Abstract
The BRCA1-associated protein-1 (BAP1) tumor predisposition syndrome (BAP1-TPDS) is a recently identified hereditary cancer syndrome. Germline mutations in this tumor suppressor gene predispose families to the development of various malignancies. The molecular functions of the gene as well as the clinical phenotype of the syndrome are still being clarified. We sought to conduct a comprehensive review of published research into BAP1-TPDS to more thoroughly delineate the clinical implications of germline BAP1 mutations. We also report two additional families with germline BAP1 mutations. Current evidence demonstrates that germline BAP1 mutations predispose families to uveal melanoma, renal cell carcinoma, malignant mesothelioma, cutaneous melanoma, and possibly to a range of other cancers as well. Some of these cancers tend to be more aggressive, have a propensity to metastasize, and onset earlier in life in patients with BAP1 mutations as compared to non-predisposed patients with equivalent cancers. Although further research is necessary, this information can aid in the management, diagnosis, and therapy of these patients and their families, and highlights the importance of genetic counseling.
Keywords: BAP1, cutaneous melanoma, germline, mesothelioma, renal cell carcinoma, uveal melanoma
Germline mutation in BRCA1-associated protein-1 (BAP1) underlies the recently identified tumor predisposition syndrome (BAP1-TPDS) OMIM 614327 (1). The work identifying this hereditary cancer syndrome came simultaneously from three independent research groups focused on uveal melanoma (UM), mesothelioma (MMe), and cutaneous melanoma (CM) (2–4). Shortly after this, renal cell carcinoma (RCC) was also associated with the syndrome (5). An increasing number of patients with germline BAP1 mutations have been reported since (Appendix S1, Supporting Information). Although the full phenotype of the syndrome is still being characterized, the literature suggests that several other cancers reported in patients and relatives of patients with germline BAP1 mutations may also be associated and that some cancers associated with this syndrome may have a poorer prognosis. A complete understanding of the disease penetrance as well as gene function and phenotypic spectrum of BAP1 has also not yet been established.
BAP1 is a deubiquitinating hydrolase identified in 1998 that binds to the RING finger domain of the BRCA1 protein (6). Although earlier reports suggested that the BAP1 tumor suppressor function was through its deubiquitinating activity upon BRCA1, this was refuted and later studies have indicated that it is an independent tumor suppressor (7). Further studies revealed its involvement in various biologic processes including chromatin dynamics, DNA damage response, cell cycle regulation, and cell growth (8–10). Several recent reviews have detailed the different molecular functions of BAP1 (8, 11–15). The complex functional roles of BAP1 are partially through its deubiquitinase activity and interactions with several other proteins, in particular HCFC1, YY1, OGT, ASXL1/2 and FOXK1/2 (15–17). However, the structural architecture and stoichiometry of BAP1 complexes have not been fully characterized. The impact of different BAP1 mutations on the function of these complexes and whether genotype–phenotype correlation exists is still unclear.
The aim of this review was to compile all reported research into BAP1-TPDS to help establish counseling, testing, and management guidelines. Further, we introduce two new families with germline BAP1 mutations and provide updates on several previously reported families.
Materials and methods
Literature review
A literature review was conducted on all peer-reviewed English language articles on BAP1 and its Drosophila homolog, Calypso, published through 18 May 2015. A search on PubMed was directed with the keywords ‘BRCA1 associated protein-1’, ‘BAP1’, and ‘Calypso’. Unpublished material was not included. Eighty-seven articles pertaining to BAP1 and its association with cancer were obtained. Of these, 27 articles described patients with germline BAP1 mutations. The articles were collated and data were extracted via an article-by-article systematic review. Online supplemental material was consulted if available. Data extracted from the review included clinical information, molecular testing results, and method of molecular analysis. All reported mutations were reviewed and updated to the current standard nomenclature. All data were analyzed and calculated by the authors to produce relevant statistics. Cancer frequency statistics in the general population were derived from the Surveillance, Epidemiology and End Results (SEER) database for comparison.
Screening guidelines from syndromes with similar phenotypes were adapted to develop management recommendations for BAP1-TPDS. Interval follow-up data from our previously reported families are included herein (2, 18, 19).
Germline mutation detection
Data on two new families identified by the authors are included. Sequencing was done according to our previously reported protocol and aligning with the reference sequence provided by Genebank accession number NM_004656.2 (2). Confirmation of the splice variant detected in one family was carried out using a primer set with forward primer 5′TGCTCGTG GAAGATTTCGGTG in exon 3 and reverse primer 5′GGCATGGCTATTATGGGCCT in exon 6. RNA was extracted from peripheral blood leukocytes utilizing Trizol reagent with phenol chloroform precipitation according to the manufacturer’s protocol (Life Technologies, Grand Island, NY). Reverse transcription was carried out utilizing SuperScript VILO cDNA synthesis Kit (Life Technologies). Splice variants detected by reverse transcription polymerase chain reaction (RT-PCR) were individually isolated and purified from agarose gel using a gel purification kit according to manufacturer’s protocol (Qiagen, Valencia, CA), and then sequenced using forward and reverse primers. The research on our patients was approved by the Institutional Review Board of The Ohio State University and informed consent was obtained from all subjects before testing.
