Abstract
Ophthalmic gene therapy is an intellectual and intentional manipulation of desired gene expression into the specific cells of an eye for the treatment of ophthalmic (ocular) genetic dystrophies and pathological conditions. Exogenous nucleic acids such as DNA, small interfering RNA (siRNA), micro RNA (miRNA), etc., are used for the purpose of managing expression of the desired therapeutic proteins in ocular tissues. The delivery of unprotected nucleic acids into the cells is limited due to exogenous and endogenous degradation modalities. Nanotechnology, a promising and sophisticated cutting edge tool, works as a protective shelter for these therapeutic nucleic acids. They are able to be safely delivered to the required cells in order to modulate anticipated protein expression. To this end, nanotechnology is seen as a potential and promising strategy in the field of ocular gene delivery. This review focused on current nanotechnology modalities and other promising non-viral strategies being used to deliver therapeutic genes in order to treat various devastating ocular diseases.
Keywords: Ocular disorder, nanotechnology, nanoparticle, non-viral gene therapy
1. Introduction
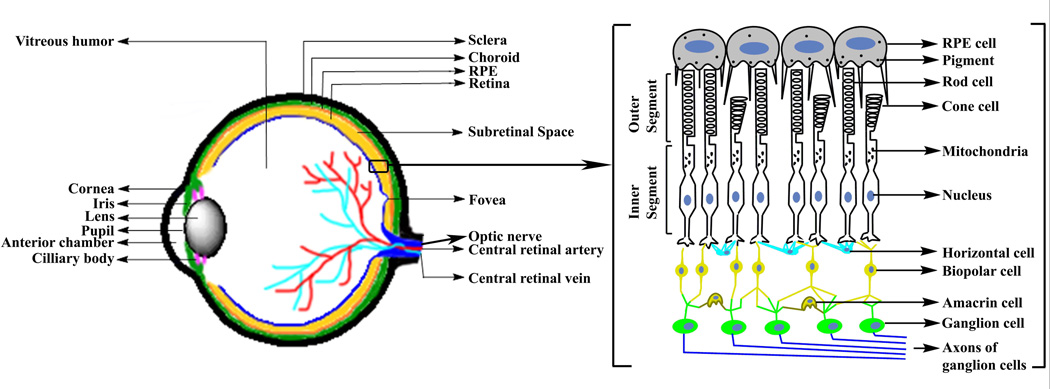
Over the last few decades, gene based therapeutic clinical strategies have been extensively explored for the treatment of ocular diseases1–8. Gene therapy, from its inception, aims to repair or replace the disease-causing mutations by delivering the suitable therapeutic genetic materials along with the rational regulatory elements into the desired cells to express the deficient protein at normal levels. For therapeutic purposes, it has already been established that the genomic and the coding and/or non-coding RNA sequences can be modulated by introducing nucleic acids into the ocular tissues (Fig. 1). The effectiveness of inserting therapeutic genes into the desired cell is not only manipulated by DNA designing strategies, but also significantly depends on the efficiency and safety of its delivery vehicle(s). This review will summarize details on several ocular dystrophies of the leading causes of blindness in the world. In addition, major focus will be on the existing preclinical studies on nanotechnology-motivated synthetic non-viral gene delivery vehicles. These vehicles have already resulted in the improvements of structural and functional recovery of several major retinal disorders in animal models, and might have promising clinical significance in future human ocular gene therapy.
Fig. 1.
Diagrams of vertebrate eye (left) and the retina (right).
2. Ocular disorders
2.1. Retinitis pigmentosa
Retinitis pigmentosa (RP) is a group of clinically and genetically heterogeneous disorders3, 9. RP has a worldwide prevalence of 1 in 3000 to 7000 people10. This is characterized by initial night blindness (tunnel vision) due to the loss of rod cells in the periphery of the retina. As the disease progresses, cone cells start to degenerate, which leads to complete physical blindness of the patient via loss of central vision. The reason behind the loss of cone cells in RP cases is not yet clear, although rod cells possess defective genes. In this context, it is assumed that these cone cell dystrophies might be due to loss of rod cell-based supporting factors11. Multiple genetic inheritance patterns were found in RP patients. Among these patients, 15–25% of cases were autosomal dominant (adRP), 5–25% of cases showed autosomal recessive (arRP), and 5–15% showed X-linked traits10, 12. In general, RP is divided into two categories: one is syndromic (40%), and the other one is non-syndromic (or simple, 60%). The non-syndromic, or simple RP, is limited to the eye. The syndromic RP is beyond the eye, which also affects other organs and tissues in the body. The most frequent and studied syndromic disease is Usher syndrome (hearing loss followed by RP)10.
2.1.1. Rhodopsin (Rho) mutations
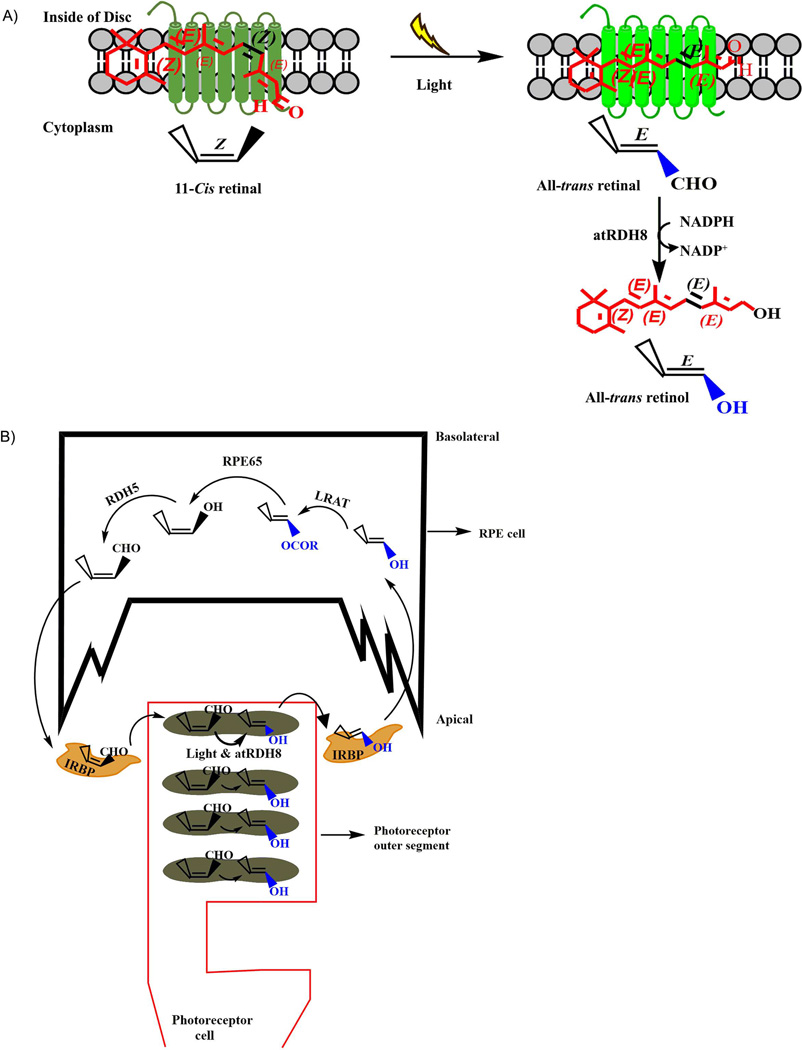
RP is associated with more than 100 mutations12 in the different regions of the rhodopsin (Rho) gene that accounts for 30–40% of adRP10, and thus is genetically heterogeneous3, 5, 10. Rhodopsin protein helps to keep the structural integrity of the rod outer segments, as it was observed that Rho−/− mice were not able to extend rod outer segments. Rhodopsin protein is covalently associated with the 11-cis retinal chromophore to form a visual pigment, G protein-coupled receptor (GPCR), and plays a vital role in the conversion of electromagnetic radiation (light) to electrical signal in the retina, which is further processed in the brain as an image10. Upon absorption of a photon, the chromophore (11-cis retinal) is isomerized to all-trans (Fig. 2a) and causes conformational changes in the visual pigment (GPCR), which triggers a signal transduction cascade. This cascade results in the closure of nonselective cyclic guanosine 3'-5'-monophosphate (cGMP)-gated cation channels in the photoreceptor (PR) outer segment and hyperpolarization of the PR plasma membrane that generates receptor potential at PR synapse. Rod cells are responsible for night vision and remain sensitive as they are activated by single photons.
