 and enhances the DAN-binding ability and transcriptional activity of p65 | [70] | |
| Vpr | HIV-1 | Vpr promotes the phosphorylation of p65 | [68] | ||
| Unknown | mu2 | Reovirus | Unknown | [81] | |
| N | Porcine epidemic diarrhea virus | Unknown | [82] | ||
| N | Porcine reproductive and respiratory syndrome virus (PRRSV) | Unknown | [83] | ||
| Nsp2 | PRRSV | Unknown | [84] | ||
4.1 PRRs
A few viral proteins directly engage TLR2 and RIG-I to elicit NF-κB activation. Recent studies have shown that glycoproteins gH/gL of HSV directly interacts with TLR2 and is sufficient to activate NF-κB [63]. Similarly, HSV gB associates with TLR2 and purified gB activates NF-κB in cells expressing TLR2 [64]. We have identified viral glutamine amidotransferases (vGATs) of γHV68 and KSHV that activate NF-κB via RIG-I. vGATs physically associate with and activate RIG-I in the absence of any viral RNA [28]. Though lacking intrinsic enzyme activity, vGATs recruit their cellular homologue, phosphoribosylformylglycinamidine synthase (PFAS), to deamidate RIG-I, thereby activating RIG-I independent of RNA. When exogenously expressed, deamidated RIG-I is competent to trigger NF-κB and IRF activation without viral RNA.
4.2 Kinases
IKKα/β, IKKε and TAK1 are targeted by viral proteins to stimulate NF-κB activation. KSHV GPCR (vGPCR) requires both IKKε and TAK1 to activate NF-κB [65,66]. vGPCR interacts with and activates both IKKε and TAK1, and loss or depletion of IKKε and TAK1 impairs vGPCR-induced NF-κB activation [65,66]. While TAK1 phosphorylation and lysine 63-linked polyubiquitination are induced by vGPCR [65], IKKε also promotes p65 phosphorylation and so promotes tumor formation [66]. The link between TAK1 and IKKε in vGPCR-induced NF-κB activation remains undefined. The cytoplasmic domain of HIV-1 glycoprotein gp41 (gp41CD) induces NF-κB activation via TAK1 [67]. gp41CD physically interacts with TAK1 and TAK1 activity is essential for gp41CD-induced NF-κB activation. Additionally, HIV-1 Vpr targets both IKKα/β and TAK1 to activate NF-κB [68,69]. Vpr interacts with and activates IKKα/β through enhancing the phosphorylation of IKKα/β, and Vpr also directly promotes the phosphorylation of p65 to activate NF-κB [68]. Both IKKα/β and TAK1 are required for Vprinduced NF-κB activation. In activating TAK1, Vpr interacts with TAK1 and enhances the TAB3-TAK1 interaction, thereby increasing TAK1 polyubiquitination, phosphorylation and consequent activation.
4.3 Other signaling components
HIV-1 Tat interacts with the p65 subunit of NF-κB, thus enhancing the DNA-binding ability of p65 and the transcription of HIV-1 LTR by p65 [70]. This is consistent with the corollary that HIV utilizes activated NF-κB to promote viral gene expression. Tat also associates with IκBα and prevents its binding to the NF-κB complex. Hepatitis B virus X protein (HBx) physically associates with the cleaved p22 form of cFLIP (p22-FLIP) and NEMO to form a ternary complex [71], which is required for HBx-induced NF-κB activation. This result is consistent with that p22-FLIP associates with and activates IKKα/β to instigate NF-κB activation. Human papillomavirus E6 activates NF-κB through targeting NFX1-91 for degradation [72]. NFX1-91 binds to the promoter of the NF-κB inhibitor p105 and up-regulates its expression.