Results
Report of two new families
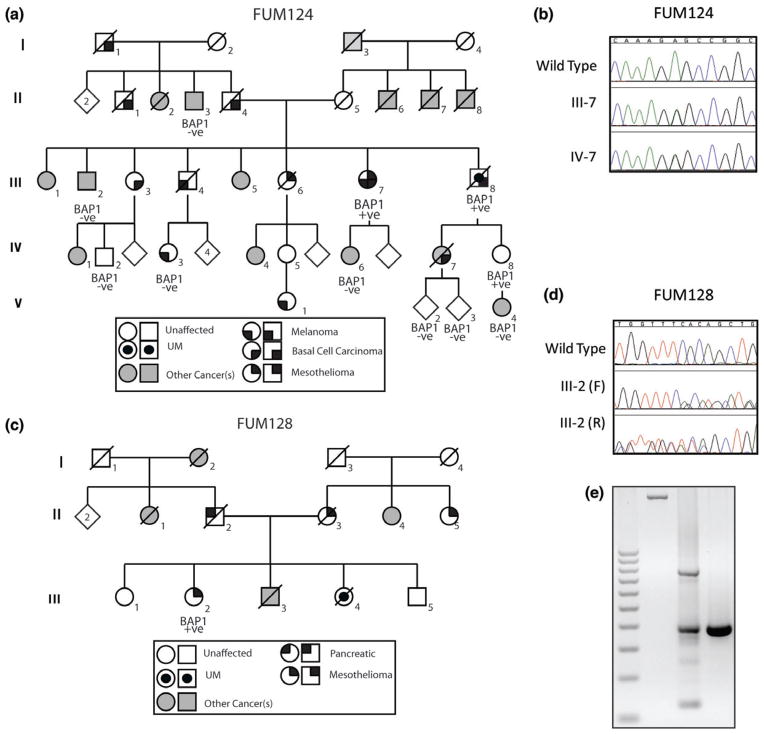
Germline BAP1 mutations were detected in two new families. The proband of FUM124 presented with MMe between the rib cage and chest wall at age 71, multiple basal cell carcinomas (BCC) beginning age 56, and CM of the hand diagnosed at age 72. Family history of UM, CM, BCC and multiple other cancers was reported (Fig. 1). A germline missense mutation (c.539T>C, p.L180P) was detected in the proband and an unaffected niece (59 years). The mutation, located in the ubiquitinase domain of BAP1, is predicted to be deleterious by both SIFT and POLYPHEN protein modeling software (Fig. 1). According to ACMG guidelines, the mutation classification is most consistent with ‘likely pathogenic’ (20). The proband’s brother (an obligate mutation carrier) was diagnosed with UM at age 60 and with several BCC on his face starting age 61, but passed away from liver metastasis at age 65 without being tested. Ten other family members tested negative for the mutation including five unaffected relatives and five relatives with CM, breast carcinoma, liposarcoma, schwannoma, and prostate cancer. Because the mutation does not unconditionally track with the disease, it is possible that there is a modifier or another gene mutation concurrently affecting this family’s cancer risk.
Fig. 1.
Two new families with germline BAP1 mutation. (a, b) In FUM124, the proband (III-7) and his niece IV-8 showed a germline heterozygous c.539T>C, p.L180P. The proband presented with pleural malignant mesothelioma (MMe), basal cell carcinomas (BCC) and cutaneous melanoma (CM), a brother (obligate carrier) had uveal melanoma (UM) and BCC while the niece was unaffected at age 59. Five other individuals in the family with cancer (II-3: prostate Ca, III-2: liposarcoma, IV-3: CM, IV-6: Breast Ca and V-4: Schawannoma) tested negative for the mutation. Five unaffected relatives also tested negative for mutation (only three are shown). (c) In FUM, 128 the proband (III-2) presented with peritoneal MMe, other cancer in the families were peritoneal MMe, UM, pancreatic and bladder cancers. (d) Germline 2 bp deletion (c.256-4_256-2del) in intron 4. (e) The deletion causes aberrant splicing of the BAP1 (first lane genomic DNA, second lane patients cDNA and third lane a control individual cDNA). In both families, BAP1 mutation status is denoted as mutated (BAP1 +ve) or wild-type (BAP1 −ve). Some of the unaffected BAP1 −ve individuals are not shown.
The proband of FUM128 presented with epithelioid peritoneal MMe at age 60 and family history of UM, MMe, and other cancers (Fig. 1). A germline intronic variant (c.256-4_256-2del) was detected. No family members were available for testing. Both NetGene 2 version 2.42 and NNSPLICE version 0.9 software indicated that this variant is a potential splice-site mutation. Sequencing of the RT-PCR product utilizing primers in exons 3 and 6 confirmed the aberrant splicing with formation of two splice products, one with splicing-out of 253 base pairs of exons 4–5 and the other with inclusion of intron 4 (393 base pairs) (Fig. 1; Fig. S1).
Clinical findings in families with germline BAP1 mutation
Including the two new families reported herein, there have been 57 families with 174 individuals reported to carry BAP1 mutations; 67 are male (39%), 95 are female (55%), and 12 patients have no gender information reported. UM is the most common cancer diagnosed with a total of 54 patients (31%) (2–5, 18, 19, 21–31). Fifty-six of the 57 families presented with one or more of four main cancers (UM, MMe, CM, and RCC). The distribution of these cancers in these families is summarized with Venn diagrams (Fig. 2). Median age of cancer diagnosis in these patients was age 50 (range 16–85). It should be noted that many reported cancers were not confirmed by records review. In addition, a comparison of cancer frequency is imperfect as our cohort includes patients reported internationally while SEER compiles data from patients in the United States (Appendix S1).
Fig. 2.
Venn diagrams of the cancers identified in BAP1 patients and families. (a) Individuals with BAP1 mutations diagnosed with uveal melanoma (UM), renal cell carcinoma (RCC), cutaneous melanoma (CM), and/or malignant mesothelioma (MMe). There are 118 patients who were diagnosed with at least one of the above cancers. Thirty individuals were only diagnosed with another cancer uncertain to be BAP1 related. Twelve individuals were diagnosed with atypical Spitz tumors (ASTs) only. Fourteen individuals were unaffected. (b) Families with BAP1 mutations with mutation-positive members diagnosed with UM, RCC, CM, and MMe. There were 57 families reported, of which 54 had at least member with one of the above diagnoses testing positive. One family presented with only ASTs and another cancer uncertain to be BAP1 related. Two families had a history of one of the above diagnoses, but those members were not tested.