Fig. 2.
A) Photo activation leads to an isomerization of 11-cis retinal molecule to all-trans retinal (atRAL) in rhodopsin (conjugation of rod opsin + 11-cis retinal) pigment in outer segment disc. The atRAL is now converted to all-trans retinol (atROL) by all-trans retinol dehydrogenase (atRDH8) and initiates visual photo transduction processes. B) Simple representation of the visual cycle in vertebrate eye. In rod cell, retinal chromophore (11-cis retinal) binds to the rod opsin protein and forms GPCR. Absorption of light causes activation of this photopigment, and leads to isomerization of the 11-cis retinal to atRAL that is subsequently reduced by atRDH8 to atROL in photoreceptor outer segment. This atROL is now transferred to RPE cells via IRBP carrier enzymes, where it is esterified to long-chain fatty acids (all-trans retinyl esters) by Lecithin retinol acyltransferase (LRAT). All-trans retinyl esters are then enzymatically isomerized and hydrolysed to the 11-cis retinol by retinal pigment epithelium-specific 65 kDa (RPE65) isomerohydrolase. This 11-cis retinol is then finally converted to 11-cis retinal, a universal chromophore for visual pigment, by 11-cis retinol dehydrogenase (RDH5), and is consequently shuttled back to photoreceptor cells by IRBP to reconstitute rhodopsin pigment in photoreceptor disc, where it completes the visual cycle.
Mutation in the Rho gene can affect the rod cell functions at different severity levels. The same RP phenotype is observed from mutations in different regions of the rhodopsin gene that can cause different amino acid substitutions, and therefore leads to different rates of progression of this disorder. When the interdiscal protein mutates, it remains less severe than that in the cytoplasmic or retinal binding site domains. Mutated rhodopsin protein in the cytoplasm possesses inappropriate transport properties towards the outer segment of rod cells.
The adRP currently does not have any therapeutic support. Rho mutations are classified as Class I and Class II based on tissue culture studies13–15. In Class I mutation, opsin expresses wild type level and binds to 11-cis retinal to form retinal photopigment (Fig. 2a) along with proper folding.
Class II mutation is the major cause of RP in North America15. Class II mutants accumulate and remain misfolded in the endoplasmic reticulum (ER). The misfolded protein causes activation of unfolded protein response (UPR), and leads to the cellular dystrophies associated with various ocular diseases. The first identified, and so far the most important Class II, RP is associated with the single missense mutation in the codon 23 in the human opsin gene. This mutation results in one proline substitution by histidine (RhoP23H), and is responsible for the most frequent cause of adRP15. The cause related to the RhoP23H toxicity remains unclear. Due to the dominant nature of the disorder, suppression and replacement of the gene are considered a useful and logical therapeutic approach in the treatment of the mutational heterogeneity of these adRP cases.
2.1.2. Phosphodiesterase 6b (PDE6b)
Rod cyclic guanosine 3'-5'-monophosphate (cGMP)-specific phosphodiesterase-6 (PDE6) enzyme is responsible for the hydrolysis of cytoplasmic cGMP in photoreceptor cells of the retina. PDE6 is a peripheral membrane heterotetramer enzyme that is composed of two inhibitory gamma (γ) subunits and two catalytic homologous α and β subunits in rods (PDE6αβγγ), and related α’ subunits in cones (PDE6 α’α’γγ). PDE6 plays a key role in the photo-transduction cascade by regulating cGMP levels in the photoreceptor cells. Guanylate cyclase synthesizes cGMP from GTP and regulates the ion channel in the plasma membrane. During dark adaptation, cGMP keeps the ion channels open, thus allowing the influx of calcium ions into the outer segment of photoreceptors that causes overall depolarization of these cells. In light adaptation, PDE6 hydrolyses the cGMP and thus the cGMP amount is reduced, which leads to closing of cGMP gated ion channels and overall hyperpolarization of the photoreceptor cells. The beta subunits of PDE6 are encoded by the PDE6B gene, and therefore mutations in this PDE6B gene lead to malfunctioning of the PDE6 enzyme activity. In the light and dark adapted mechanism, the non-functional PDE6 enzyme is not able to hydrolyse cGMP, which results in accumulation of cGMP without closing the ion channels. This leads to the accumulation of excess calcium in cytoplasm that finally induces the degeneration of photoreceptor cells, resulting in blindness16. The mutation in the PDE6B gene is responsible for the arRP in humans. This earliest and most severe form of the disease is contributing to 5% of all arRP cases.
2.2. Usher syndrome
Usher syndrome (USH) is a genetically heterogeneous group of autosomal recessive genes that affects both hearing and vision (related to inner ear and retina, the most sensitive neurosensory organs in mammals), along with occasional loss of balance. This syndrome was first discovered by Scottish ophthalmologist Charles Usher in 1914, upon examination of over 69 patients who were dealing with deafness with RP. There is a predictable occurrence in which 3–6 per 100,000 human patients are observed with both blindness and deafness17, 18. Clinically, there are three types of Usher syndrome (USH1, USH2 and USH3)3, 19, 20. Regarding the onset of hearing loss, USH1 is the most severe19. In USH1, a child is born with severe to profound deafness. The vision problem starts within 10 years of age, and progresses quickly to complete blindness. The child with USH1 might also have more balance problems associated with sitting and walking than normal. USH2 is comparatively less severe than USH1. The child is born with moderate to severe hearing loss and normal balance. Night vision problems appear in late childhood or teens with slow progressive loss of vision19. In USH3 cases, the child is born with normal hearing and balance, but there is an advanced loss of hearing by adolescence19. Vision loss varies in severity, and night vision problems develop during the teenage years. Night blindness and loss of peripheral vision are the early symptoms of RP due to the loss of rod photoreceptor cells. As the disease progresses, the cone photoreceptor cells also start to degenerate, leading to loss in the central vision that finally results in blindness of USH patients.
Significant amounts of research are currently underway to identify the genes related to USH syndrome. There are six genes that have been found so far to be associated with USH1, and these are MYO7A (myosin VIIA), USH1C (harmonin), CDH23 (cadherin 23), PCDH15 (protocadherin 15), SANS (scaffold protein containing ankyrin repeats and sam domain) and CIB2 (calcium- and integrin-binding protein 2) 18. The proteins, encoded by these genes, are expressed in the cochlear hair cells in the inner ear, as well as in the photoreceptor cells of the retina. These USH proteins are helping the inner ear hair cell bundles in their development and maintenance of their function and stability. Mutations in these genes can result in the loss of protein functions that finally lead to the prevention of hair cell developments and loss in hearing. In mice, MYO7A has also been found in the RPE (retinal pigmented epithelium) cells, along with different sections of photoreceptor cells like connecting cilia, inner segment, and synapse. The USH2 (usherin, VLGR1 and whirlin) and USH3 (clarin-1) proteins are also found in the same sections of photoreceptor cells in the mouse retina. Literature shows that MYO7A functions the same way in both human and mouse RPE cells with respect to melanosome mortality21.