5. Hijacking NF-κB signaling events by virus
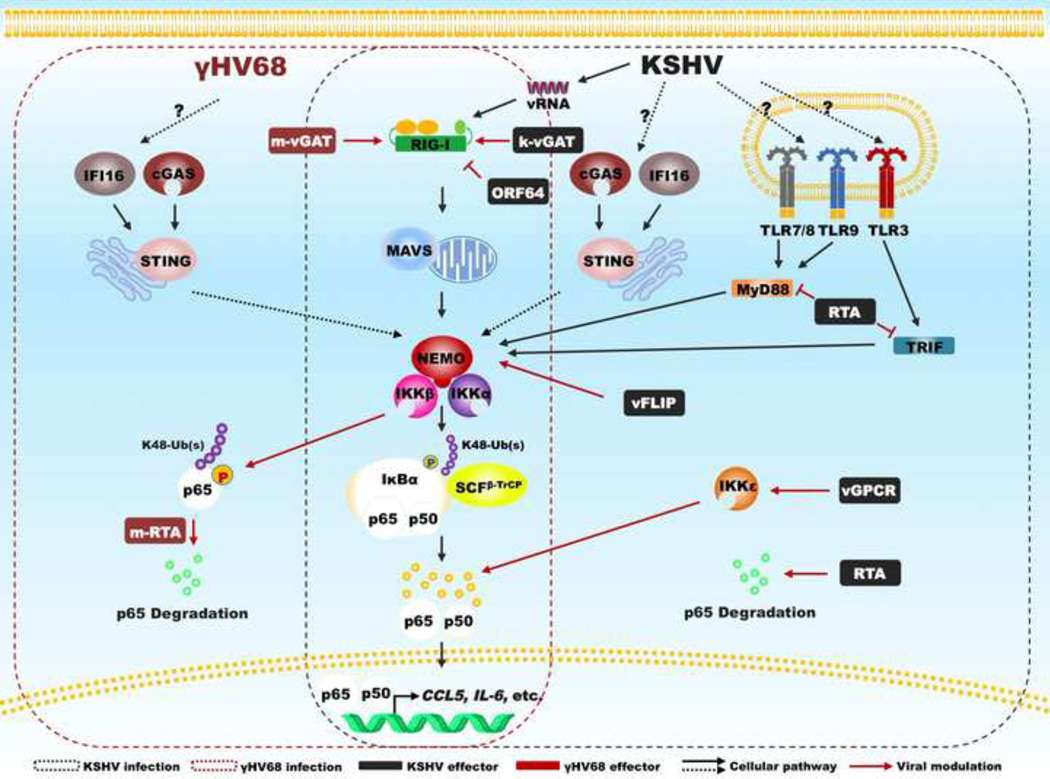
A recurring theme is that virus hijacks the NF-κB pathway to promote infection by using NF-κB TFs to enhance viral gene expression. Examples include HIV [73], HSV-1 [24] and bovine foamy virus [74]. This has been extensively reviewed previously [62]. Our recent studies demonstrate that murine γHV68 exploits the activation of MAVS and IKKβ to promote viral lytic replication [25]. Specifically, γHV68 activates IKKβ in a MAVS-dependent manner and activated IKKβ phosphorylates viral replication transactivator (RTA), an essential TF for gamma herpesvirus replication, thereby elevating the expression of viral lytic genes. On the other hand, γHV68 directs the IKKβ kinase to phosphorylate p65, the critical subunit for the transcriptionally active NF-κB dimer, and induces p65 degradation in conjunction with the RTA E3 ligase [26,27]. As such, MAVS and IKKβ are necessary to abrogate antiviral cytokine production and loss of MAVS results in the secretion of increased cytokines (Figure 2). We have discovered a family of viral pseudo enzymes that activate RIG-I via deamidation, a molecular action distinct from RNA-induced RIG-I activation [28]. Our study illustrates an intriguing mechanism whereby innate immune activation is intercepted by a viral TF/E3 ligase to enable the expression of viral lytic genes while disabling the production of host antiviral cytokines.
Figure 2. Modulators of NF-κB encoded by human KSHV and murine γHV68.
In response to viral infection, pattern recognition receptors activate the NF-κB pathway, while viruses target key signaling components to modulate NF-κB activation. Highlighted components are diagrammed in conjunction with viral modulators of human KSHV and murine γHV68.
