Abstract
The therapeutic approach to congenital diaphragmatic hernia (CDH) has shifted from one of immediate repair to management of pulmonary hypertension, physiologic stabilization, and delayed surgical repair. Lung hypoplasia, remodeled pulmonary vasculature, and ventricular dysfunction all contribute to the high morbidity and mortality associated with CDH. In addition, genetic syndromes associated with CDH can increase the incidence of serious anomalies and hence impact survival. Prenatal and postnatal management of infants with CDH is challenging in the best of circumstances and need multidisciplinary teams for optimal outcomes. However, advances using ultrasound and fetal MRI can predict prognosis and survival and plan for postnatal management. Survival rates for patients with CDH have increased for the past decade with better management at resuscitation; implementation of gentle ventilation strategies; and medical management of pulmonary hypertension, physiologic stabilization, and extracorporeal membrane oxygenation. However, follow-up of these infants for long-term morbidities is essential for optimal outcomes after discharge.
Keywords: CDH, Pulmonary hypertension, Lung hypoplasia, ECMO, Neonate
Congenital diaphragmatic hernia (CDH) is a developmental defect in the diaphragm which occurs in approximately 1 in 3000 live births [1]. Although advances in medical and surgical management have improved outlook, survival of CDH infants remains at 60–70 % [1]. Lung hypoplasia and pulmonary hypertension are key factors which contribute to the morbidity and mortality associated with CDH.
Etiopathogenesis of CDH
CDH is a complex developmental defect that is etiologically heterogeneous (multifactorial), and in a majority of cases (~80 %), the cause is unknown [2]. Most cases are isolated; however, numerous genetic syndromes are associated with CDH [2]. The most co-occurring defect is congenital heart defect which is present in ~20 % of patients. Among the syndromes associated with CDH include Cornelia de Lange, Pallister-Killian, and Marfan syndrome. Even though no specific environmental factor has been clearly associated with CDH, the involvement of retinol signaling pathway in the development of the diaphragm has been described [3]. In case-control studies, infants with CDH had low levels of retinol and retinol-binding protein compared to controls [4, 5], suggesting abnormal retinoid homeostasis may contribute to the development of CDH.
The diaphragm is a complex structure made up of the septum transversum, pleuro-peritoneal folds, esophageal mesentery, and musculature. Failure of the diaphragm to fully develop during embryogenesis or weakening of one its parts or failure to connect with one another could predispose to the development of CDH. This could be precipitated by the premature return of the midgut into the abdominal cavity with the resultant increase in intra-abdominal pressure, disrupting diaphragmatic development. Whatever the cause results in a defect in the diaphragm, commonly on the left side (85–90 %), but can occur on the right or bilaterally and may be associated with other anomalies. The commonest (90 %) defect involves the posterolateral region of the diaphragm (Bochdalek hernia) but the anterior (Morgagni hernia) or central regions can also be affected [1].
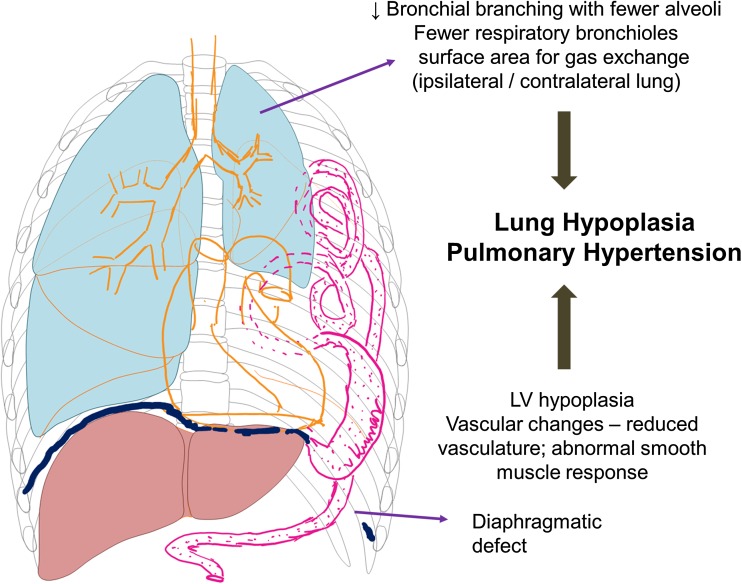
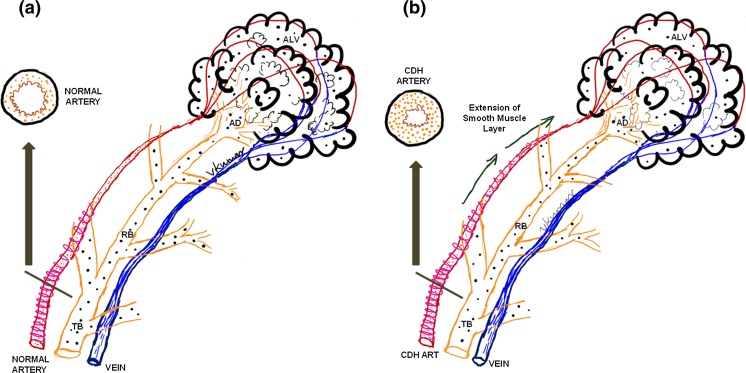
The pathology of CDH comprise of three elements: the diaphragmatic hernia, pulmonary hypoplasia, and herniation of the abdominal organs into the thorax (Fig. 1). Lung hypoplasia and arrested lung development are the essential features of CDH, manifesting histologically as fewer alveoli and decreased surface area for gas exchange. Arrested alveolar development is more conspicuous on the ipsilateral side, although it is seen on the contralateral side. This differential pathophysiology in the lung is thought to be due to a dual-hit hypothesis [6], with the initial insult occurring before the development of the diaphragm at 8–9 weeks from a genetic or environmental cause and the later insult from the physical compression of the lung by the abdominal contents in the thorax. Terminal bronchioles are decreased and alveolar septa are thickened with decreased complexity of respiratory acinus resulting in decreased surface area for gas exchange. Vascular changes occurs secondary to epithelial-dependent vascular development resulting in reduced vasculature, abnormal vascular smooth muscle response, and vascular remodeling. The pulmonary arteries are hypertrophied with medial and adventitial thickening with extension of smooth muscle cells distally beyond the terminal bronchioles (Fig. 2). The critical determinant factor is the magnitude of lung vasculature that is “responsive” (dynamic or reactive vasculature) to therapy compared to non-responsive vasculature (rigid vasculature), as this may impact both morbidity and survival of infants with CDH. This can be complicated in severe cases by left ventricular hypoplasia [7] secondary to transcriptional and cardiovascular responses to changes in the lung and also secondary to the physical presence of hernia in the thorax during fetal development.