2.3. Stargardt’s disease
Stargardt’s is an autosomal recessive juvenile disorder22 which mainly occurs in children between the ages of 6–16 years, with a prevalence of 1 in 8000 to 10000 patients23. This common genetic macular disorder presents throughout the world, due to the mutation in a gene that encodes a photoreceptor ATP binding cassette (ABC) lipid transporter protein (more commonly known as ABCA4)1, 24, 25. ABCA4 is mainly expressed in photoreceptor cells and remains in the outer segment disc membrane25. In dark adaptation, opsin protein remains covalently conjugated with the 11-cis retinal chromophore. During light activation, this 11-cis retinal is isomerized to the all-trans retinal (atRAL) form that isomerizes back to 11-cis retinal in RPE cells to reconstitute the photopigment in maintaining the visual cycle (Fig. 2b). In this process, atRAL is converted to all-trans retinol (atROL) by all-trans retinol dehydrogenase 8 (atRDH8), and is transported to the RPE cells by interphotoreceptor retinoid-binding proteins (IRBP). Through some enzymatic pathways, atROL is now converted back to 11-cis retinal in RPE, and is immediately shuttled back to the photoreceptor outer segment by IRBP to reconstitute rhodopsin photopigment. During this process, there is another pathway where a fraction of atRAL is conjugated with phosphatidylethanolamine (PE) to form N-retinylidenephosphatidylethanolamine (N-ret-PE) in the disc membrane lumen side24. The flipase ABCA4 protein binds with N-ret-PE and flips it from the lumen side to the cytoplasmic side of the disc membrane using ATP energy source24. Once this N-ret-PE is brought to the cytoplasmic side, it is hydrolysed by all-trans retinol dehydrogenase 8 (RDH8) to atROL and PE. Therefore, ABCA4 makes sure the disc membrane atRAL is converted to atROL in cytoplasm, and is shuttled towards RPE to regenerate 11-cis retinal.
Mutation in the ABCA4 gene can cause the accumulation of N-ret-PE in the lumen side of the disc membrane, which facilitates further possible reaction with another molecule of atRAL in the disc membrane to form a phosphatidyl pyridinium diretinoid derivative A2PE (2: 1 ratio)25. Under normal phagocytocis of photoreceptor cells, A2PE is engulfed by RPE and degraded to A2E by lysomal enzymes. RPE cells are not able to metabolize the A2E that leads to a high amount of A2E accumulation as fluorescent lipofusin25. This A2E accumulation causes excessive generation of reactive oxygen species (ROS) and leads to degeneration of RPE cells. The macula also has a high density of photoreceptor cells, and therefore causes high levels of A2E accumulation. RPE, just underlying the macula (which possesses the highest density of cone photoreceptor cells), plays a key role in providing the structural and functional integrity of photoreceptor cells. The loss of RPE cells causes damage to the photoreceptor cells in the macula, which finally leads to Stargardt’s disease.
2.4. Leber congenital amaurosis (LCA)
Leber congenital amaurosis (LCA) is an autosomal recessive inheritance pattern which was found by Theodor Leber in 1869. So far, there are 14 genes evaluated which are responsible for this severe congenital blindness that presents in early childhood26. The worldwide prevalence of this disease is 1 out of 30,000 cases 27, 20% of all congenital blindness, and 5% of all inherited retinal dystrophies. One of the most important mutations is the RPE65 gene that encodes RPE65 (RPE-specific 65 KDa) protein in RPE cells 6, 8, 28, 29. This protein works as a retinoid isomerohydrolase in RPE cells to convert all-trans retinoid to 11-cis retinal in the reconstruction process of photopigments in the photoreceptor cells, which completes the visual cycle6 (Fig. 2b). Therefore, mutation in the RPE65 gene can cause a generation of non-functional RPE65 protein that will not be able to regenerate 11-cis retinal, and causes genetically heterogeneous and severe visual impairment at birth or during the first month of life. The RPE65 thus causes the disturbances in the visual cycle at this early stage of life.
Another form of severe LCA gene is the retina specific guanylate cyclase-1 (GUCY2D), which encodes guanylate cyclase-1 (GC-1) in the photoreceptor cells that convert GTP to cGMP and control the cyclic nucleotide-gated (CNG) ion channels in the photoreceptor cell plasma membrane28, 30. Therefore, GC-1 plays a vital role in the visual photo-transduction process and conversion of electromagnetic radiation to chemical signals. The mutation in this gene causes malfunctioning of GC-1 protein, which impairs the production of cGMP that leads to a permanent closure of the cGMP gated channel.
Proto-oncogene tyrosine-protein kinase (MER)31 is a transmembrane protein that is encoded by the MERTK gene in humans. This protein has an intracellular tyrosine kinase domain. The extracellular signaling domain of this protein remains on the apical portion of RPE cells32. MERTK signaling plays an important role in the daily clearance of shed photoreceptor outer segment debris by RPE phagocytosis and survival of photoreceptor cells of the retina32. Therefore, it helps to inhibit the intracellular antigen-induced inflammation and autoimmune responses. It is observed that 0.6% of LCA is caused by the mutation of this MERTK gene, which encodes non-functional MER protein. Mutation in this gene causes the reduction of phagocytic activity of RPE cells that results in the accumulation of photoreceptors shed in the subretinal space of the retina. As a consequence, the subretinal debris generates inflammation and autoimmune diseases and blocks the oxygen and nutrition supply to photoreceptor cells. These detrimental effects lead to rapid loss of vision due to photoreceptor degenerations.
2.5. Choroideremia
Choroideremia (CHM), an X-linked inheritance, was found in 1871 by Mauthner, and is the leading rare inheritance with a prevalence of 1 in 50,000 patients33. This disease causes a progressive degeneration of the choroid, retinal pigment epithelium, and retina that leads to loss of peripheral vision (night blindness) followed by loss of central vision in middle age. Female carriers have a rare progression of degeneration, but the males are affected by severe damage of RPE cells and choriocapillaries that develop night blindness during adolescence and complete loss of visual acuity by late adulthood. Choroideremia is caused by a mutation in the CHM gene that encodes ubiquitously expressed Rab-escort protein-1 (REP-1)33. REP-1 plays a key role in intracellular trafficking of vesicles to the cellular compartments. The mutation in the CHM gene remains null most of the time, and absence of this protein results in the severe effect of trafficking of the opsin protein to photoreceptor outer segment, migration of melanosome to the apical part of RPE cells, and phagocytosis activity of RPE cells.
2.6. Diabetic retinopathy (DR)
There are 93 million people suffering from diabetic retinopathy (DR) worldwide34. Diabetes can cause damage to the retinal blood vessels that feed the retina. The leaky blood and other fluids can cause thickening and swelling of the retina35. Fluid is accumulated by chronic high blood sugar levels, which cause blurred vision. Hyperglycemia is assumed to be the main reason for microvascular complications in DR, where generation of reactive oxygen species, vascular growth factor, and increase in vascular permeability play a vital role. Retinal vasculature plays a supporting role in the health of retinal neuronal and glial cells, and they are degenerated as the microvascular complications begin. DR can sometimes be controlled if the blood glucose level is stabilized, but not in all cases. For example, DR associated with gene alteration may not always be controlled by the long-term management of blood sugar by using insulin therapy. Currently, multidisciplinary strategies are being evaluated, such as laser photocoagulation, anti-vascular endothelial growth factor (anti-VEGF), and intravitreal steroid therapies. With the progression of DR, proliferative DR generates as the retinal blood vessels proliferate towards the retinal cells and vitreous. This leads to the generation of new blood vessels and results in vitreous hemorrhage, retinal detachments, and increase in the permeability of retinal blood vessels.
2.7. Age-related macular degeneration (AMD)
Symptoms of age-related macular degeneration generally present around age 60, and are caused by damage to the macula, according to a National Eye Institute (NEI, USA) report 15 million Americans and millions of people around the world are affected by this devastating retinal disorder. Macular degeneration does not affect the majority of patients until old age, and it is therefore difficult to study the sequential pattern in family members. There are two forms of AMD; one is “DRY” (non-nonvascular) and the other is “WET” (neovascular) AMD. The majority of AMD presents in dry form. Dry AMD is associated with the deposition of yellowish lipid proteins known as drusen under the retina that develop slowly and lead to gradual loss in central vision. Dry AMD can progress to geographic atrophy or the more devastating wet form. In wet AMD, an abnormal angiogenesis quickly leads to the choroidal neovascularization (CNV) within the retina and degenerates photoreceptor cells in the macula. This is responsible for 90% of AMD related blindness. There are some treatments available for wet AMD that involve inhibiting the growth of new blood vessels. Several delivery approaches of anti-angiogenic drugs like bevacizumab (trade name: Avastin) or ranibizumab (trade name: Lucentis) have been studied to inhibit vascular endothelial growth factor A (VEGF-A), which is responsible for the proliferation and growth of new blood vessels 36. There is no specific gene candidate established for AMD, and thus gene therapy remains an unpredictable therapeutic approach so far.