When in vivo function of NF-κB signaling events is considered, γHV68 infection in mice contributes substantially to our understanding in host immune defense and viral manipulation, Although blockade of NF-κB activation by the IκBα super-suppressor did not impact γHV68 lytic replication [75], γHV68 exploits signaling events upstream of NF-κB to facilitate lytic gene transcription and productive infection [25]. On the other hand, inhibition of NF-κB activation via host factors or engineered recombinant γHV68 demonstrated crucial roles of NF-κB activation in viral latent infection [75,76]. Interestingly, activation of NF-κB appeared to originate from CpG DNA-triggered TLR9 signaling. Despite that loss of TLR9 expression increased γHV68 reactivation from latently-infected B cells, the frequency of latently-infected B cells was comparable in wild-type and Tlr9−/− mice [77]. MyD88-deficiency or expression of the IκBα super-suppressor, but not MAVS-deficiency, reduced the frequency of γHV68 latently-infected splenocytes [25,75,76], supporting the corollary that TLR-dependent NF-κB activation facilitates the establishment of latent infection. In conclusion, signaling events upstream of NF-κB are important for γHV68 lytic replication, whereas NF-κB activation per se is critical for γHV68 latent infection.
6. Concluding remarks
We anticipate that the list of viral factors modulating cellular NF-κB activation will rapidly expand with research into innate immune signaling. Studies on viral modulation of NF-κB-dependent gene expression in the nucleus, such as epigenetic modifications, will likely uncover fundamental principles governing the specificity and coordination of NF-κB-mediated transcription. PRRs constitute another research frontier in virus-host interaction and viral manipulation of PRRs should elucidate significant mechanisms of host defense and viral evasion. Virus-host interactions shaped by millions of years of co-evolution are sure to surprise us with elegant examples of viral manipulation of both host NF-κB activation and related signaling pathways.
Highlight.
NF-κB transcription factors regulate the expression of hundreds of genes in immune response.
NF-κB activation by PRRs constitutes a research frontier in infection and immunity.
Viruses have evolved distinct and intricate strategies to modulate NF-κB signaling.
Viral modulation offers mechanistic view into key regulation governing NF-κB activation.
Acknowledgement
we apologize to our colleagues for whose work has not been cited in this review due to limited space. Research of the Feng laboratory is supported by grants from NIH (CA134241 and DE021445) and ACS (RSG-11-162-01-MPC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 2.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nature Reviews Immunology. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 3.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 4.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Sylla BS, Hung SC, Davidson DM, Hatzivassiliou E, Malinin NL, Wallach D, Gilmore TD, Kieff E, Mosialos G. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kappaB through a pathway that includes the NF-kappaB-inducing kinase and the IkappaB kinases IKKalpha and IKKbeta. Proc Natl Acad Sci U S A. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun SC, Ballard DW. Persistent activation of NF-kappaB by the tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 7.Sun SC, Cesarman E. NF-kappaB as a target for oncogenic viruses. Curr Top Microbiol Immunol. 2011;349:197–244. doi: 10.1007/82_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 12.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 13.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 15.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira DE, Ballon G, Cesarman E. NF-kappaB signaling modulation by EBV and KSHV. Trends Microbiol. 2010;18:248–257. doi: 10.1016/j.tim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Varin A, Manna SK, Quivy V, Decrion AZ, Van Lint C, Herbein G, Aggarwal BB. Exogenous Nef protein activates NF-kappa B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells. Role in AIDS pathogenesis. J Biol Chem. 2003;278:2219–2227. doi: 10.1074/jbc.M209622200. [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 25.Dong X, Feng H, Sun Q, Li H, Wu TT, Sun R, Tibbetts SA, Chen ZJ, Feng P. Murine gamma-herpesvirus 68 hijacks MAVS and IKKbeta to initiate lytic replication. PLoS Pathog. 