Fig. 1.
Schematic diagram of congenital diaphragmatic hernia with lung hypoplasia, left ventricular hypoplasia, and abdominal contents in the left thorax due to the diaphragmatic defect in the left side. The pathology of lung hypoplasia is described and is suspected to be secondary to “dual-hit hypothesis” (see text for details). (Copyright: Vasanth HS Kumar)
Fig. 2.
Anatomy of pulmonary vasculature in normal (a) and CDH (b) infants. Arterioles in the pulmonary vasculature have smooth muscles in the medial layer; however, the lumen is of fairly decent size and the vascular smooth muscles do not extend beyond the terminal bronchioles in control infants (a). Vascular remodeling in CDH infants (b) results in two significant changes—the vascular smooth muscle layer is hypertrophied (on cross-section) and it extends into the vasculature beyond the terminal bronchioles into the respiratory bronchioles towards the alveolar duct. TB terminal bronchiole, RB respiratory bronchiole, AD alveolar duct, ALV alveolus. (Copyright: Vasanth HS Kumar)
Prenatal Imaging
Approximately two of three patients with CDH will be diagnosed on prenatal ultrasound (US). Ultrasound is the standard prenatal imaging modality for detection of congenital anomalies including CDH. However, the diagnosis is particularly difficult before 24 weeks of gestation (22–52 % of the cases), and even after 24 weeks of gestation, the prenatal diagnosis is a challenge, with more than 25 % of cases missed and 11 % diagnosed postnatally [8]. The diagnosis of CDH is usually suspected by the absence of stomach in the normal intra-abdominal location; intrathoracic mass containing the liver, bowel, or stomach; or by indirect evidence such as abnormal cardiac axis, mediastinal shift, or polyhydramnios. Despite the widespread use of US, the role of fetal MRI is rapidly expanding. MRI is useful to confirm the diagnosis in cases of equivocal US findings; however, both US and MRI can be used in the assessment of prognosis in CDH.
The presence of liver herniation into the chest (liver up) is considered to be a poor prognostic indicator. In a recent systematic review which included 407 fetuses with liver up and 303 with the liver down infants, statistically worse survival was found in liver up infants (45.4 %) compared with liver down infants (73.9 %) [9]. Also, fetuses with the liver up required extracorporeal membrane oxygenation (ECMO) in 80 %, compared with 25 % for those with liver down, and overall survival rate was 45 %, compared with 93 % for those with liver down [10].
As lung hypoplasia is one of the poor prognostic markers, studies have tried to quantify this in fetuses with CDH. Lung hypoplasia is seen in both the lungs; however the ipsilateral lung is more affected than the contralateral lung. Sonographic measurement of contralateral lung area-to-head ratio (LHR) has been used to assess lung volume and hence mortality. There were no survivors with an LHR <0.6, whereas survival was 100 % if the LHR was >1.35 [11]. In another study, low lung-to-head ratio (<1.0) predicted increased incidence of extracorporeal membrane oxygenation (75 %) and lower survival (35 %) [10]. However, when measured at <24 weeks of gestation, LHR was not predictive of outcome. As LHR is a variable across gestational age, this can be corrected by measuring expected LHR-to-observed LHR (O/E LHR) ratio, and several studies have used this to predict mortality in fetuses with CDH. Liver position is the best prenatal predictor of outcome in isolated left congenital diaphragmatic hernia. Lung-to-head ratio alone should not be used to counsel families regarding mid gestational management choices [10].