Common BAP1-TPDS tumors
Uveal melanoma
Both germline and somatic BAP1 mutations have been found in patients with UM, the most common ocular malignancy in adults (Table 1). Fifty-four of 174 (31%) reported BAP1 mutation carriers had a diagnosis of UM (2–5, 18, 19, 21–31). The incidence of UM in the general population is 5.1 diagnoses per million population (32).
Table 1.
Summary of clinical and molecular feature of tumor types reported more than once in patients with germline BAP1 mutations
| Tumor type | Tumor features in patients with BAP1 germline mutation
|
Tumor characteristics in general population
|
Sporadic tumors Somatic mutation frequency (32) | |||||
|---|---|---|---|---|---|---|---|---|
| Reported frequency | Sex ratio M:F | Median age of onset in BAP1 patients (range) | Tumor biallelic inactivation reported (n) | References | Lifetime risk (reference) | Median age of onset (reference) | ||
| Uveal melanoma | 54/174 | 23:25 | 51 (16–72) | Yes (7) | 2–5, 18, 19, 21–31 | 0.00051% (31) | 62 | 54/233 |
| Mesothelioma | 39/174 | 12:27 | 56 (34–85) | Yes (7) | 2, 3, 5, 18, 30, 31, 33–35 | 0.13% (33)a | 74 (36) | 26/153 |
| Cutaneous melanoma | 23/174 | 13:10 | 46 (25–72) | Yes (4) | 2, 4, 5, 22, 24, 27, 29–31, 35 | 3.25% (37)a | 58 (37) b | 15/820 |
| Renal cell carcinoma | 18/174 | 8:7 | 47 (36–72) | Yes (5) | 3, 5, 18, 25, 29, 38–40 | 1.60% (37)a | 64 (37)b | 138/1508 |
| Abdominal cancer | 2/174 | 0:2 | 64 (57–64) | N/A | 18, 22 | – | – | 21/1934 |
| Basal cell carcinoma | 11/174 | 4:7 | 50 (42–65) | Yes (9) | 3, 5, 22, 24, 31 | – | – | – |
| Breast cancer | 9/95 | 9 | 58 (37–85) | Yes (2) | 3, 5, 18, 29–31 | 12.33% (37)a | 61 (37)b | 11/1688 |
| Cholangiocarcinoma | 4/174 | 2:2 | 66 (47–71) | N/A | 18, 28, 29, 31 | 0.83% (37)a | 50 (41) | 16/113 |
| Colorectal cancer | 2/174 | 1:1 | 62 (53–71) | N/A | 18, 25 | 4.96% (37)a | 71 (37)b | 42/1132 |
| Lung cancer | 6/174 | 3:3 | 56 (46–59) | Yes (1) | 2, 22, 29, 30 | 6.99% (37)a | 70 (37)b | 9/1031 |
| Meningioma | 3/174 | 0:3 | 44 (35–52) | Yes (2) | 2, 31, 42 | – | 65 (43) | 0/65 |
| Neuroendocrine cancer | 2/174 | 1:1 | 47 (42–52) | Yes (1) | 2, 30 | – | – | 5/2545 |
| Ovarian cancer | 3/95 | 3 | 59 (34–69) | N/A | 2, 3 | 1.12% (37)a | 63 (37)b | 6/890 |
| Prostate cancer | 2/67 | 2 | 65 (63–67) | N/A | 25, 27 | 16.15% (37)a | 68 (37)b | 5/861 |
| Sarcoma | 4/174 | 1:3 | 45 (42–64) | N/A | 3, 18, 30 | – | – | 0/353 |
| Thyroid cancer | 2/174 | 0:2 | 38 (34–41) | No | 5, 42 | 0.90% (37)a | 47 (37)b | 1/535 |
F, female; M, male; N/A, not applicable; SEER, Surveillance, Epidemiology and End Results.
Age-adjusted SEER data from 2007 to 2009.
SEER data from 2000 to 2003.
The earliest reported age of cancer onset in a germline BAP1 mutation carrier is for UM at age 16, and there have been a total of four UM diagnosed by age 20 (19, 22, 27, 30). Median age of onset is earlier in UM patients with germline BAP1 mutation (51 years, range 16–72) compared with the general population (62 years) (32).
Beyond epidemiologic data, there is good molecular evidence of UM’s association with the BAP1-TPDS. Genetic analysis of UM tumor tissue from seven patients with germline BAP1 mutations showed loss-of-heterozygosity (LOH) or loss of expression of the wild-type allele (2, 4, 22, 26, 27, 30). Somatic BAP1 mutations have also been widely reported in sporadic UM, and protein expression studies have also shown lack of BAP1 expression in UM tumors (Table 1) (44–47).
Malignant mesothelioma
A total of 39/174 patients (22%) with germline mutations have been diagnosed with MMe with median age of onset earlier than that in the general population (Table 1) (2, 3, 5, 18, 30, 31, 33–36). Twenty-six patients (67%) had pleural MMe, 12 patients (31%) had peritoneal MMe, and 1 patient had diagnoses of both. Interestingly, all 13 patients reported to have diagnoses of peritoneal MMe are female, while peritoneal MMe is more commonly diagnosed in males in the general population (48).
Another finding in this subset of patients is the high frequency of multiple cancers. Excluding cases of multiple MMe, 14 patients (36%) have been diagnosed with another primary cancer in addition to MMe. Of the second cancers, UM occurred in 5/39 (13%) of the MMe patients.