2.8. Glaucoma
Glaucoma is the second leading cause of blindness in the world. Overall, 9% to 12% of blindness is attributed to glaucoma. Damage to the optic nerve causes irreversible dystrophy in the eye, leading to blindness37. Increased intraocular pressure (IOP) is a key risk factor associated with this disease. However, this is not a guaranteed cause of glaucoma, as it is observed that 25% of glaucoma patients do not have elevation in their IOP37. In the anterior chamber, the aqueous humor forms by the ciliary body and is removed by the trabecular meshwork outflow pathways. IOP is based on the rate of removal of this aqueous humor from the interior chamber, which under normal conditions remains balanced with the rate of formation. The loss of retinal ganglion cells (RGCs) leads to damage in the ganglion cell axons, which finally degenerates the optic nerve. There are several therapeutic approaches developed so far to reduce the IOP by using drugs or surgery. The neuroprotection of these RGC cells is established as another well-studied therapeutic approach using different neuroprotective agents.
3. Ocular gene delivery
The success of retinal gene therapy primarily depends on the efficiency of the delivery vehicle to the targeted cells. The monogenic nature of retinal diseases is the basis for using the gene replacement therapeutic strategies. Two main approaches have been shown to be promising for the delivery of therapeutic genes to the targeted cells. One approach is viral vector, and the other is a non-viral synthetic cargo-based gene delivery vehicle.
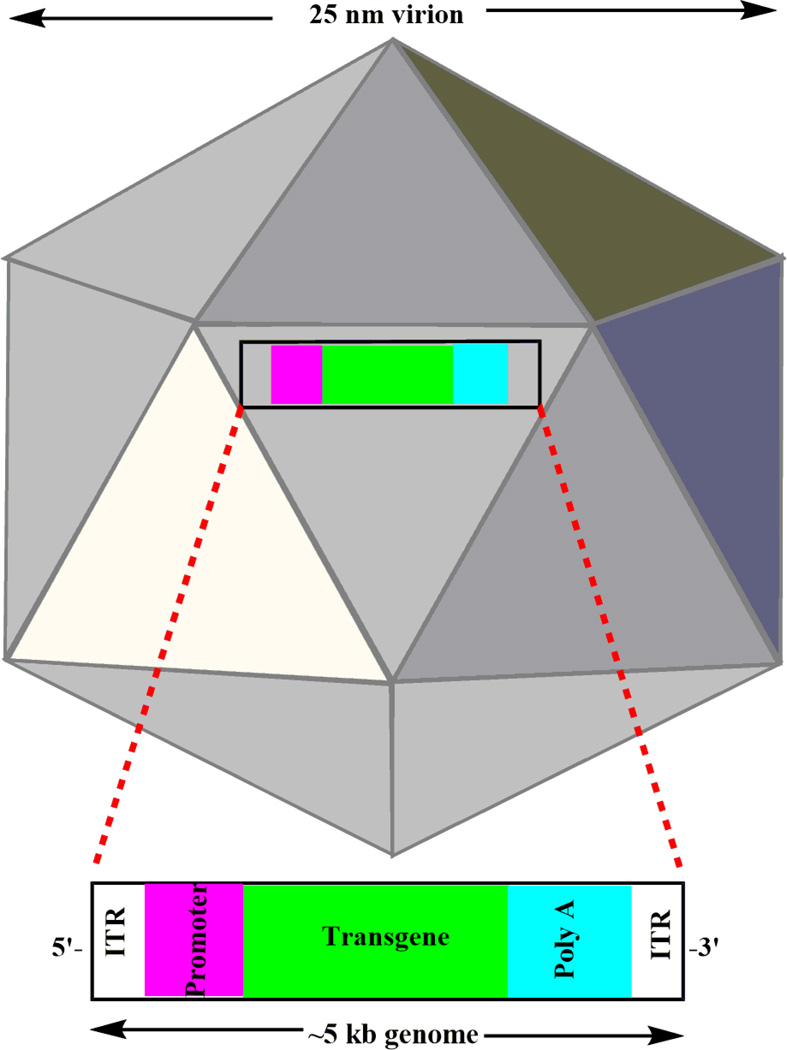
The targeting and delivery efficiency of the viral vectors depends on the vector serotype and nature of targeting tissues. Different types of viral vectors, including adenovirus, adeno-associated virus (AAV, a non-pathogenic), and lentivirus, were demonstrated to be efficient for the retinal tissues in in vivo animal models and in vitro tissue cultures. To date, viral vectors are among the most commonly used vectors for gene therapy. Among these, AAVs (Fig. 3) are currently the most frequently used viral vectors and AAV2 is the most widely used AAV serotype38. Significant progress has been made using viral vector for gene targeting. For example, in 2001, the successful viral gene replacement therapy using AAV was carried out in three Briard dogs with RPE65 mutation39. Delivery of the MYO7A gene (~9kb), packaged in AAV2 and AAV5, was injected into the subretinal space of the shaker 1 mice of a USH1 model40. By splitting into two separate AAV virions that contained splice donor and splice acceptor independently, they were able to efficiently express large MYO7A cDNA (~6.7 kb) in vitro and in vivo 40. The use of EIVA (equine infectious anemia virus) lentivirals mediated delivery of large wild type ABCR genes (~6.8kb) into the subretinal space of ABCR−/− mice, which increased the transduction efficiency of both rod and cone photoreceptor cells and decreased the toxic A2E levels in RPE cells41, 42, etc. However, the packaging capacities of AAV and lentivirus vectors are limited to ~5 and 9 kb, respectively. This size capacity restricts their delivery efficiency for large therapeutic genes. Therefore, this can also limit the delivery of large genes which might be composed of non-coding elements (e.g. intron) required for the improvement of in vivo gene expression43. In addition, the major drawbacks are their potential immunogenicity, carcinogenesis, broad tissue tropism, and genomic insertional mutagenesis that generate severe patient outcomes.
Fig. 3.
Representative 25 nm icosahedral capsid of AAV virion. The ~5 kb AAV genome is packaged within the non-enveloped capsid. A gene of interest is inserted between the ITRs under the control of promoter at upstream. ITR: Inverted Terminal Repeat.
Based on these disadvantages of viral vectors, synthetic non-viral gene delivery and replacement methods have been evaluated as promising gene delivery alternatives. This synthetic field has several important advantages, which include nonimmunogenecity, ease and low production costs, simplicity in manipulating molecular structure according to requirements, and most importantly, safety to the genome. Therefore, this area of research has been growing as an attractive and opportunistic field for the development of promising synthetic lipid based liposomes, polymers (linear and branched dendrimers and polysaccharides), and polypeptide based gene carriers. Next, we will review recent progress for using non-viral nanoparticles (NPs) to carry out gene targeting in ocular cells.
3.1 Lipid based liposomes vehicles
Lipid based liposomes are widely applied as non-viral gene delivery vectors. It was first discovered in the 1980s when phosphatidylserine phospholipid was utilized to compact and deliver SV40 DNA to the monkey kidney cells44. The constituent lipid molecules are composed of a cationic or neutral or zwitterionic head group, a hydrophobic tail group, and a linker group in between them. When these lipid molecules come into contact with negatively charged DNA, they form a complex (lipoplex, schematically presented in Fig. 5a) with the dimension of 100–300 nm. The shape and overall charge of the complex depends on the structure of the lipid and conditional adjustments. The overall charge of the lipoplex remains positive, which helps it to interact with the cell surface, followed by the fusion with cell membrane and internalization into the endosomal compartment45. To enhance the NP stability and transfection efficiency, a neutral/zwitterionic lipid such as fusogenic phospholipid DOPE (1, 2-dioleoyl-3-phosphatidylethanolamine) or membrane component cholesterol are introduced in the lipoplex formulation (Fig. 4a). DNA gets released into the cytosol generally via an endosomal escape pathway.