Other parameters that can be used in predicting the severity of lung disease include measuring fetal lung volume (FLV) at 34 week gestation on fetal MRI. Again, this can be assessed as observed to expected FLV based on normograms [12]. If fetal lung volumes are >20–25 ml and in the absence of bad prognostic indicator such as liver up, spontaneous delivery should be the mode of treatment [12]. Infants who survived had higher FLV compared to those who died (20.7 ± 9.3 vs 8.9 ± 4.0 ml), and similarly, infants who underwent ECMO had lower fetal lung volumes compared to infants who did not undergo the procedure (14.3 ± 7.5 vs 20.4 ± 10.2 ml) [13].
Fetal lung-to-thorax ratio and cardiac axis have been used to predict survival in addition to the above methods. However, liver position is thought to have a greater value in predicting survival. However, in some cases of fetal CDH with worse outcomes (intrathoracic herniation of liver with LHR ≤1.0), antenatal therapy has been considered as an option to improve lung development. The principle of tracheal occlusion is to improve lung development and this can be achieved by fetal endoscopic tracheal occlusion (FETO). This is a highly specialized procedure done at around 28 weeks of gestation, wherein the trachea is plugged with a balloon for about 6 weeks to increase lung volume and then unplugged prior to delivery [14]. Currently this is being evaluated in a multicenter trial, and initial reports suggest that temporary FETO in fetuses with left CDH increased survival from 24 to 49 % (left-sided) and from 0 to 35 % in right-sided CDH [14]. However, this was associated with a higher incidence of PPROM and preterm birth despite a substantial improvement in survival [14]. If there is risk of preterm delivery ≤34 week gestation, antenatal steroids should be given according to NIH guidelines [15]. Prenatal glucocorticoids have been shown to decrease hypertrophy of pulmonary arterioles in combination with prolonged tracheal occlusion in a lamb model of severe CDH [16].
It is essential to make an early diagnosis of CDH, so that a thorough evaluation is done to look for associated anomalies, which are present in 40–60 % of cases with CDH. The associated anomalies are widespread including cardiac, gastrointestinal, renal, and central nervous system. The need for amniocentesis for chromosomal defects and the type of cardiac defects will impact the outcome in individual cases. The presence of hydrops fetalis is also a significant factor in determining cardiac dysfunction and poor prognosis. As the management of antenatal CDH can be complicated, this involves management by a multidisciplinary team including genetics evaluation and antenatal counselling. In those cases with poor prognosis and with other congenital anomalies, termination of pregnancy should be sensitively considered. Expectant management and regular ultrasounds for fetal growth and follow-up should be done in most cases. Delivery should be planned in a tertiary care center whenever possible and obstetric decisions should guide the mode of delivery.
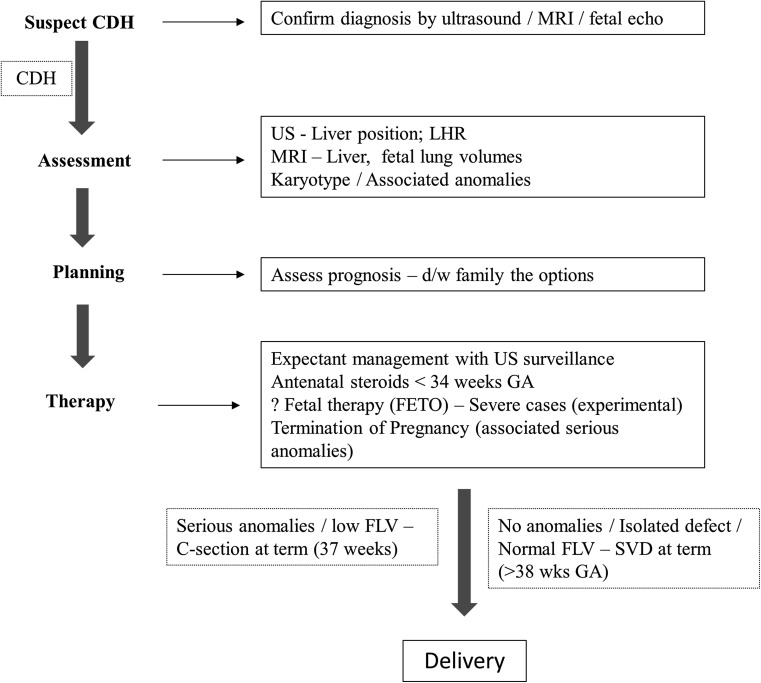
The timing and the mode of delivery for CDH patients is not clear. Among term infants with CDH receiving ECMO, ELSO registry reported that, late-term delivery (40 0/7–41 6/7 weeks) compared with early-term delivery (38 0/7–39 6/7 weeks) is associated with improved survival, shorter ECMO duration, shorter hospital length of stay, and fewer complications on ECMO [17]. However, among fetuses with prenatally diagnosed CDH and without major associated anomalies, early-term delivery (37–38 weeks) was independently associated with survival [18]. Cesarean delivery was associated with a slightly better outcome in terms of a significantly higher survival without the use of ECMO, although there was no significant difference in total survival [19]. Because this study was not randomized, it is not possible to determine if elective cesarean delivery was the cause for the better outcome or if centers favoring elective cesarean delivery by protocol were more skillful in the management of patients with CDH. Mode of delivery for term and near-term infants with CDH deserves further prospective study, as all the quoted studies are retrospective studies. Figure 3 broadly outlines the prenatal management of CDH; however, this has to put in the context of fetal MRI being not widely available and fetal therapy which is experimental at this time in the management of CDH.