Asbestos exposure is a strong predisposing factor for MMe development in the general population, but its role in BAP1-TPDS is unclear. Testa et al. detected asbestos traces in homes of all affected family members in both of the families they report with germline mutations (3). Arzt et al., however, found no statistically significant effect of asbestos exposure on BAP1 protein expression in vitro; any potential mechanism of how asbestos and BAP1 may interact is unknown (49).
MMe tumors from seven germline BAP1 mutation carriers have been shown to have loss of the wild-type BAP1 allele or its expression (3, 35). Somatic BAP1 mutations and loss of BAP1 expression have also been reported in presumably sporadic MMe (Table 1) (3, 44, 50–52).
Cutaneous melanoma
There are 23/174 (13%) reported patients with germline BAP1 mutations diagnosed with CM; median age of onset is earlier than in the general population (Table 1) (2, 4, 5, 22, 24, 27, 29–31, 35, 37). Notably, 5/23 (22%) of these patients had multiple primary CM with a maximum of seven in a single patient (5, 29, 30). Further, 11 (48%) had CM diagnosed in addition to another cancer (4, 5, 24, 29, 31, 35). Tumors from four of these patients show LOH and/or loss of expression of BAP1 as well (4, 29, 35). Njauw et al. noted three families carrying germline mutations had diagnoses of ‘nevoid CM’, a rare subtype. Analysis of these irregular tumors in one family revealed semitranslucent orange-red pigmentation and high levels of Ki67 staining. These lesions were distinct from traditional CM and may be related to atypical Spitz tumors (ASTs) (29).
Somatic mutations and lack of staining for BAP1 via immunohistochemistry have also been noted in CM tissues from patients without germline mutations, indicating BAP1 may be involved in some aspects of CM pathogenesis (Table 1) (4, 44, 53, 54).
Renal cell carcinoma
Eighteen of 174 (10%) of the reported patients with germline BAP1 mutations had RCC with median age of diagnosis earlier than that seen in the general population (Table 1) (3, 5, 18, 25, 29, 37–40). Tumor tissue studies demonstrating LOH, somatic BAP1 mutations, and IHC staining all support an association between germline BAP1 mutation and RCC (Table 1) (39, 40, 44, 55–60).
Cutaneous melanocytic lesions
BAP1-TPDS is associated with a distinct subset of benign skin lesions located on the head and neck, trunk, and limbs. A range of names has been given to these lesions, including melanocytic BAP1-mutated atypical intradermal tumors (MBAITs), ASTs, Wiesner nevi, and nevoid melanoma-like melanocytic proliferations (NEMMPs) (29, 61–63). Herein, we will refer to them as ASTs as these lesions fit closest clinically and pathologically to this designation. These lesions are well-circumscribed dome shaped, skin-colored or reddish-brown nodules, with average sizes of 5 mm, and range widely in number. Morphologically, the lesions are mostly intradermal with occasional involvement of the junctional epidermis, and show cytological features resembling atypical Spitz nevi (64). They are characterized by biallelic inactivation of BAP1 and frequent BRAFV600E mutation; both of which can be reliable markers for aiding in the diagnosis (63).
The prevalence of ASTs in BAP1-TPDS is unclear as they were not assessed in a majority of reported patients. Of 43 germline BAP1 patients who were assessed via full body skin exams with biopsy of suspicious lesions, 31 patients (72%) were diagnosed with ASTs (4, 27–31, 35, 61, 65). Eleven of 31 (35%) of these patients had multiple lesions, with a range of 2 to over 50 (4, 5, 24, 29, 35, 65). Median age of diagnosis was 42 years. Although their natural history is unknown, Busam et al. found ASTs to be present since childhood in one germline BAP1 mutation carrier (65, 66). LOH in the tumor was confirmed in 22 ASTs in 3 germline mutation carriers from one family (4).
Lesions with similar morphological and molecular characteristics have also been reported in patients without germline BAP1 mutation (4, 14, 63). The clinical significance of these lesions and any possible relation to CM is yet to be determined.
Possible BAP1-TPDS tumors
A number of other tumor types including a range of sarcomas and carcinomas have been reported in germline BAP1 mutation carriers; however, there are limited data supporting their inclusion in BAP1-TPDS (Table 1; Appendix S1) (2, 3, 5, 18, 22, 24, 25, 27–31, 41–44, 67–75). The most commonly reported cancers include breast cancer and BCC with 9 and 11 patients with germline mutations diagnosed, respectively. Tumor testing in these patients has shown LOH. Although BCC is common in the general population, these molecular data suggest an association. BAP1’s association with breast cancer has been suspected, given its interaction with the BRCA1 tumor suppressor. Conflicting reports regarding the association have been published, however, as germline BAP1 mutations are not common in breast cancer, including in patients with family histories consistent with hereditary cancer predisposition (5, 76, 77). The recent addition of BAP1 to multigene panels offered by several clinical laboratories, including those for breast cancers, will help elucidate any association.
Germline BAP1 mutation and tumor aggressiveness
Current data suggest that germline mutations in patients with UM, CM, and RCC are associated with more aggressive disease, while in MMe they are associated with less aggressive disease, as discussed below.
In UM, patients with germline BAP1 mutations tend to have more aggressive cancers with higher tumor staging and a greater likelihood of metastasis (29). Tumors with somatic BAP1 mutations have also demonstrated larger tumor sizes, more aggressive disease with poorer outcomes, and higher rates of metastasis, and they may represent a precursor event to the more aggressive ‘class 2’ UM (26, 39, 55, 59, 78). Mean survival in UM patients with tumors that lacked BAP1 protein expression was 4.74 years as compared to 9.97 years in patients with BAP1 protein expression (45).