2010;6:e1001001. doi: 10.1371/journal.ppat.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong X, Feng P. Murine gamma herpesvirus 68 hijacks MAVS and IKKbeta to abrogate NFkappaB activation and antiviral cytokine production. PLoS Pathog. 2011;7:e1002336. doi: 10.1371/journal.ppat.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X, He Z, Durakoglugil D, Arneson L, Shen Y, Feng P. Murine gammaherpesvirus 68 evades host cytokine production via replication transactivator-induced RelA degradation. J Virol. 2012;86:1930–1941. doi: 10.1128/JVI.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He S, Zhao J, Song S, He X, Minassian A, Zhou Y, Zhang J, Brulois K, Wang Y, Cabo J, et al. Viral Pseudo-Enzymes Activate RIG-I via Deamidation to Evade Cytokine Production. Mol Cell. 2015;58:134–146. doi: 10.1016/j.molcel.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulhern O, Harrington B, Bowie AG. Modulation of innate immune signalling pathways by viral proteins. Adv Exp Med Biol. 2009;666:49–63. doi: 10.1007/978-1-4419-1601-3_4. [DOI] [PubMed] [Google Scholar]
- 30.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MR, Loo YM, Horner SM, Gale M, Jr, Malik HS. Convergent evolution of escape from hepaciviral antagonism in primates. PLoS Biol. 2012;10:e1001282. doi: 10.1371/journal.pbio.1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee A, Morosky SA, Delorme-Axford E, Dybdahl-Sissoko N, Oberste MS, Wang T, Coyne CB. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011;7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Z, Li L, Lei X, Zhou H, Zhou Z, He B, Wang J. Enterovirus 68 3C protease cleaves TRIF to attenuate antiviral responses mediated by Toll-like receptor 3. J Virol. 2014;88:6650–6659. doi: 10.1128/JVI.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu L, Feng Z, Yamane D, Liang Y, Lanford RE, Li K, Lemon SM. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 2011;7:e1002169. doi: 10.1371/journal.ppat.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, Knipe DM, Kurt-Jones EA. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J Virol. 2010;84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bussey KA, Reimer E, Todt H, Denker B, Gallo A, Konrad A, Ottinger M, Adler H, Sturzl M, Brune W, et al. The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response. J Virol. 2014;88:9245–9259. doi: 10.1128/JVI.00841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis ME, Wang MK, Rennick LJ, Full F, Gableske S, Mesman AW, Gringhuis SI, Geijtenbeek TB, Duprex WP, Gack MU. Antagonism of the phosphatase PP1 by the measles virus V protein is required for innate immune escape of MDA5. Cell Host Microbe. 2014;16:19–30. doi: 10.1016/j.chom.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesman AW, Zijlstra-Willems EM, Kaptein TM, de Swart RL, Davis ME, Ludlow M, Duprex WP, Gack MU, Gringhuis SI, Geijtenbeek TB. Measles virus suppresses RIGI-like receptor activation in dendritic cells via DC-SIGN-mediated inhibition of PP1 phosphatases. Cell Host Microbe. 2014;16:31–42. doi: 10.1016/j.chom.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao X, Kolli D, Ren J, Liu T, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G disrupts mitochondrial signaling in airway epithelial cells. PLoS One. 2013;8:e62568. doi: 10.1371/journal.pone.0062568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol. 2013;87:13466–13479. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Fang L, Wei D, Zhang H, Luo R, Chen H, Li K, Xiao S. Hepatitis A virus 3C protease cleaves NEMO to impair induction of beta interferon. J Virol. 2014;88:10252–10258. doi: 10.1128/JVI.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C, Zhang Q, Guo XK, Yu ZB, Xu AT, Tang J, Feng WH. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-kappaB essential modulator. J Virol. 2014;88:10934–10945. doi: 10.1128/JVI.01396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S, Song L, Li J, Zhang Z, Peng H, Jiang W, Wang Q, Kang T, Chen S, Huang W. Influenza A virus-encoded NS1 virulence factor protein inhibits innate immune response by targeting IKK. Cell Microbiol. 2012;14:1849–1866. doi: 10.1111/cmi.12005. [DOI] [PubMed] [Google Scholar]