Fig. 3.
Algorithm of prenatal management of CDH. US ultrasound, LHR lung area-to-head circumference ratio, FLV fetal lung volume, SVD spontaneous vaginal delivery, FETO fetal endoscopic tracheal occlusion. See text for details
Postnatal Management of CDH
The guidelines mentioned here are drawn mainly from the consensus statement of the CDH EURO Consortium prepared with the aim of achieving standardized postnatal treatment, which might help in reducing morbidity and mortality associated with CDH [20].
Delivery Room Management
The infant’s heart rate, preductal and postductal saturations, and blood pressure (invasive or non-invasive) need to be monitored. The key principles of successful delivery room resuscitation and stabilization are the avoidance of high airway pressures and high oxygen concentration as this will facilitate in minimizing ventilation-induced lung injury.
After delivery, the infant should be intubated immediately without bag and mask ventilation. Ventilation by bag and mask may cause distention of the stomach and must be avoided as it may limit expansion of the hypoplastic lung. Ventilation in the delivery suite may be done with a ventilation bag or Neopuff with a peak pressure as low as possible, preferably ≤25 cm of H2O to avoid lung damage.
Oxygen saturations—the goal is to achieve acceptable preductal saturations levels of 80–95 %. FiO2 should be started at 1.0 and then adjusted downwards to achieve SpO2 targets.
Immediate placement of oro- or nasogastric tube with continuous or intermittent suction will help to decompress the bowel and facilitate lung expansion.
A central or peripheral venous line should be inserted to allow for administration of fluids and inotropes if necessary. Arterial line is to be placed for monitoring of blood pressure and for blood sampling after initial stabilization.
Arterial blood pressure to be maintained at acceptable levels for gestational age. In case of hypotension or poor perfusion volume bolus (NaCl 0.9 % at 10–20 ml/kg) should be administered and inotropes should be considered.
No routine use of surfactant in either term or premature infants is recommended as surfactant therapy is associated with higher mortality rate, greater use of ECMO, and more chronic lung disease with CDH [21].
Ventilation Management in the ICU
Ventilation strategy should aim for preductal saturations between 85 and 95 % with a targeted postductal saturation of ≥70 % and a PaCO2 between 45 and 60 mmHg (permissive hypercapnia). In exceptional cases, however, preductal saturations down to 80 % may be accepted, provided end organs are well perfused as indicated by a pH ≥7.2, lactate levels <5 mmol/l, and urinary output ≥1 ml/kg/h. Weaning oxygen to maintain SpO2 in the targeted range will facilitate avoiding oxygen toxicity.
The objective of ventilator management is to minimize ventilator-induced lung injury and this can be achieved by permissive hypercapnia and gentle ventilation. Towards that goal, it does not matter whether the infant is ventilated with conventional ventilation or high-frequency oscillatory ventilation (HFOV). The European consortium recommends that the infant be ventilated with PIP ≤25 cm H2O and a PEEP of 2–5 cm H2O and adjust the rate to achieve PaCO2 of 45 to 60 mmHg. A randomized controlled trial to compare initial ventilator management with HFOV or conventional ventilation in infants with CDH is underway [22].
Even though the indications for HFOV in CDH are not defined, any infant with relatively high settings on the conventional ventilator is a candidate for HFOV to minimize lung injury. Mean airway pressure is set to optimize lung recruitment (MAP of 13–17). This is accomplished by a chest X-ray, to confirm contralateral lung expansion of eight-rib expansion above the diaphragm. Avoiding alveolar overdistension and hence lung injury should be the goal of lung recruitment.
Hemodynamic Monitoring
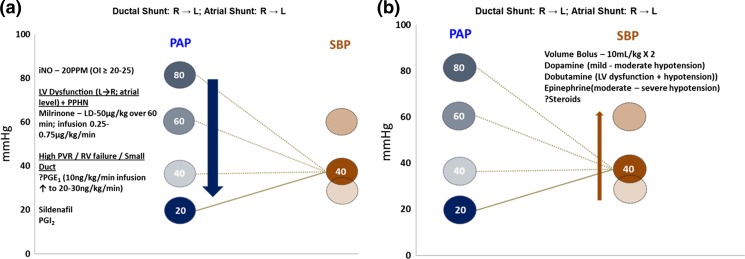
Hemodynamic monitoring should aim at end-organ perfusion such as heart rate, capillary refill, urine output, and lactate levels. Any signs of poor perfusion should facilitate intervention that should include volume bolus at first (NaCl 0.9 % × 2; 10 ml/kg) followed by maintaining systemic blood pressures that are appropriate for gestational age. However, hypoxemia may be influenced by right-to-left shunting across the ductus in the presence of pulmonary hypertension and hence the need to maintain systemic BP at a relatively higher level to decrease shunting. Inotropes such dopamine, dobutamine, and epinephrine are frequently used to raise systemic BP (Fig. 4b).
Fig. 4.