Similar somatic findings were reported in RCC as Hakimi et al. found an average of 31.2 month survival in RCC patients with somatic BAP1 mutations compared to 78.2 month survival in patients without in a cohort of 421 patients (56). Kapur et al. determined similar survival rates with an average of 1.9-year survival in RCC patients with tumors with somatic BAP1 mutations vs 5.4-year survival in patients with tumors with PBRM1 mutations in a cohort of 327 patients (79). A smaller cohort in the study showed 4.6-year survival in BAP1 mutant tumors vs 10.6-year survival in PBRM1 mutant tumors.
In CM, a small pilot study has suggested the tumors with low BAP1 expression tends to have shorter disease-free survival and melanoma-specific survival (53). However, in vitro and in vivo studies of primary melanomas and non-tranformed melanoma cell lines suggested a pro-survival role of BAP1. This could be partially explained through its effect on survivin protein expression (80).
In contrast, a cohort of 23 MMe patients with germline BAP1 mutations appeared to have sevenfold longer overall survival as compared with sporadic MMe patients (81). Patients with germline BAP1 mutations had improved survival in both peritoneal and pleural MMe (81). de Reynies et al. found a distinct, less aggressive subset of MMe through molecular profiling that was characterized by a high prevalence of somatic BAP1 mutation (82).
The molecular mechanism of such variation in aggressiveness between BAP1-associated cancers is not clear. A potential explanation could be differences in treatment response in tumors with and without BAP1 inactivation. Another explanation could be the well-differentiated morphology of some MMe in patients with germline BAP1 mutations in contrast to high grade tumors in other patients, although the mechanism for this is unknown. Also, the increased rate of peritoneal MMe in BAP1 carriers, which has a better prognosis than pleural MMe, may be causing the increase in overall survival (83).
Penetrance of BAP1-TPDS
Current evidence suggests that the penetrance of BAP1 mutations is high with 148/174 (85%) reported mutation carriers affected with cancer. Although ages for unaffected individuals were not reported, estimates based on the pedigrees placed an average unaffected age in the late 50’s (5, 18, 27, 29, 30). It should be noted, however, that several unaffected carriers are young and therefore may yet develop cancer. One BAP1 patient (FUM036, III.9) previously reported as unaffected by our group subsequently developed MMe at age 60 and passed away (2). Additionally, two newly tested members of families previously reported by our group were found to carry the family mutations and remain unaffected at ages 47 and 35, respectively (FUM103, III.3; FUM152, III.2) (18, 19).
It is likely, however, that these penetrance data are inflated by test bias since the patients and family members who get tested are generally those affected by cancer. Of the 57 reported families, only the affected proband was tested in 30, and only 17 families had more than 3 individuals tested. Thus, the true penetrance for BAP1 mutations may well be lower than currently estimated.
Genotype–phenotype correlation
Of 57 families, 46 had unique mutations, two families had the c.2050C>T, p.Q684* mutation, two families had the c.1882_1885delTCAC, p.S628Pfs*8 mutation, two families had the c.1717delC, p.L573Wfs*3 mutation, two families had the c.588G>A, p.Trp196* mutation, while three families had the c.178C>T, p.R60* mutation. Discussions between the authors concluded that the families carrying the c.2050C>T, p.Q684*and c.1882_1885delTCAC, p.S628Pfs*8 mutations were unrelated. However, discussions and haplotype analysis suggested that the two families from Denmark carrying the c.178C>T, p.R60* mutation were related, whereas the family carrying this mutation from the United States was not (3, 18, 19, 29, 31).
The majority (32/51, 63%) of the reported mutations were truncating, 11 (22%) were missense mutations thought to be pathogenic, and 8 (15%) were splice-site variations. Five of the splice-site variations also caused protein truncation. Thus, 22% of the reported germline mutations are missense mutations and 73% cause protein truncation. All the truncating mutations were proximal to the nuclear localizing regions of BAP1.
With the exception of one patient, all reported pathogenic mutations in BAP1, including truncating, missense, and splice-site mutations, were associated with at least one of the four main cancers in the family (Fig. 3). All four cancers were observed with all classes of mutations. Taken together, the available data suggest no clear genotype–phenotype correlation between type or location of the mutation and the type of cancer in patients.
Fig. 3.
Gene location and mutation type of the reported germline pathogenic variants in BAP1 in the four main cancers associated with BAP1-TPDS. No genotype–phenotype correlation was observed. One mutation was found in a family not reporting a uveal melanoma (UM), malignant mesothelioma (MMe), cutaneous melanoma (CM), or renal cell carcinoma (RCC). Two family mutations were not tested in a member with a personal history of UM, MMe, CM, or RCC. ▲, splice-site mutation; ★, truncating mutation; ●, missense mutation; UCH, ubiquitin C-terminal hydrolase; BARD1, BARD1 binding domain; HCF1, HCF1 binding motif; BRCA1, BRCA1 binding domain; N, nuclear localization signal.
Discussion
Genetic counseling and patient management
Of the 174 reported patients with BAP1 mutations 130 (75%) were diagnosed with UM, MMe, CM, RCC, and/or BAP1-deficient ASTs, and 90% of these families had at least two of these tumors in first or second-degree relatives. Five families reported histories of only one of the main tumors. Thus, we recommend that genetic assessment and testing for BAP1 mutations should be considered in patients with two or more of these tumors in themselves and/or first or second-degree relatives, with the exclusion of families with only multiple CM cases, given its frequency in the general population. We recognize, however, that if a melanoma-only family is undergoing CDKN2A testing for hereditary melanoma, it may be reasonable and cost effective to also test them for mutations in BAP1 at the same time. Although it has been suggested elsewhere that BAP1 testing be conducted in families with histories of one UM and two or more primary cancers of any type, we feel this is overly broad as there is not sufficient evidence supporting the involvement of other cancers (84).
