Abstract
The basidiomycete Gloeophyllum trabeum KU-41 can degrade Japanese cedar wood efficiently. To construct a strain better suited for biofuel production from Japanese cedar wood, we developed a gene transformation system for G. trabeum KU-41 using the hygromycin phosphotransferase-encoding gene (hpt) as a marker. The endogenous laccase candidate gene (Gtlcc3) was fused with the promoter of the G. trabeum glyceraldehyde-3-phosphate dehydrogenase-encoding gene and co-transformed with the hpt-bearing pAH marker plasmid. We obtained 44 co-transformants, and identified co-transformant L#61, which showed the highest laccase activity among all the transformants. Moreover, strain L#61 was able to degrade lignin in Japanese cedar wood-containing medium, in contrast to wild-type G. trabeum KU-41 and to a typical white-rot fungus Phanerochaete chrysosporium. By using strain L#61, direct ethanol production from Japanese cedar wood was improved compared to wild type. To our knowledge, this study is the first report of the molecular breeding of lignin-degrading brown-rot fungus and direct ethanol production from softwoods by co-transformation with laccase overproduction constructs.
Electronic supplementary material
The online version of this article (doi:10.1186/s13568-015-0173-9) contains supplementary material, which is available to authorized users.
Keywords: Gloeophyllum trabeum KU-41, Laccase gene, Homologous expression, Lignin degradation
Introduction
Gloeophyllum trabeum, along with Postia placenta, is a well-characterized brown-rot fungus used as a model laboratory organism. Brown-rot fungi are important recyclers in coniferous forest ecosystems and cause a highly destructive type of wood decay. Thus, elucidating the mechanisms employed by brown-rot fungi in the biodegradation of lignified plant cell walls is very important. A Fenton system is proposed to play an important role in cellulose decomposition by brown-rot fungi (Arantes et al. 2012). This system recently has been receiving increased attention because utilization of this process could facilitate the cost-effective transformation of lignocellulose biomass into biofuel or renewable chemicals (Kerem et al. 1999; Jensen et al. 2001). Therefore, many studies have been conducted to understand the lignocellulose breakdown mechanisms in G. trabeum. In addition, decoding of the genomic sequences of brown-rot fungi, including those of G. trabeum, has facilitated the identification of key enzymes involved in lignocellulose metabolism (Floudas et al. 2012).
Ethanol is the most widely utilized liquid biofuel alternative to fossil fuels. Cellulosic biomass has been widely regarded as a readily available sugar source to replace starch materials in fermentation. Use of a single microorganism to produce ethanol from lignocellulosic biomass is expected to be especially cost-efficient; such an organism would have to possess the combined abilities to degrade lignin, hydrolyze cellulose, and ferment monosaccharides to ethanol (Lynd et al. 2005). Basidiomycetes play important roles in the carbon cycle as decomposers in forest ecosystems. Recently, we have proposed a new process of unified aerobic delignification and anaerobic saccharification and fermentation of hardwood by a single microorganism, the white-rot fungus Phlebia sp. MG-60 (Kamei et al. 2012). In contrast to hardwoods, softwoods such as Japanese cedar are more difficult to degrade by microbial activity (Katagiri et al. 1997). Brown-rot fungi have different mechanisms for the wood degradation with white-rot fungi, which are rapidly depolymerize the cellulose and hemicellulose in wood with modified lignin in the brown residue (Goodell et al. 1997; Hyde and Wood 1997). Brown-rot fungi are accessible to cellulose due to the high xylanase activity (Kim et al. 2014). The accessibility of cellulose increases in G. trabeum due to the removal of xylan (Gao et al. 2012). Furthermore, G. trabeum has the ability to produce ethanol from sugar under anaerobic or stressed conditions (Rasmussen et al. 2010). Thus, to address this challenge, we are investigating G. trabeum KU-41 as a model for the fungal degradation of Japanese cedar wood. Specifically, we hypothesized that it would be possible to develop this strain as a host basidiomycete capable of integrating the delignification, saccharification, and fermentation of softwoods. In this context, the construction of a novel genetic transformation system for G. trabeum was expected to permit the construction of strains with improved combinations of these properties. T. versicolor, Pycnoporus coccineus, T. hirsute were used as the laccase-producing wood rot fungi for the control in this study, because these fungi with high laccase production have been widely reported (Bourbonnais et al. 1995; Jaouani et al. 2005; Wu et al. 2011). Phanerochaete chrysosporium is a model white-rot fungus, used for comparing the lignin degradation in this study.
In the present study, we developed a novel genetic transformation system for G. trabeum KU-41, and demonstrated the molecular breeding of ligninolytic G. trabeum KU-41 by the homologous expression of an endogenous gene encoding a putative laccase activity.
Materials and methods
Strains and media
G. trabeum KU-41 (NBRC 111644) was described previously (Gao et al. 2012). The G. trabeum ATCC11539 strain was purchased from the American Type Culture Collection. The typical white-rot basidiomycetes Trametes versicolor, Pycnoporus coccineus, and T. hirsuta were purchased from NITE (National Institute of Technology and Evaluation). The strains used in this study are listed in Additional file 1: Table S1. All strains were maintained on semi-solid CYM medium (Yamagishi et al. 2007) at 28 °C.