Schematic representation of management of systemic blood pressure (SBP) (b) and pulmonary arterial pressure (PAP) (a) in infants with CDH. Decreasing R → L shunting at the ductal level often includes raising systemic blood pressure (systolic or mean), managing systemic hypotension, or decreasing pulmonary hypertension. The lines shown show how changes in PAP and SBP could alter hemodynamics and oxygenation. The therapies to increase SBP and to decrease PAP are shown. (Copyright: Vasanth HS Kumar)
Management of Pulmonary Hypertension
Elevated pulmonary vascular resistance from vascular remodeling results in right-to-left shunting of blood after birth. This results in hypoxemia and differences between pre- and postductal oxygen saturations. However, the absence of pre- and postductal gradient in oxygenation does not exclude the diagnosis of pulmonary hypertension (PH), as shunting can occur predominantly intracardiac through the foramen ovale, rather than the PDA. Consequently, 2D echocardiography performed within the first 24 h is the best real-time assessment of pulmonary arterial pressure and right heart function in CDH infants. Right ventricular diastolic dysfunction is associated with disease severity and this may impact the duration of respiratory support in CDH [23]. Left ventricular dysfunction can result secondary to RV dysfunction or due to underdevelopment of left ventricle and is also associated with poor prognosis [24]. Treatment of RV and/or LV dysfunction and the associated pulmonary hypertension should take preference over surgical repair.
Treatment of PH in CDH is challenging to say the least, as this is backed by limited evidence. In a large randomized trial, early inhaled nitric oxide (iNO) failed to improve survival or reduce the need for ECMO in newborns with CDH [25]; however, positive response to iNO is observed in some cases. Despite the lack of response to iNO, its use in neonates with CDH is widespread and has increased in many US tertiary pediatric hospitals and is currently the first therapy of choice. At an oxygenation index of ≥20, iNO may be administered for at least an hour and the infant assessed for response to treatment (an increase in PaO2 by 10–20 mmHg or a 10 % response in SpO2) [26]. Serial echocardiography will help in assessing the response to therapy. Optimal lung inflation and maintaining adequate and a relatively higher systemic blood pressure with inotropes will minimize the effects of PH to some extent (Fig. 4b). However, in cases of severe PH not responsive to iNO, assessment of the heart by echo should be done frequently to assess both RV and LV function. Prostaglandin E1 can be considered, to maintain ductal patency, as a “blow-off valve” for the right ventricle, especially in the presence of RV dysfunction or RV failure [27]. The role of PGE1 in the management of pulmonary hypertension in maintaining ductal patency in the absence of RV dysfunction is not clear [28]. Opening of the ductus helps to decrease stress and vent the right ventricle from severe PH (Fig. 4a).
Milrinone, a PDE3 inhibitor, is a pulmonary vasodilator, is an inotrope, and improves ventricular systolic and diastolic function in low-cardiac output syndromes [29]. In a small case series, it was associated with improvement in systolic and diastolic function of the right ventricle with corresponding improvement in clinical status in infants with CDH [30]. Because of its inotropic (improved systolic function) and lusitropic (improved diastolic function) effects, it has been used in the presence of LV dysfunction in CDH (Fig. 4a). However, systemic hypotension and arrhythmias are a source of concern and it has to be used with caution in infants. Sildenafil, a PDE5 inhibitor, improves both cardiovascular function and oxygenation in patients with CDH [31]. Moreover, sildenafil may increase the efficacy of iNO and may prevent rebound pulmonary hypertension during weaning of iNO and hence help in the management of chronic PH [32]. Prostacyclin (PGI2), which acts via the cAMP pathway, has been used in isolated cases of CDH [33]; however, its effects have not been studied systematically. Newer prostanoids such as iloprost that can be delivered via aerosol are used in the management of chronic PH in adults and children; however, these have yet to be studied in infants with CDH. Bosentan, a non-selective endothelin receptor antagonist, has been used in the management of pulmonary hypertension in infants; however, liver toxicity is an important adverse side effect [34]. No randomized controlled trials has been carried out yet to test the effect of ET receptor antagonists in neonates. Management of pulmonary and systemic hemodynamics should be often simultaneous, to avoid hypoxemia and acidosis, which is essential to maximize effect on “reversible” pulmonary hypertension in these infants (Fig. 4).