Families in which a germline BAP1 mutation is found should receive counseling regarding cancer risk management options and risks to family members. Testing of at-risk family members is indicated because the syndrome follows an autosomal dominant inheritance pattern. Although evidence-based management recommendations have not been established, the cancer risks in these families cannot be ignored. As such, regular examinations, particularly for eye and skin tumors, to facilitate early diagnosis, and thereby improved prognosis, are necessary. Although imperfect, we recommend that screenings follow guidelines based on other TPDS with similar phenotypes (Table 2) (53, 85–91). The recommended ages to begin screening given are based on currently reported ages of diagnoses; however, if an individual family has members diagnosed at an even earlier age for a specific cancer, screening for that cancer should begin for other members of that family about 5 years before that age of diagnosis. ASTs have a unique clinical, morphological, and molecular characterization and their high frequency in patients with germline BAP1 mutation indicate they might serve as a potential marker of BAP1-TPDS. It is highly recommended that ASTs, in particular those with a prominent epithelioid component, be screened for BAP1 status by immunohistochemistry. If BAP1 loss is detected, referral for genetic counseling and germline BAP1 testing should be considered (14). There are currently no Food and Drug Administration (FDA)-approved targeted treatments for BAP1-driven malignancies. Intriguingly, preliminary attempts at discovering therapies have shown modest success in UM in vitro (90).
Table 2.
Proposed management recommendations for families with germline BAP1 mutation
| Risk | Age of earliest reported case | Screening recommendations |
|---|---|---|
| Uveal melanoma (UM) | 16 | |
| Malignant mesothelioma | 34 |
|
| Cutaneous melanoma, basal cell carcinoma and atypical Spitz tumors | 25 | |
| Renal cell carcinoma | 36 |
|
MRI, magnetic resonance imaging.
Five years earlier than earliest reported case.
Conclusions
UM, MMe, CM, RCC, and ASTs are clearly established as part of the phenotypic spectrum associated with germline BAP1 mutations. Although other cancers, in particular breast cancer and BCC might be associated, further studies are needed. Although UM, CM, and RCC tumors in patients with germline BAP1 mutations tend to be more aggressive and have poorer outcomes, patients with MMe appear to have longer survival. There appears to be no genotype–phenotype correlation with this syndrome. Penetrance for the gene appears to be high, but ascertainment bias makes it difficult to currently establish accurate estimates of cancer risk. Nonetheless, increased screening is indicated, particularly for skin and eye cancers.
Acknowledgments
This work was supported by the Patti Blow Research Fund in Ophthalmology, Grant #IRG-67-003-47 from the American Cancer Society, and funding from the Ohio Lions Eye Research Foundation, Ocular Melanoma Foundation, Melanoma Know More Foundation, the R21CA191943 Grant from the National Cancer Institute (PI: Abdel-Rahman, MH), and the National Eye Institute grant K08EY022672 (C. M. C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Cancer Institute, or National Eye Institute. We thank Brandon Massengill for his work in sample sequencing and other investigative experiments.
Footnotes
Conflict of interest
No conflicts of interest exist for any author.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s website.
References
- 1.Online Mendelian Inheritance in Man, OMIM®. Baltimore, MD: Johns Hopkins University; 2014. MIM Number: 614327. [Google Scholar]
- 2.Abdel-Rahman MH, Pilarski R, Cebulla CM, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesner T, Obenauf AC, Murali R, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popova T, Hebert L, Jacquemin V, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen DE, Proctor M, Marquis ST, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 7.Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eletr ZM, Wilkinson KD. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem Biophys. 2011;60:3–11. doi: 10.1007/s12013-011-9184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eletr ZM, Yin L, Wilkinson KD. BAP1 is phosphorylated at serine 592 in S-phase following DNA damage. FEBS Lett. 2013;587:3906–3911. doi: 10.1016/j.febslet.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Pak H, Hammond-Martel I, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci USA. 2014;111:285–290. doi: 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen DE, Rauscher FJ., 3rd BAP1, a candidate tumor suppressor protein that interacts with BRCA1. Ann N Y Acad Sci. 1999;886:191–194. doi: 10.1111/j.1749-6632.1999.tb09414.x. [DOI] [PubMed] [Google Scholar]
- 12.Jensen DE, Rauscher FJ., 3rd Defining biochemical functions for the BRCA1 tumor suppressor protein: analysis of the BRCA1 binding protein BAP1. Cancer Lett. 1999;143 (Suppl 1):S13–S17. doi: 10.1016/s0304-3835(99)90004-6. [DOI] [PubMed] [Google Scholar]
- 13.Carbone M, Yang H, Pass H, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013;45:116–126. doi: 10.1097/PAT.0b013e32835d0efb. [DOI] [PubMed] [Google Scholar]
- 15.White AE, Harper JW. Cancer. Emerging anatomy of the BAP1 tumor suppressor system. Science. 2012;337:1463–1464. doi: 10.1126/science.1228463. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Mashtalir N, Daou S, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey A, Seshasayee D, Noubade R, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 2012;337:1541–1546. doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilarski R, Cebulla CM, Massengill JB, et al. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer. 2014;53:177–182. doi: 10.1002/gcc.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cebulla CM, Binkley EM, Pilarski R, et al. Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet. 2015;36 (2):126–131. doi: 10.3109/13816810.2015.1010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoude LG, Vajdic CM, Kricker A, Armstrong B, Hayward NK. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res. 2013;26:278–279. doi: 10.1111/pcmr.12046. [DOI] [PubMed] [Google Scholar]