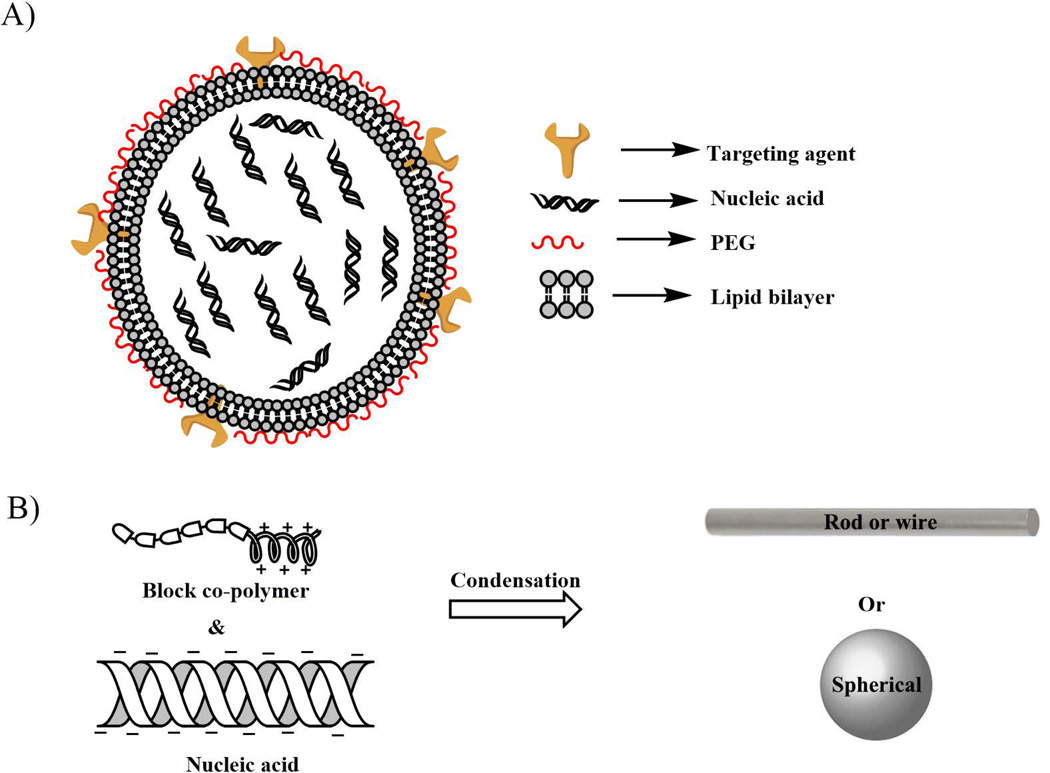
Fig. 5.
Simple representation of A) lipoplex, where lipid molecules can form bilayer structures and are thus able to encapsulate hydrophilic nucleic acids inside the nanoparticle core. The lipid coating can be used with different targeting agents. The polyethylene glycol (PEG) can also work as a shielding element to protect the nucleic acids from harsh extracellular and intracellular nuclease, as well as lysomal environments, and B) polyplex, where morphology of the nano-composites depend primarily on the chemical structure and charge of the constituent polymer compound (s). The negatively charged nucleic acids and positively charged polymers (via electrostatic interactions) constitute the compacted charge-neutral DNA nanoparticles. The PEG block shields the nanocompactions and protects it from nuclease and other degradative pathways.
Fig. 4.
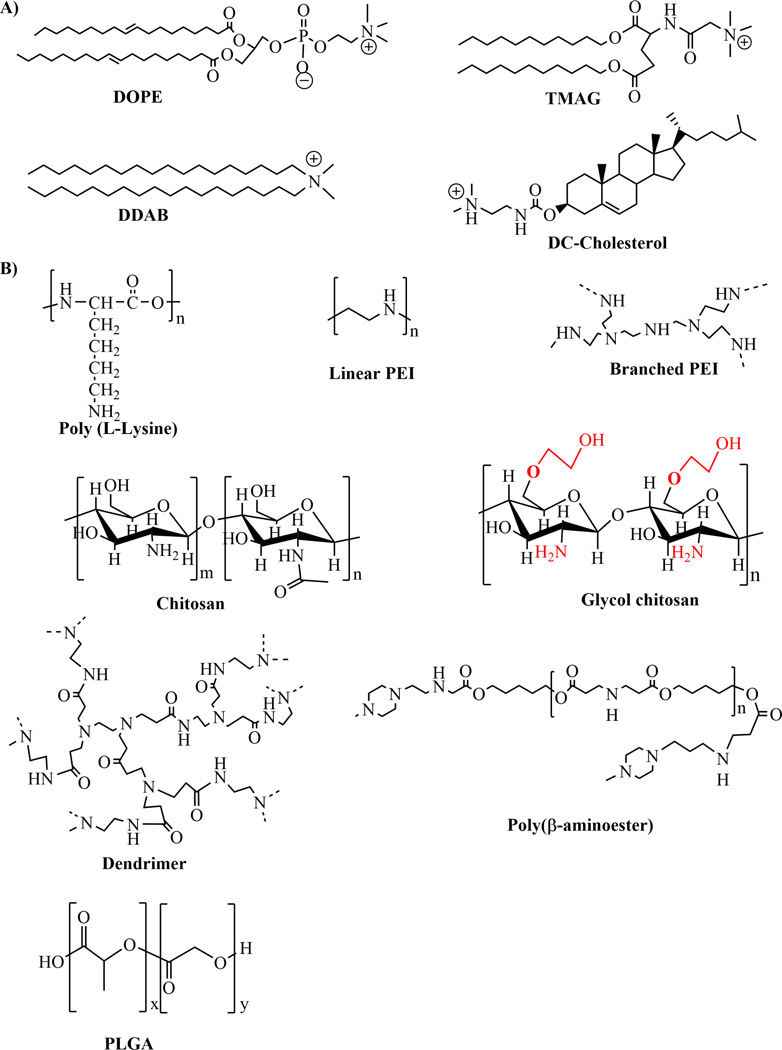
Chemical structures of commonly used non-viral compounds for ocular gene delivery. A) Chemical structures of lipid based compounds and B) chemical structures of some frequently used polymeric compounds.
For the first time in 1996, the beta galactosidase reporter gene, under the control of cytomegalovirus (CMV) promoter, was administered in the adult Wistar rats using three cationic liposomes via topical, anterior chamber, vitreous, and subretinal space routes46. The three liposomes were TMAG (N-(triethylamminoacetyl)-didodecyl-D-gluamate), DDAB (dimethyldioctadecylammonium bromide), and DC-Cholesterol (3-beta [N-(N’-N’-dimethylaminoethane)-carbamyl] cholesterol) (Fig. 4a). The gene was expressed in the RGCs on topical administration using TMAG and DC-cholesterol, but not by using DDAB up to 1 month. The TMAG liposome directed delivery of the gene and exhibited its highest level of expression among all three liposomes, irrespective of administrative route. The major problem associated with this technique was that the expression pattern was seen only in RGCs and RPE cells, whereas no expression was found in photoreceptor cells.
In 2005, Kachi and colleagues showed that the LacZ gene under the control of vitelliform macular dystrophy 2 (VMD2) promoter transduced to the RPE cells by using commercial 40% lipofectamine 2000 (Lf)47. Use of another lipid carrier, NeuroPorter, was tolerated well47. At two weeks, the scotopic a- and b- wave electroretinography (ERG) values were reduced by 40% and 8%, respectively, after subretinal injection of Lf in adult bulb/c mice. On the other hand, after 10% of NeuroPorter injection in the subretinal space, normal retinal morphology and functions were revealed for up to 14 days, and expressed in the RPE cells without any noticeable toxicity to retinal cells. Due to phagocytic activity of RPE cells, the transfer of reporter or therapeutic genes using lipid complex (lipoplex) is feasible (Fig. 5a), and may be a future therapeutic approach for RPE cell-related human ocular diseases.