Extracorporeal Membrane Oxygenation
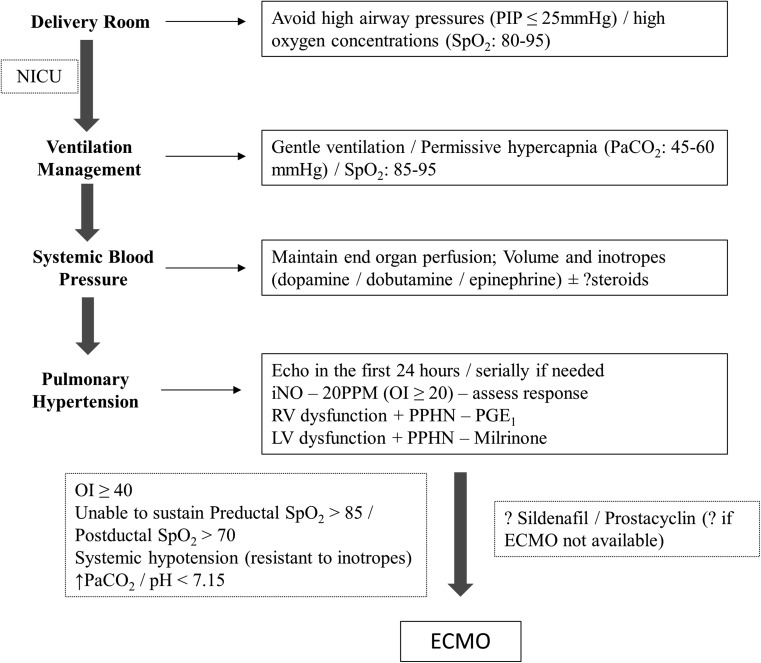
With advances in postnatal management of CDH, the use of ECMO as a rescue therapy in CDH has decreased over the years [35]. ECMO has been shown to improve survival rates and its utilization ranges from 15 to 40 %, with higher rates in right-sided CDH [35, 36]. Most candidates with CDH qualify for ECMO if they do not have major congenital anomalies and fail medical management after birth. Criteria for ECMO include inability to maintain preductal saturations >85 % or postductal saturations >70 %; oxygenation index (mean airway pressure × FiO2 × 100 / PaO2) ≥40; systemic hypotension resistant to fluid and inotropic support with signs of poor perfusion; and increasing PaCO2 and pH <7.15 despite optimal management on the ventilator. However, selection criteria may vary as each center has their own set of guidelines for entry into ECMO. The use of ECMO has reduced mortality in CDH [37], with overall survival rates following ECMO around 50 % [38]. A synopsis of postnatal management of infants with CDH is proposed in an algorithm (Fig. 5).
Fig. 5.
Algorithm of postnatal management of CDH. OI oxygenation index, ECMO extracorporeal membrane oxygenation. The algorithm is by no means a complete representation of postnatal management of CDH, as the therapy for CDH is still evolving. See text for details
Surgical repair of the defect in the diaphragm should be performed after a period of physiologic stabilization defined as stable mean blood pressure for gestational age, preductal saturations of 85 to 95 % in ≤50 % oxygen, urine output of >2 ml/kg/h, and blood lactate of <3 mmols/l. Repair can be done post-ECMO; however, repair can be done while on ECMO with particular attention to bleeding issues.
General Management
Infants with CDH need to be well sedated with opioid analgesics such as morphine or fentanyl both pre- and postoperatively as this facilitates the management of pulmonary hypertension and pain associated with the condition. Neuromuscular blocking agents are avoided, whenever possible, in view of side effects. Infants need to be monitored for strict fluid intake and output, and they benefit from restricted fluid intake in the first few days of life (40–60 ml/kg/day). Early TPN from day 1 of life that maximizes nutrition should be given priority, as these infants will be dependent on TPN for a prolonged period of time.
Surgical Repair
There are no standard criteria to define physiologic stabilization, and hence, this may vary with the severity of the defect, and most surgeons wait for a few days for the period of stabilization to occur prior to surgery. The standard surgical approach to repair the diaphragmatic defect consists of the subcostal incision with removal of the abdominal contents from the thorax and complete closure of the defect. For defects that are too large to be closed by primary repair, a number of reconstructive techniques such as prosthetic patches have been evolved to close the gap [39], and this can be accomplished by synthetic non-absorbable material (Gore-Tex) or by natural absorbable patch (Surgisis) [39]. Approximately half of all the repairs that require some type of patch may result in recurrent diaphragmatic herniation. A recent meta-analysis comparing the use of Gore-Tex and Surgisis in infants with CDH patch repair showed no differences between the two prosthetic materials in the incidence of hernia recurrence and small bowel obstruction rate [40]. The use of minimally invasive surgery techniques for repair of CDH is gaining popularity; however, they have a significantly higher recurrence risk of herniation [41]. Even though early repair of CDH in neonates on ECMO can be accomplished [42], CDH repair after ECMO therapy is associated with improved survival compared to repair on ECMO after controlling for factors associated with severity of CDH [43].
Long-Term Prognosis in CDH
Prematurity, lung hypoplasia, mechanical ventilation, oxygen toxicity, and vascular abnormalities all play a role in the morbidity associated with CDH. Overall survival rates in CDH is around 60–70 % and as high as 90 % in few high-volume centers. Infants who survive need to have a comprehensive plan for the detection of associated morbidities that includes respiratory, nutritional and reflux issues, growth failure, hearing loss, neurodevelopmental issues, and recurrence of hernia for optimal outcomes [44]. A multidisciplinary team involved in long-term issues will help in maintaining the high quality of life in these infants. It is important to develop a consensus among the specialties involved about the timing for optimal follow-up to include cardiac echo/catheterization, lung function tests, and feeding-related and developmental assessment. Despite the challenges for pediatric surgeons and critical care specialists, advances in acute and chronic management of pulmonary hypertension and other issues have led to better outcomes for these infants.
Acknowledgments
Conflict of Interest
The author declares that he has no competing interests.