- 22.Aoude LG, Wadt K, Bojesen A, et al. A BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancers. PLoS One. 2013;8:e72144. doi: 10.1371/journal.pone.0072144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung M, Talarchek J, Schindeler K, et al. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet. 2013;206:206–210. doi: 10.1016/j.cancergen.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 24.de la Fouchardiere A, Cabaret O, Savin L, et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet. 2014 doi: 10.1111/cge.12472. Epub 31 July 2014. [DOI] [PubMed] [Google Scholar]
- 25.Gupta MP, Lane AM, DeAngelis MM, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol. 2015 doi: 10.1001/jamaophthalmol.2015.1119. Epub 14 May 2015. [DOI] [PubMed] [Google Scholar]
- 26.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoiom V, Edsgard D, Helgadottir H, et al. Hereditary uveal melanoma: a report of a germline mutation in BAP1. Genes Chromosomes Cancer. 2013;52:378–384. doi: 10.1002/gcc.22035. [DOI] [PubMed] [Google Scholar]
- 28.Maerker DA, Zeschnigk M, Nelles J, et al. BAP1 germline mutation in two first grade family members with uveal melanoma. Br J Ophthalmol. 2014;98:224–227. doi: 10.1136/bjophthalmol-2013-303814. [DOI] [PubMed] [Google Scholar]
- 29.Njauw CN, Kim I, Piris A, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One. 2012;7:e35295. doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadt K, Choi J, Chung JY, et al. A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment Cell Melanoma Res. 2012;25:815–818. doi: 10.1111/pcmr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadt KA, Aoude LG, Johansson P, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet. 2014 doi: 10.1111/cge.12501. Epub 15 September 2014. [DOI] [PubMed] [Google Scholar]
- 32.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Betti M, Casalone E, Ferrante D, et al. Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosomes Cancer. 2015;54:51–62. doi: 10.1002/gcc.22218. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro C, Campelos S, Moura CS, Machado JC, Justino A, Parente B. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol. 2013;24:2147–2150. doi: 10.1093/annonc/mdt135. [DOI] [PubMed] [Google Scholar]
- 35.Wiesner T, Fried I, Ulz P, et al. Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol. 2012;30:e337–e340. doi: 10.1200/JCO.2011.41.2965. [DOI] [PubMed] [Google Scholar]
- 36.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 37.Surveillance, Epidemiology, and End Results (SEER) Program; Surveillance Systems Branch, editor. National Cancer Institute D, Surveillance Research Program. 2013. [DOI] [PubMed] [Google Scholar]
- 38.Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11:1061–1071. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gossage L, Murtaza M, Slatter AF, et al. Clinical and pathological impact of VHL, PBRM1, BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma. Genes Chromosomes Cancer. 2014;53:38–51. doi: 10.1002/gcc.22116. [DOI] [PubMed] [Google Scholar]
- 40.Pena-Llopis S, Vega-Rubinde-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 42.de la Fouchardiere A, Cabaret O, Petre J, et al. Primary leptomeningeal melanoma is part of the BAP1-related cancer syndrome. Acta Neuropathol. 2015;129:921–923. doi: 10.1007/s00401-015-1423-2. [DOI] [PubMed] [Google Scholar]
- 43.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalirai H, Dodson A, Faqir S, Damato BE, Coupland SE. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111:1373–1380. doi: 10.1038/bjc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koopmans AE, Verdijk RM, Brouwer RW, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. 2014;27:1321–1330. doi: 10.1038/modpathol.2014.43. [DOI] [PubMed] [Google Scholar]
- 47.Shah AA, Bourne TD, Murali R. BAP1 protein loss by immunohistochemistry: a potentially useful tool for prognostic prediction in patients with uveal melanoma. Pathology. 2013;45:651–656. doi: 10.1097/PAT.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 48.Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol. 2007;18:985–990. doi: 10.1093/annonc/mdl345. [DOI] [PubMed] [Google Scholar]
- 49.Arzt L, Quehenberger F, Halbwedl I, Mairinger T, Popper HH. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res. 2014;20:145–151. doi: 10.1007/s12253-013-9677-2. [DOI] [PubMed] [Google Scholar]
- 50.Alakus H, Yost SE, Woo B, et al. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J Transl Med. 2015;13:122. doi: 10.1186/s12967-015-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21. 1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10:565–576. doi: 10.1097/JTO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murali R, Wilmott JS, Jakrot V, et al. BAP1 expression in cutaneous melanoma: a pilot study. Pathology. 2013;45:606–609. doi: 10.1097/PAT.0b013e3283653818. [DOI] [PubMed] [Google Scholar]