More recently in 2014, Puras and colleague showed that a novel noisome composed of cationic lipid 2, 3-di (tetradecyloxy) propan-1-amine with polysorbate 80 additive, could deliver pCMS-EGFP plasmid DNA into the rat retina48. Injection into the subretinal space transduced both photoreceptor and RPE cells. Intravitreal injection expressed a uniform distribution of the reporter EGFP gene throughout the inner retina. Also in 2014, a novel strategy was discovered when liposome-proteamine decorated with cell-penetrating nuclear localizing signal peptides entrapped functional DNA and expression in the RPE cells49. So far, all of these ocular gene therapeutic approaches are limited to proof-of-principle steps and in the near future we can expect more novel lipid based formulations for human use.
3.2 Polymer based vehicles
A cationic polymer combines with negatively charged anionic DNA and forms a polyplex (Fig. 4b) of different surface charges45. The well-known polymers used for ocular gene delivery are composed of biopolymers (proteins), dendrimers, polysaccharides, polylysin, polyethyleneimines and small organic biocompatible lactic acid (e.g. PLA) and/or glutamic acid (e.g. poly (lactic-co-glycolic acid) molecules (Fig. 4b). In 2013, Puras and colleagues evaluated the gene delivery efficiency of low molecular weight oligochitosan (Fig. 4b) and pCMS-EGFP polyplex in vitro and in vivo50. This polyplex protected plasmid DNA from nuclease degradation and transfected well to the ARPE 19 cells at pH 7.1. Subretinal injection expressed EGFP to the RPE cells, while intravitreal injections exhibited transfection to the inner nuclear layer, plexiform layer, and primarily to RGCs in rat retina.
In 2014, Mitra and colleagues synthesized and characterized an ethylene glycol modified chitosan (glycol chitosan, GCS, Fig. 4b) and pCBA-EGFP polyplex that protected plasmid DNA from nuclease, which expressed reporter EGFP gene in the RPE cells when injected into the subretinal space of adult Balb/c mice without affecting the morphology and function (using ERG) of retinal cells51. In 2012, Klausner and colleagues evaluated the enhancement of transgene expression using chitosan DNA nanoformulation on topical administration on the rat cornea52. At 1 day post injection of chitosan DNA complex, it was observed that expression of CpG free pCpG-Luc plasmid DNA enhanced by 7.1, 116.8, and 76.8 folds, compared to commercially available gWiz-CMV-Luc, pPEI-CMV, and pPEI-UbC plasmid DNAs respectively, and demonstrated the development of effective vectors for corneal gene therapy52. Another biopolymer gelatin has also been investigated to deliver and significantly express mucin MUC5AC (responsible for dry eye syndrome) transgene into the cornea and conjunctiva in vivo53. In 2012, Delgado and colleagues reported the ocular gene therapy using solid lipid NPs (SLN) composed of biocompatible dextran, protamine, and a plasmid DNA (pCMS-EGFP or pCEP4-RS1)54. Dextran is a neutral nonionic polysaccharide and combines with cationic polymer to entrap, stabilize, and protect negatively charged DNA. On topical administration of SLN to the rat eye, reporter EGFP gene was expressed in the cornea and provided a strategic opportunity to deal with various ocular surface diseases54.
In 2014, Alqawlaq and colleagues demonstrated the localization of Cy5 labelled pCMV-GFP plasmid DNA into the nerve fiber layer of the retina by intravitreal administration using Gemini surfactant, whereas the same happened to anterior chamber tissues including limbus, iris, and conjunctiva on topical administration to the 4 week old C57BL/6 mice55. Intravitreal route of injection is promising, as this reduces the induction of photoreceptor cell degeneration seen during subretinal injections. However, this route possesses huge viscosity, and thus provides resistance to the mobility of cationic polyplexes (Fig. 4b). Therefore, when the polyplexes are strategically coated with the hyaluronic acid (HA, component of vitreous matrix), they are less aggregated and able to flow more easily through the vitreous space. Most recently in 2015, Martens and colleagues have demonstrated that in an ex vivo experiment where cationic N, N′-cystaminebisacrylamide-4-aminobutanol (p (CBA-ABOL) vector was coated with the HA, there was a significant amount of enhancement in the gene expression in the retina via intravitreal route of administration 56.
Albumin, a widely used protein carrier, is retained with a high percentage (60–70%) among all the proteins in the vitreous compartment 57. Therefore, this biocompatible and biodegradable component is safe to use for in vivo ocular gene therapy. In 2007, Mo and colleagues found enhanced Cu, Zn superoxide dismutase (SOD1) gene expression by 5 fold, compared to the untreated control via intravitreal injection in the mouse eye when entrapped in human serum albumin NP (HSA NP)58. In another separate study in 2009, Kim and colleagues evaluated the potential of anionic HSA NP as a promising gene delivery tool for RPE cells via the subretinal space59. A category of highly branched nanodimension molecules made of central core, interior branches, and exterior surface functional groups, known as dendrimers, were also evaluated as potential gene delivery vehicles for in vitro and in vivo models. When dendrimer is composed of a high number of surface amine functional groups, it condenses negatively charged DNA and protects it from external nuclease degradation. Polyethylenimine (PEI) and polyamidoamine (PAMAM) are commonly used dendrimers for retinal gene delivery applications due to their “proton sponge” mediated endosomal escape attitude. Oligonucleotide polyethylenimin (ODN/PEI) complex has been observed to be efficient in transfecting retinal glial cells at 72 hours after intravitreal injection in normal rat eyes without any detectable toxicity 60. In another attempt, PEI condensed shRNA plasmid DNA to target melanopsin in Balb/c mice, and was able to knock down melanopsin in RGC cells at 16 hrs of intravitreal injection, which lasted for 2 months61. In 2015, Mastorakos and colleagues showed the combined effect of hydroxyl-terminated PAMAM and triamcinolone acetonide (TA) in enhancing transfection of dendrimer-gene complex into the most challenging human RPE cells in vitro62. In 2012, Sunshine and colleagues synthesized a novel poly (β-amino ester, Fig. 4b), which exhibited the expression of reporter GFP gene into the RPE and choroid at post injection of 72 hrs via subretinal delivery63. In 2004, Marano and colleagues evaluated for the first time the intravitreal delivery of anti-vascular endothelial growth factor (VEGF) oligonucleotide (ODN-1) into the rat eyes using the lipophilic amino-acid dendrimer, which significantly inhibited laser-induced choroidal neovascularization (CNV) for 4–6 months by 95% in the initial stage of CNV development, thereby paving the way for the treatment of angiogenic eye disorders 64.
Polycationic compounds (Fig. 4b) are generally limited by their high positive charge and significant toxicities. These toxicities are strategically eliminated by encapsulating them in conjunction with DNA into the neutrally charged (at physiological pH) biocompatible and biodegradable poly (lactic-co-glycolic acid) (PLGA) molecules. PLGA has been widely used for drug delivery and approved by both the US Food and Drug Administration (FDA) and the European Medicine Agency. In 2010, Zhang and colleagues developed hypoxia-inducible factor 1α (HIF-1α) shRNA and GFP co-expressed plasmid DNA-loaded PLGA NPs (pshHIF-1α NPs) 65. The result showed that the intravitreal injection of these NPs reduced the mean area of CNV in the rat laser photocoagulation model without any deleterious effects on the retina. Another study by Bejjani and colleagues showed the expression of reporter RNFP (red nuclear fluorescence) in RPE cells up to 14 days post intraocular injection without any apparent structural damage or toxicity66.