References
- 1.Kotecha S, Barbato A, Bush A, Claus F, Davenport M, Delacourt C, et al. Congenital diaphragmatic hernia. Eur Respir J. 2012;39:820–9. doi: 10.1183/09031936.00066511. [DOI] [PubMed] [Google Scholar]
- 2.Wynn J, Yu L, Chung WK. Genetic causes of congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:324–30. doi: 10.1016/j.siny.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallot D, Marceau G, Coste K, Hadden H, Robert-Gnansia E, Laurichesse H, et al. Congenital diaphragmatic hernia: a retinoid-signaling pathway disruption during lung development? Birth Defects Res Part A Clin Mol Teratol. 2005;73:523–31. doi: 10.1002/bdra.20151. [DOI] [PubMed] [Google Scholar]
- 4.Beurskens LW, Tibboel D, Lindemans J, Duvekot JJ, Cohen-Overbeek TE, Veenma DC, et al. Retinol status of newborn infants is associated with congenital diaphragmatic hernia. Pediatrics. 2010;126:712–20. doi: 10.1542/peds.2010-0521. [DOI] [PubMed] [Google Scholar]
- 5.Major D, Cadenas M, Fournier L, Leclerc S, Lefebvre M, Cloutier R. Retinol status of newborn infants with congenital diaphragmatic hernia. Pediatr Surg Int. 1998;13:547–9. doi: 10.1007/s003830050399. [DOI] [PubMed] [Google Scholar]
- 6.Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karamanoukian HL, Glick PL, Wilcox DT, O’Toole SJ, Rossman JE, Azizkhan RG. Pathophysiology of congenital diaphragmatic hernia. XI: anatomic and biochemical characterization of the heart in the fetal lamb CDH model. J Pediatr Surg. 1995;30:925–8. doi: 10.1016/0022-3468(95)90314-3. [DOI] [PubMed] [Google Scholar]
- 8.Gallot D, Coste K, Francannet C, Laurichesse H, Boda C, Ughetto S, et al. Antenatal detection and impact on outcome of congenital diaphragmatic hernia: a 12-year experience in Auvergne, France. Eur J Obstet Gynecol Reprod Biol. 2006;125:202–5. doi: 10.1016/j.ejogrb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Mullassery D, Ba’ath ME, Jesudason EC, Losty PD. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol: Off J Int Soc Ultrasound Obstet Gynecol. 2010;35:609–14. doi: 10.1002/uog.7586. [DOI] [PubMed] [Google Scholar]
- 10.Hedrick HL, Danzer E, Merchant A, Bebbington MW, Zhao H, Flake AW, et al. Liver position and lung-to-head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. Am J Obstet Gynecol. 2007;197:422 e1–4. doi: 10.1016/j.ajog.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31:148–51. doi: 10.1016/S0022-3468(96)90338-3. [DOI] [PubMed] [Google Scholar]
- 12.Waag KL, Loff S, Zahn K, Ali M, Hien S, Kratz M, et al. Congenital diaphragmatic hernia: a modern day approach. Semin Pediatr Surg. 2008;17:244–54. doi: 10.1053/j.sempedsurg.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Busing KA, Kilian AK, Schaible T, Dinter DJ, Neff KW. MR lung volume in fetal congenital diaphragmatic hernia: logistic regression analysis—mortality and extracorporeal membrane oxygenation. Radiology. 2008;248:233–9. doi: 10.1148/radiol.2481070934. [DOI] [PubMed] [Google Scholar]
- 14.Jani JC, Nicolaides KH, Gratacos E, Valencia CM, Done E, Martinez JM, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol: Off J Int Soc Ultrasound Obstet Gynecol. 2009;34:304–10. doi: 10.1002/uog.6450. [DOI] [PubMed] [Google Scholar]
- 15.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus statement. 1994;12:1-24 [PubMed]
- 16.Davey M, Shegu S, Danzer E, Ruchelli E, Adzick S, Flake A, et al. Pulmonary arteriole muscularization in lambs with diaphragmatic hernia after combined tracheal occlusion/glucocorticoid therapy. Am J Obstet Gynecol. 2007;197:381 e1–7. doi: 10.1016/j.ajog.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 17.Stevens TP, Chess PR, McConnochie KM, Sinkin RA, Guillet R, Maniscalco WM, et al. Survival in early- and late-term infants with congenital diaphragmatic hernia treated with extracorporeal membrane oxygenation. Pediatrics. 2002;110:590–6. doi: 10.1542/peds.110.3.590. [DOI] [PubMed] [Google Scholar]
- 18.Stevens TP, van Wijngaarden E, Ackerman KG, Lally PA, Lally KP, Congenital Diaphragmatic Hernia Study G Timing of delivery and survival rates for infants with prenatal diagnoses of congenital diaphragmatic hernia. Pediatrics. 2009;123:494–502. doi: 10.1542/peds.2008-0528. [DOI] [PubMed] [Google Scholar]
- 19.Frenckner BP, Lally PA, Hintz SR, Lally KP, Congenital Diaphragmatic Hernia Study G Prenatal diagnosis of congenital diaphragmatic hernia: how should the babies be delivered? J Pediatr Surg. 2007;42:1533–8. doi: 10.1016/j.jpedsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–64. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 21.Van Meurs K, Congenital Diaphragmatic Hernia Study G Is surfactant therapy beneficial in the treatment of the term newborn infant with congenital diaphragmatic hernia? J Pediatr. 2004;145:312–6. doi: 10.1016/j.jpeds.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 22.van den Hout L, Tibboel D, Vijfhuize S, te Beest H, Hop W, Reiss I, et al. The VICI-trial: high frequency oscillation versus conventional mechanical ventilation in newborns with congenital diaphragmatic hernia: an international multicentre randomized controlled trial. BMC Pediatr. 2011;11:98. doi: 10.1186/1471-2431-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moenkemeyer F, Patel N. Right ventricular diastolic function measured by tissue Doppler imaging predicts early outcome in congenital diaphragmatic hernia. Pediatr Crit Care Med: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2014;15:49–55. doi: 10.1097/PCC.0b013e31829b1e7a. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal S, Stockmann P, Klein MD, Natarajan G. Echocardiographic measures of ventricular function and pulmonary artery size: prognostic markers of congenital diaphragmatic hernia? J Perinatol: Off J Calif Perinatal Assoc. 2011;31:561–6. doi: 10.1038/jp.2011.3. [DOI] [PubMed] [Google Scholar]