- 54.Piris A, Mihm MC, Jr, Hoang MP. BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol. 2015;46:239–245. doi: 10.1016/j.humpath.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Hakimi AA, Chen YB, Wren J, et al. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol. 2013;63:848–854. doi: 10.1016/j.eururo.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakimi AA, Ostrovnaya I, Reva B, et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network. Clin Cancer Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho TH, Kapur P, Joseph RW, et al. Loss of PBRM1 and BAP1 expression is less common in non-clear cell renal cell carcinoma than in clear cell renal cell carcinoma. Urol Oncol. 2015;33:23 e29–14. doi: 10.1016/j.urolonc.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph RW, Kapur P, Serie DJ, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer. 2014;120:1059–1067. doi: 10.1002/cncr.28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapur P, Christie A, Raman JD, et al. BAP1 immunohistochemistry predicts outcomes in a multi-institutional cohort with clear cell renal cell carcinoma. J Urol. 2014;191:603–610. doi: 10.1016/j.juro.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 60.Shinagare AB, Vikram R, Jaffe C, et al. Radiogenomics of clear cell renal cell carcinoma: preliminary findings of The Cancer Genome Atlas-Renal Cell Carcinoma (TCGA-RCC) Imaging Research Group. Abdom Imaging. 2015 doi: 10.1007/s00261-015-0386-z. Epub 10 March 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Llamas-Velasco M, Perez-Gonzalez YC, Requena L, Kutzner H. Histopathologic clues for the diagnosis of Wiesner nevus. J Am Acad Dermatol. 2014;70:549–554. doi: 10.1016/j.jaad.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 63.Wiesner T, Murali R, Fried I, et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol. 2012;36:818–830. doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martorano LM, Winkelmann RR, Cebulla CM, Abdel-Rahman MH, Campbell SM. Ocular melanoma and the BAP1 hereditary cancer syndrome: implications for the dermatologist. Int J Dermatol. 2014;53:657–663. doi: 10.1111/ijd.12386. [DOI] [PubMed] [Google Scholar]
- 65.Busam KJ, Wanna M, Wiesner T. Multiple epithelioid Spitz nevi or tumors with loss of BAP1 expression: a clue to a hereditary tumor syndrome. JAMA Dermatol. 2013;149:335–339. doi: 10.1001/jamadermatol.2013.1529. [DOI] [PubMed] [Google Scholar]
- 66.Busam KJ, Sung J, Wiesner T, von Deimling A, Jungbluth A. Combined BRAF(V600E)-positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional nevomelanocytes. Am J Surg Pathol. 2013;37:193–199. doi: 10.1097/PAS.0b013e318263648c. [DOI] [PubMed] [Google Scholar]
- 67.Chan-On W, Nairismagi ML, Ong CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 68.Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Je EM, Lee SH, Yoo NJ. Somatic mutation of a tumor suppressor gene BAP1 is rare in breast, prostate, gastric and colorectal cancers. APMIS. 2012;120:855–856. doi: 10.1111/j.1600-0463.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 70.Jhunjhunwala S, Jiang Z, Stawiski EW, et al. Diverse modes of genomic alteration in hepatocellular carcinoma. Genome Biol. 2014;15:436. doi: 10.1186/s13059-014-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luscan A, Just PA, Briand A, et al. Uveal melanoma hepatic metastases mutation spectrum analysis using targeted next-generation sequencing of 400 cancer genes. Br J Ophthalmol. 2015;99(4):437–439. doi: 10.1136/bjophthalmol-2014-305371. [DOI] [PubMed] [Google Scholar]
- 73.Mochel MC, Piris A, Nose V, Hoang MP. Loss of BAP1 expression in basal cell carcinomas in patients with germline BAP1 mutations. Am J Clin Pathol. 2015;143:901–904. doi: 10.1309/AJCPG8LFJC0DHDQT. [DOI] [PubMed] [Google Scholar]
- 74.Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839–2852. doi: 10.18632/oncotarget.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang J, Xi S, Wang G, et al. Prognostic significance of BRCA1-associated protein 1 in colorectal cancer. Med Oncol. 2013;30:541. doi: 10.1007/s12032-013-0541-8. [DOI] [PubMed] [Google Scholar]
- 76.Coupier I, Cousin PY, Hughes D, et al. BAP1 and breast cancer risk. Fam Cancer. 2005;4:273–277. doi: 10.1007/s10689-005-2833-4. [DOI] [PubMed] [Google Scholar]
- 77.Guenard F, Labrie Y, Ouellette G, Beauparlant CJ, Durocher F INHERIT BRCAs. Genetic sequence variations of BRCA1-interacting genes AURKA, BAP1, BARD1 and DHX9 in French Canadian families with high risk of breast cancer. J Hum Genet. 2009;54:152–161. doi: 10.1038/jhg.2009.6. [DOI] [PubMed] [Google Scholar]
- 78.Karlo CA, Di Paolo PL, Chaim J, et al. Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology. 2014;270:464–471. doi: 10.1148/radiol.13130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kapur P, Pena-Llopis S, Christie A, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar R, Taylor M, Miao B, et al. BAP1 has a survival role in cutaneous melanoma. J Invest Dermatol. 2015;135:1089–1097. doi: 10.1038/jid.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36:76–81. doi: 10.1093/carcin/bgu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Reynies A, Jaurand MC, Renier A, et al. Molecular classification of malignant pleural mesothelioma: identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clin Cancer Res. 2014;20:1323–1334. doi: 10.1158/1078-0432.CCR-13-2429. [DOI] [PubMed] [Google Scholar]
- 83.Faig J, Howard S, Levine EA, Casselman G, Hesdorffer M, Ohar JA. Changing pattern in malignant mesothelioma survival. Transl Oncol. 2015;8:35–39. doi: 10.1016/j.tranon.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121:1281–1288. doi: 10.1016/j.ophtha.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolff H, Vehmas T, Oksa P, Rantanen J, Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41:5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 86.Kefford R, Bishop JN, Tucker M, et al. Genetic testing for melanoma. Lancet Oncol. 2002;3:653–654. doi: 10.1016/s1470-2045(02)00894-x. [DOI] [PubMed] [Google Scholar]
- 87.Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a consensus statement of the Melanoma Genetics Consortium. J Clin Oncol. 1999;17:3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- 88.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 89.Kaya H, Demir M, Taylan M, et al. Fibulin-3 as a diagnostic biomarker in patients with malignant mesothelioma. Asian Pac J Cancer Prev. 2015;16:1403–1407. doi: 10.7314/apjcp.2015.16.4.1403. [DOI] [PubMed] [Google Scholar]
- 90.Landreville S, Agapova OA, Matatall KA, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber DG, Casjens S, Johnen G, et al. Combination of MiR-103a–3p and mesothelin improves the biomarker performance of malignant mesothelioma diagnosis. PLoS One. 2014;9:e114483. doi: 10.1371/journal.pone.0114483. [DOI] [PMC free article] [PubMed] [Google Scholar]