3.3 Polypeptide based vehicles
In the pipeline of developing gene delivery vehicles, polypeptide based systems are evaluated as a promising tool for ocular gene delivery. A novel cell penetrating peptide, peptide for ocular delivery (POD, CGGG(ARKKAAKA)4), was able to transduce GFP protein under the control of CMV promoter, which was expressed into the RPE and photoreceptor cells via subretinal injection in C57BL6/J mice67. On intravitreal injection, the POD compacted GFP plasmid DNA (POD-GFP) transduced retinal ganglion cells, with limited expression in the inner nuclear layers and lens capsule68. The topical administration of POD-GFP expressed GFP protein in the corneal epithelium cells. In 2014, Read and colleagues demonstrated that the pegylation of POD peptide was able to increase pCAGLuc expression by 215-fold, compared to naked plasmid DNA in the RPE cells by subretinal injection in Balb/c mice69. These PEG-POD NPs were also able to protect photoreceptor cells by delivering glial cell line-derived neurotrophic factor (GDNF) into the subretinal space of the blue light-induced photoreceptor apoptosis adult murine model at the 14th day post light treatment70. Based on polylysin and DNA compaction strategy, a novel cationic CK30-PEG polymer71 was developed to compact plasmid DNA to form an NP formulation (Fig. 5b) that could transfect therapeutic genes to the retinal cells in different mice models. The shape of these NPs determines the targets in retinal tissues71. The rod shaped NPs transfect the RPE and photoreceptor cells, while ellipsoidal NPs transfect only RPE cells in vivo. The major advantage of these NPs is the compaction efficiency of plasmid DNA with long molecular range (from 5.3 kb to 20.2Kb), while in all cases the charge of the NPs remains neutral, minimizing toxicity72. Subretinal injection of these NPs didn’t leave the eye, compared to the AAV mediated ocular gene delivery approach. No apparent toxicity to the retinal cells was seen, which makes this NP a promising alternative as a non-viral gene delivery vehicle to ocular tissues72. The CK30-PEG compacted GFP reporter gene (driven by CMV promoter) was able to highly express to RPE and photoreceptor cells by subretinal injection, while intravitreal route of injection exhibited GFP expression mainly in ganglion cells along with less amounts in the cornea, trabecular meshwork, and lens. The CK30-PEG compacted NPs highly expressed human RPE65 gene driven by VMD2 promoter (RPE cell specific) in the RPE cells of RPE65−/− mice model of LCA disease, with long term phenotypic rescue (up to 2 years)73, 74. Human photoreceptor cell specific ATP-binding cassette transporter gene (ABCA4) gene was expressed by subretinal injection of this compacted NP (by CK30-PEG polypeptide), under the control of IRBP promoter (photoreceptor cells specific), into the retina of the ABCA4−/−- knockout mice model of Stargardt’s disease, with a persistent expression of up to 8 months post injection75. A significant amount of retinal degeneration slow (RDS) gene expression with partial rescue of photoreceptor cell function was observed when compacted with CK30-PEG and delivered into the subretinal space of Rds+/− mice 76. This CK30-PEG has not shown any apparent toxicity towards retinal cells, even with a second set of subretinal injection of NPs77, and can drive gene expression levels on the same scale and duration as AAV78. These NPs have so far been evaluated as a promising ocular gene delivery vehicle, and can be the potential tool for ocular gene delivery with some modifications in their chemical structures to target primary and secondary retinal neuronal cells.
Conclusion
Despite this promising non-viral delivery vehicle development for ocular tissues, achievements primarily have been limited to transfection efficiency into RPE cells, but not towards photoreceptor and other neuronal tissues in the eye, which is the origin of major retinal disorders. Polypeptide-based vehicles have demonstrated some success, but rational chemical modifications of these compounds might develop smart gene delivery cargos as a promising alternative to viral vectors and are very appealing for human ocular gene therapy in the near future.
Acknowledgements
The authors thank Sandy Janowski Barnhart, MPH (Department of Ophthalmology, University of North Carolina at Chapel Hill) for her critical reading of the manuscript. This work was supported by the U.S. National Institutes of Health, National Eye Institute (R21 EY024059-ZH), the Oklahoma Center for the Advancement of Science and Technology (ZH), the Knights Templar Eye Foundation (ZH), the Carolina Center of Cancer Nanotechnology Excellence and the North Carolina Translational and Clinical Sciences (ZH), Fight for Sight (RNM), and the Research to Prevent Blindness to the University of North Carolina Department of Ophthalmology.
Footnotes
The authors declare no competing financial interests.
References
- 1.Solinis MA, Del Pozo-Rodriguez A, Apaolaza PS, Rodriguez-Gascon A. Treatment of ocular disorders by gene therapy. Eur J Pharm Biopharm. 2014 doi: 10.1016/j.ejpb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Schon C, Biel M, Michalakis S. Retinal gene delivery by adeno-associated virus (AAV) vectors: Strategies and applications. Eur J Pharm Biopharm. 2015 doi: 10.1016/j.ejpb.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Lipinski DM, Thake M, MacLaren RE. Clinical applications of retinal gene therapy. Prog Retin Eye Res. 2013;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Charbel Issa P, MacLaren RE. Non-viral retinal gene therapy: a review. Clin Experiment Ophthalmol. 2012;40:39–47. doi: 10.1111/j.1442-9071.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- 5.Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm. 2015 doi: 10.1016/j.ejpb.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–1197. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- 8.Smith AJ, Bainbridge JW, Ali RR. Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther. 2012;19:154–161. doi: 10.1038/gt.2011.161. [DOI] [PubMed] [Google Scholar]
- 9.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12:238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leveillard T, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010;2:26ps16. doi: 10.1126/scitranslmed.3000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahim AT, Daiger SP, Weleber RG. Retinitis Pigmentosa Overview. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 13.Sung CH, Davenport CM, Nathans J. Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa. Clustering of functional classes along the polypeptide chain. J Biol Chem. 1993;268:26645–26649. [PubMed] [Google Scholar]
- 14.Kaushal S, Khorana HG. Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochemistry. 1994;33:6121–6128. doi: 10.1021/bi00186a011. [DOI] [PubMed] [Google Scholar]
- 15.Maubaret C, Kosmaoglou M, Low S, Chakarova CF, Bidot S, Thauvin-Robinet C, Robson AG, Waseem N, Cheetham ME, Bhattacharya SS. Functional characterization of a novel c.614-622del rhodopsin mutation in a French pedigree with retinitis pigmentosa. Mol Vis. 2012;18:581–587. [PMC free article] [PubMed] [Google Scholar]
- 16.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 17.Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36:595–603. doi: 10.1016/0021-9681(83)90147-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Li P, Liu Y, Li W, Wong F, Du R, Wang L, Li C, Jiang F, Tang Z, et al. Novel compound heterozygous mutations in MYO7A in a Chinese family with Usher syndrome type 1. Mol Vis. 2013;19:695–701. [PMC free article] [PubMed] [Google Scholar]
- 19.Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Petit C. Usher syndrome: from genetics to pathogenesis. Annu Rev Genomics Hum Genet. 2001;2:271–297. doi: 10.1146/annurev.genom.2.1.271. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs D, Diemer T, Khanobdee K, Hu J, Bok D, Williams DS. Function of MYO7A in the human RPE and the validity of shaker1 mice as a model for Usher syndrome 1B. Invest Ophthalmol Vis Sci. 2010;51:1130–1135. doi: 10.1167/iovs.09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 23.Yatsenko AN, Shroyer NF, Lewis RA, Lupski JR. An ABCA4 genomic deletion in patients with Stargardt disease. Hum Mutat. 2003;21:636–644. doi: 10.1002/humu.10219. [DOI] [PubMed] [Google Scholar]