- 25.The Neonatal Inhaled Nitric Oxide Study Group (NINOS) Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997;99:838–45. doi: 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 26.Kinsella JP. Inhaled nitric oxide in the term newborn. Early Hum Dev. 2008;84:709–16. doi: 10.1016/j.earlhumdev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Buss M, Williams G, Dilley A, Jones O. Prevention of heart failure in the management of congenital diaphragmatic hernia by maintaining ductal patency. A case report. J Pediatr Surg. 2006;41:e9–11. doi: 10.1016/j.jpedsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Shiyanagi S, Okazaki T, Shoji H, Shimizu T, Tanaka T, Takeda S, et al. Management of pulmonary hypertension in congenital diaphragmatic hernia: nitric oxide with prostaglandin-E1 versus nitric oxide alone. Pediatr Surg Int. 2008;24:1101–4. doi: 10.1007/s00383-008-2225-6. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.CIR.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 30.Patel N. Use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatology. 2012;102:130–6. doi: 10.1159/000339108. [DOI] [PubMed] [Google Scholar]
- 31.Noori S, Friedlich P, Wong P, Garingo A, Seri I. Cardiovascular effects of sildenafil in neonates and infants with congenital diaphragmatic hernia and pulmonary hypertension. Neonatology. 2007;91:92–100. doi: 10.1159/000097125. [DOI] [PubMed] [Google Scholar]
- 32.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–10. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 33.De Luca D, Zecca E, Vento G, De Carolis MP, Romagnoli C. Transient effect of epoprostenol and sildenafil combined with iNO for pulmonary hypertension in congenital diaphragmatic hernia. Paediatr Anaesth. 2006;16:597–8. doi: 10.1111/j.1460-9592.2006.01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Goissen C, Ghyselen L, Tourneux P, Krim G, Storme L, Bou P, et al. Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. Eur J Pediatr. 2008;167:437–40. doi: 10.1007/s00431-007-0531-y. [DOI] [PubMed] [Google Scholar]
- 35.Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44:1691–701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Bryner BS, Kim AC, Khouri JS, Drongowski RA, Bruch SW, Hirschl RB, et al. Right-sided congenital diaphragmatic hernia: high utilization of extracorporeal membrane oxygenation and high survival. J Pediatr Surg. 2009;44:883–7. doi: 10.1016/j.jpedsurg.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg: Off J Aust Assoc Pediatr Surg [et al] = Zeitschrift fur Kinderchirurgie. 2006;16:385–91. doi: 10.1055/s-2006-924751. [DOI] [PubMed] [Google Scholar]
- 38.Sluiter I, van de Ven CP, Wijnen RM, Tibboel D. Congenital diaphragmatic hernia: still a moving target. Semin Fetal Neonatal Med. 2011;16:139–44. doi: 10.1016/j.siny.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Zani A, Zani-Ruttenstock E, Pierro A. Advances in the surgical approach to congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:364–9. doi: 10.1016/j.siny.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Romao RL, Nasr A, Chiu PP, Langer JC. What is the best prosthetic material for patch repair of congenital diaphragmatic hernia? Comparison and meta-analysis of porcine small intestinal submucosa and polytetrafluoroethylene. J Pediatr Surg. 2012;47:1496–500. doi: 10.1016/j.jpedsurg.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Lansdale N, Alam S, Losty PD, Jesudason EC. Neonatal endosurgical congenital diaphragmatic hernia repair: a systematic review and meta-analysis. Ann Surg. 2010;252:20–6. doi: 10.1097/SLA.0b013e3181dca0e8. [DOI] [PubMed] [Google Scholar]
- 42.Dassinger MS, Copeland DR, Gossett J, Little DC, Jackson RJ, Smith SD, et al. Early repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. 2010;45:693–7. doi: 10.1016/j.jpedsurg.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Congenital Diaphragmatic Hernia Study G. Bryner BS, West BT, Hirschl RB, Drongowski RA, Lally KP, et al. Congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: does timing of repair matter? J Pediatr Surg. 2009;44:1165–71. doi: 10.1016/j.jpedsurg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Academy of Pediatrics Section on S, American Academy of Pediatrics Committee on F. Newborn, Lally KP, Engle W. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics. 2008;121:627–32. doi: 10.1542/peds.2007-3282. [DOI] [PubMed] [Google Scholar]