- 47.Reis AL, McCauley JW. The influenza virus protein PB1-F2 interacts with IKKbeta and modulates NF-kappaB signalling. PLoS One. 2013;8:e63852. doi: 10.1371/journal.pone.0063852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D, Wu A, Cui L, Hao R, Wang Y, He J, Guo D. Hepatitis B virus polymerase suppresses NF-kappaB signaling by inhibiting the activity of IKKs via interaction with Hsp90beta. PLoS One. 2014;9:e91658. doi: 10.1371/journal.pone.0091658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 51.Sonnberg S, Seet BT, Pawson T, Fleming SB, Mercer AA. Poxvirus ankyrin repeat proteins are a unique class of F-box proteins that associate with cellular SCF1 ubiquitin ligase complexes. Proc Natl Acad Sci U S A. 2008;105:10955–10960. doi: 10.1073/pnas.0802042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansur DS, Maluquer de Motes C, Unterholzner L, Sumner RP, Ferguson BJ, Ren H, Strnadova P, Bowie AG, Smith GL. Poxvirus targeting of E3 ligase beta-TrCP by molecular mimicry: a mechanism to inhibit NF-kappaB activation and promote immune evasion and virulence. PLoS Pathog. 2013;9:e1003183. doi: 10.1371/journal.ppat.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morelli M, Dennis AF, Patton JT. Putative E3 ubiquitin ligase of human rotavirus inhibits NF-kappaB activation by using molecular mimicry to target beta-TrCP. MBio. 2015;6 doi: 10.1128/mBio.02490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Ni L, Wang S, Zheng C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-kappaB activation. J Virol. 2014;88:7941–7951. doi: 10.1128/JVI.03394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Wang K, Wang S, Zheng C. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. J Virol. 2013;87:12935–12948. doi: 10.1128/JVI.01952-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diel DG, Luo S, Delhon G, Peng Y, Flores EF, Rock DL. A nuclear inhibitor of NF-kappaB encoded by a poxvirus. J Virol. 2011;85:264–275. doi: 10.1128/JVI.01149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q, Burles K, Couturier B, Randall CM, Shisler J, Barry M. Ectromelia virus encodes a BTB/kelch protein, EVM150, that inhibits NF-kappaB signaling. J Virol. 2014;88:4853–4865. doi: 10.1128/JVI.02923-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22:1282–1290. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumner RP, Maluquer de Motes C, Veyer DL, Smith GL. Vaccinia virus inhibits NF-kappaB-dependent gene expression downstream of p65 translocation. J Virol. 2014;88:3092–3102. doi: 10.1128/JVI.02627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-kappaB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol. 2013;87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang LS, Wang JT, Doong SL, Lee CP, Chang CW, Tsai CH, Yeh SW, Hsieh CY, Chen MR. Epstein-Barr virus BGLF4 kinase downregulates NF-kappaB transactivation through phosphorylation of coactivator UXT. J Virol. 2012;86:12176–12186. doi: 10.1128/JVI.01918-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman MM, McFadden G. Modulation of NF-kappaB signalling by microbial pathogens. Nat Rev Microbiol. 2011;9:291–306. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J Virol. 2012;86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai M, Li M, Wang K, Wang S, Lu Q, Yan J, Mossman KL, Lin R, Zheng C. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-kappaB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One. 2013;8:e54586. doi: 10.1371/journal.pone.0054586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bottero V, Kerur N, Sadagopan S, Patel K, Sharma-Walia N, Chandran B. Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J Virol. 2011;85:1980–1993. doi: 10.1128/JVI.01911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Lu X, Zhu L, Shen Y, Chengedza S, Feng H, Wang L, Jung JU, Gutkind JS, Feng P. IKK epsilon kinase is crucial for viral G protein-coupled receptor tumorigenesis. Proc Natl Acad Sci U S A. 2013;110:11139–11144. doi: 10.1073/pnas.1219829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Postler TS, Desrosiers RC. The cytoplasmic domain of the HIV-1 glycoprotein gp41 induces NF-kappaB activation through TGF-beta-activated kinase 1. Cell Host Microbe. 2012;11:181–193. doi: 10.1016/j.chom.