- 24.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 25.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan AO, Al-Mesfer S, Al-Turkmani S, Bergmann C, Bolz HJ. Genetic analysis of strictly defined Leber congenital amaurosis with (and without) neurodevelopmental delay. Br J Ophthalmol. 2014;98:1724–1728. doi: 10.1136/bjophthalmol-2014-305122. [DOI] [PubMed] [Google Scholar]
- 27.Coppieters F, De Wilde B, Lefever S, De Meester E, De Rocker N, Van Cauwenbergh C, Pattyn F, Meire F, Leroy BP, Hellemans J, et al. Massively parallel sequencing for early molecular diagnosis in Leber congenital amaurosis. Genet Med. 2012;14:576–585. doi: 10.1038/gim.2011.51. [DOI] [PubMed] [Google Scholar]
- 28.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 30.Milam AH, Barakat MR, Gupta N, Rose L, Aleman TS, Pianta MJ, Cideciyan AV, Sheffield VC, Stone EM, Jacobson SG. Clinicopathologic effects of mutant GUCY2D in Leber congenital amaurosis. Ophthalmology. 2003;110:549–558. doi: 10.1016/S0161-6420(02)01757-8. [DOI] [PubMed] [Google Scholar]
- 31.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan AC, Winges A, DeRyckere D, Carson CC, Trembath DG, Tentler JJ, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123:2257–2267. doi: 10.1172/JCI67816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JW, Thrasher AJ, Smith AJ, Ali RR. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald IM, Smaoui N, Seabra MC. Choroideremia. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 34.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 36.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 37.Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Curr Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 40.Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods. 2014;25:166–177. doi: 10.1089/hgtb.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di Vicino U, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Z, Banworth MJ, Makkia R, Conley SM, Al-Ubaidi MR, Cooper MJ, Naash MI. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB J. 2015 doi: 10.1096/fj.15-270363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraley R, Subramani S, Berg P, Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980;255:10431–10435. [PubMed] [Google Scholar]
- 45.Elouahabi A, Ruysschaert JM. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Masuda I, Matsuo T, Yasuda T, Matsuo N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci. 1996;37:1914–1920. [PubMed] [Google Scholar]
- 47.Kachi S, Oshima Y, Esumi N, Kachi M, Rogers B, Zack DJ, Campochiaro PA. Nonviral ocular gene transfer. Gene Ther. 2005;12:843–851. doi: 10.1038/sj.gt.3302475. [DOI] [PubMed] [Google Scholar]
- 48.Puras G, Mashal M, Zarate J, Agirre M, Ojeda E, Grijalvo S, Eritja R, Diaz-Tahoces A, Martinez Navarrete G, Aviles-Trigueros M, et al. A novel cationic niosome formulation for gene delivery to the retina. J Control Release. 2014;174:27–36. doi: 10.1016/j.jconrel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Liu HA, Liu YL, Ma ZZ, Wang JC, Zhang Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Invest Ophthalmol Vis Sci. 2011;52:4789–4794. doi: 10.1167/iovs.10-5891. [DOI] [PubMed] [Google Scholar]
- 50.Puras G, Zarate J, Diaz-Tahoces A, Aviles-Trigueros M, Fernandez E, Pedraz JL. Oligochitosan polyplexes as carriers for retinal gene delivery. Eur J Pharm Sci. 2013;48:323–331. doi: 10.1016/j.ejps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Mitra RN, Han Z, Merwin M, Al Taai M, Conley SM, Naash MI. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. ChemMedChem. 2014;9:189–196. doi: 10.1002/cmdc.201300371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klausner EA, Zhang Z, Wong SP, Chapman RL, Volin MV, Harbottle RP. Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J Gene Med. 2012;14:100–108. doi: 10.1002/jgm.1634. [DOI] [PubMed] [Google Scholar]
- 53.Zorzi GK, Contreras-Ruiz L, Parraga JE, Lopez-Garcia A, Bello RR, Diebold Y, Seijo B, Sanchez A. Expression of MUC5AC in Ocular Surface Epithelial Cells Using Cationized Gelatin Nanoparticles. Molecular Pharmaceutics. 2011;8:1783–1788. doi: 10.1021/mp200155t. [DOI] [PubMed] [Google Scholar]
- 54.Delgado D, del Pozo-Rodriguez A, Solinis MA, Aviles-Triqueros M, Weber BHF, Fernandez E, Gascon AR. Dextran and Protamine-Based Solid Lipid Nanoparticles as Potential Vectors for the Treatment of X-Linked Juvenile Retinoschisis. Human Gene Therapy. 2012;23:345–355. doi: 10.1089/hum.2011.115. [DOI] [PubMed] [Google Scholar]
- 55.Alqawlaq S, Sivak JM, Huzil JT, Ivanova MV, Flanagan JG, Beazely MA, Foldvari M. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: towards non-invasive glaucoma gene therapy. Nanomedicine. 2014;10:1637–1647. doi: 10.1016/j.nano.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Martens TF, Remaut K, Deschout H, Engbersen JF, Hennink WE, van Steenbergen MJ, Demeester J, De Smedt SC, Braeckmans K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J Control Release. 2015;202C:83–92. doi: 10.1016/j.jconrel.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 57.Ulrich JN, Spannagl M, Kampik A, Gandorfer A. Components of the fibrinolytic system in the vitreous body in patients with vitreoretinal disorders. Clin Experiment Ophthalmol. 2008;36:431–436. [PubMed] [Google Scholar]
- 58.Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm Res. 2009;26:329–337. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 60.Gomes dos Santos AL, Bochot A, Tsapis N, Artzner F, Bejjani RA, Thillaye-Goldenberg B, de Kozak Y, Fattal E, Behar-Cohen F. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006;23:770–781. doi: 10.1007/s11095-006-9748-0. [DOI] [PubMed] [Google Scholar]
- 61.Liao HW, Yau KW. In vivo gene delivery in the retina using polyethylenimine. Biotechniques. 2007;42 doi: 10.2144/000112404. 285-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastorakos P, Kambhampati SP, Mishra MK, Wu T, Song E, Hanes J, Kannan RM. Hydroxyl PAMAM dendrimer-based gene vectors for transgene delivery to human retinal pigment epithelial cells. Nanoscale. 2015;7:3845–3856. doi: 10.1039/c4nr04284k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sunshine JC, Sunshine SB, Bhutto I, Handa JT, Green JJ. Poly(beta-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS One. 2012;7:e37543. doi: 10.1371/journal.pone.0037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marano RJ, Wimmer N, Kearns PS, Thomas BG, Toth I, Brankov M, Rakoczy PE. Inhibition of in vitro VEGF expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Exp Eye Res. 2004;79:525–535. doi: 10.1016/j.exer.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Wang YS, Wu H, Zhang ZX, Cai Y, Hou HY, Zhao W, Yang XM, Ma JX. Inhibitory efficacy of hypoxia-inducible factor 1alpha short hairpin RNA plasmid DNA-loaded poly (D, L-lactide-co-glycolide) nanoparticles on choroidal neovascularization in a laser-induced rat model. Gene Ther. 2010;17:338–351. doi: 10.1038/gt.2009.158. [DOI] [PubMed] [Google Scholar]
- 66.Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, Gollomb G, Behar-Cohen FF. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- 67.Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Read SP, Cashman SM, Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med. 2010;12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol Ther. 2010;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Z, Conley SM, Naash MI. AAV and compacted DNA nanoparticles for the treatment of retinal disorders: challenges and future prospects. Invest Ophthalmol Vis Sci. 2011;52:3051–3059. doi: 10.1167/iovs.10-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koirala A, Makkia RS, Conley SM, Cooper MJ, Naash MI. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum Mol Genet. 2013;22:1632–1642. doi: 10.1093/hmg/ddt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koirala A, Conley SM, Makkia R, Liu Z, Cooper MJ, Sparrow JR, Naash MI. Persistence of non-viral vector mediated RPE65 expression: case for viability as a gene transfer therapy for RPE-based diseases. J Control Release. 2013;172:745–752. doi: 10.1016/j.jconrel.2013.08.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest. 2012;122:3221–3226. doi: 10.1172/JCI64833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Z, Koirala A, Makkia R, Cooper MJ, Naash MI. Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: expression, repeat delivery and lack of toxicity. Nanomedicine (Lond) 2012;7:521–539. doi: 10.2217/nnm.11.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI. Comparative Analysis of DNA Nanoparticles and AAVs for Ocular Gene Delivery. PLoS One. 2012;7:e52189. doi: 10.1371/journal.pone.0052189. [DOI] [PMC free article] [PubMed] [Google Scholar]