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R, Tan J, Lin Y, Jia R, Yang W, Liang C, Geng Y, Qiao W. HIV-1 Vpr activates both canonical and noncanonical NF-kappaB pathway by enhancing the phosphorylation of IKKalpha/beta. Virology. 2013;439:47–56. doi: 10.1016/j.virol.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Liu R, Lin Y, Jia R, Geng Y, Liang C, Tan J, Qiao W. HIV-1 Vpr stimulates NF-kappaB and AP-1 signaling by activating TAK1. Retrovirology. 2014;11:45. doi: 10.1186/1742-4690-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiume G, Vecchio E, De Laurentiis A, Trimboli F, Palmieri C, Pisano A, Falcone C, Pontoriero M, Rossi A, Scialdone A, et al. Human immunodeficiency virus-1 Tat activates NF-kappaB via physical interaction with IkappaB-alpha and p65. Nucleic Acids Res. 2012;40:3548–3562. doi: 10.1093/nar/gkr1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim KH, Choi HS, Park YK, Park ES, Shin GC, Kim DH, Ahn SH, Kim KH. HBx-induced NF-kappaB signaling in liver cells is potentially mediated by the ternary complex of HBx with p22-FLIP and NEMO. PLoS One. 2013;8:e57331. doi: 10.1371/journal.pone.0057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu M, Katzenellenbogen RA, Grandori C, Galloway DA. NFX1 plays a role in human papillomavirus type 16 E6 activation of NFkappaB activity. J Virol. 2010;84:11461–11469. doi: 10.1128/JVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verhoef K, Sanders RW, Fontaine V, Kitajima S, Berkhout B. Evolution of the human immunodeficiency virus type 1 long terminal repeat promoter by conversion of an NF-kappaB enhancer element into a GABP binding site. J Virol. 1999;73:1331–1340. doi: 10.1128/jvi.73.2.1331-1340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Tan J, Guo H, Zhang Q, Jia R, Xu X, Geng Y, Qiao W. Bovine foamy virus transactivator BTas interacts with cellular RelB to enhance viral transcription. J Virol. 2010;84:11888–11897. doi: 10.1128/JVI.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krug LT, Moser JM, Dickerson SM, Speck SH. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 2007;3:e11. doi: 10.1371/journal.ppat.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gargano LM, Moser JM, Speck SH. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J Virol. 2008;82:3853–3863. doi: 10.1128/JVI.02577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haas F, Yamauchi K, Murat M, Bernasconi M, Yamanaka N, Speck RF, Nadal D. Activation of NF-kappaB via endosomal Toll-like receptor 7 (TLR7) or TLR9 suppresses murine herpesvirus 68 reactivation. J Virol. 2014;88:10002–10012. doi: 10.1128/JVI.01486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82:8262–8271. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saito S, Murata T, Kanda T, Isomura H, Narita Y, Sugimoto A, Kawashima D, Tsurumi T. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J Virol. 2013;87:4060–4070. doi: 10.1128/JVI.02020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sloan E, Henriquez R, Kinchington PR, Slobedman B, Abendroth A. Varicella-zoster virus inhibition of the NF-kappaB pathway during infection of human dendritic cells: role for open reading frame 61 as a modulator of NF-kappaB activity. J Virol. 2012;86:1193–1202. doi: 10.1128/JVI.06400-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stebbing RE, Irvin SC, Rivera-Serrano EE, Boehme KW, Ikizler M, Yoder JA, Dermody TS, Sherry B. An ITAM in a nonenveloped virus regulates activation of NF-kappaB, induction of beta interferon, and viral spread. J Virol. 2014;88:2572–2583. doi: 10.1128/JVI.02573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao L, Ge X, Gao Y, Ren Y, Ren X, Li G. Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2, TLR3, and TLR9 pathways in porcine intestinal epithelial cells. J Gen Virol. 2015 doi: 10.1099/vir.0.000133. [DOI] [PubMed] [Google Scholar]
- 83.Luo R, Fang L, Jiang Y, Jin H, Wang Y, Wang D, Chen H, Xiao S. Activation of NF-kappaB by nucleocapsid protein of the porcine reproductive and respiratory syndrome virus. Virus Genes. 2011;42:76–81. doi: 10.1007/s11262-010-0548-6. [DOI] [PubMed] [Google Scholar]
- 84.Fang Y, Fang L, Wang Y, Lei Y, Luo R, Wang D, Chen H, Xiao S. Porcine reproductive and respiratory syndrome virus nonstructural protein 2 contributes to NF-kappaB activation. Virol J. 2012;9:83. doi: 10.1186/1743-422X-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]