Cloning of genomic DNA fragments encoding Gtact and Gtgpd
The reagents and protocols for manipulating DNA and RNA (DNA and RNA purification, cDNA synthesis, 3′-RACE, inverse PCR procedure, error-prone PCR, cloning of PCR products) were described previously (Yamagishi et al. 2013). The G. trabeum KU-41 genomic DNAs encoding cytosolic actin (Gtact) and glyceraldehyde-3-phosphate dehydrogenase (Gtgpd) were cloned by a series of PCR procedures (Additional file 1: Figs. S1, S2). The primers that were used are listed in Additional file 1: Table S2. These primers were designed based on nucleotide sequences and intron positions obtained from the DDBJ (http://www.ddbj.nig.ac.jp/). The accession numbers for Gtact and Gtgpd are AB856051 and AB856282, respectively.
Construction of transforming plasmids
The construction of transforming plasmids pAH and pGL are depicted in Additional file 1: Figs. S3, S4, S5. The plasmids used for the transformation of G. trabeum KU-41 were purified using Quantum Prep Midi kits (Bio-Rad Laboratories KK, Tokyo, Japan).
Preparation of G. trabeum protoplasts
G. trabeum protoplasts were prepared by slight modification of the previously described procedure (Yamagishi et al. 2007). Briefly, the KU-41 strain was pre-cultured for 10 days at 28 °C in liquid CYM medium (100 mL/flask in four 500-mL flasks). The mycelium was homogenized with a Waring blender (5000 rpm, 3 min) and cultured (for 4 days at 28 °C) in liquid CYM medium (100 mL/flask in twenty 500-mL flasks). Approximately 5 g of wet mycelium was collected from these cultures and treated with 20 mL of enzyme solution [Lyzing enzymes from Trichoderma (Singma L1412-10G) 2.5 %, Cellulase Onozuka RS (Yakult) 2.5 %/0.5 M MgSO4] at 30 °C for 6 h with gentle shaking. Protoplasts were separated from undigested mycelial debris by overlaying onto SorbOsm and centrifuging (4000×g) at 4 °C for 20 min. The viability of the resulting protoplasts was estimated as described previously (Yamagishi et al. 2007).
Genetic transformation procedure
Protoplasts of G. trabeum KU-41 (0.8 × 108 cells), marker plasmid pAH (2.5 to 20-µg), co-transformed plasmid pGL (20 µg), and 40 mM CaCl2 (final concentration) were combined with SorbOsm to yield a total transformation volume of 1.6 mL. After incubation in ice for 30 min, 50 % PEG solution (1.6 mL) was added, and the combination was gently mixed before being diluted by addition of 20 mL SorbOsm. The transformation mix then was mixed with 150 mL of regeneration medium and poured into 20 Petri dishes (7.5 mL dish−1). The composition of the regeneration medium was as described previously (Yamagishi et al. 2007), except that the pH of the medium used in the present study was adjusted to 6.5 (optimal pH for hygromycin) with NaOH prior to use. Following solidification, the plates were incubated at 28 °C for 1 day, and the regenerating protoplasts then overlaid with 7.5 mL/plate of selection medium. (Selection medium consisted of regeneration medium, pH 6.5, supplemented with 40 µg mL−1 hygromycin.) Regenerated clones, which appeared in 7–14 days, were picked and subcultured in the individual wells of a 24-well micro-plate containing 1 mL/well of semi-solid CYM medium (pH 6.5) supplemented with 20 µg mL−1 hygromycin.
Amplification of transformed plasmids in regenerated clones
The direct PCR procedure for each regenerated clone was described previously (Yamagishi et al. 2013). Forward primers (hph-F1 and gpd-F12) and reverse primers (hph-R1 and lcc3-R1) were used to amplify the transformed plasmids pAH and pGL, respectively. The locations of the primers within the respective plasmids are depicted in Fig. 1.
Fig. 1.
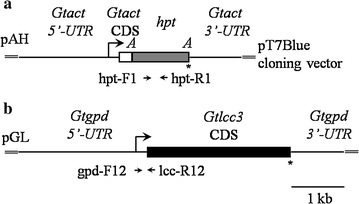
Structures of marker plasmids. a, b show the overall structures of marker plasmids pAH and pGL, respectively. Gtact and Gtgpd coding sequence (CDS) indicate Gloeophyllum trabeum KU-41 genomic DNA, including introns. The indicated constructs were cloned into the TA-cloning site of cloning vector pT7Blue. The arrows indicate the location of primers used for direct PCR. A AscI; asterisk stop codon
Measurement of laccase activities of clones co-transformed with pGL
Initial screening was performed as follows. For each transformant harboring pGL (44 clones total), two 5-mm2 pieces of mycelium were used to inoculate Kirk liquid medium (Tien and Kirk 1988) (2 mL/well in 12-well multititer plate), and plates were grown at 28 °C for 5 days. The laccase activity of each clone (each well) was measured using 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) as the substrate (Nagai et al. 2002). Eleven clones showing appreciable laccase activity were further characterized for laccase activity. For each clone, three flasks were generated as follows: five 5-mm2 pieces of mycelium were used to inoculate Kirk liquid medium (10 mL/flask in 100-mL flask), and cultures were grown at 28 °C for 5 days. The laccase activity of each clone was calculated as the mean ± standard deviation of the values from each trio of cultures. The laccase activity was also detected as zymogram. Extracellular proteins were separated by native polyacrylamide gel electrophoresis (PAGE), then visualized as zymogram by laccase active staining with 2,6-dimethoxyphenol.
Assessment of genetic stability of clones co-transformed with pGL
Transformed clone L#61 was subcultured on semi-solid CYM medium without hygromycin until the border of colony reached the edge of the Petri dish (90 × 15 mm). A piece of mycelium was punched out from the peripheral zone and subcultured on a new plate of semi-solid CYM medium without hygromycin. This process was repeated for a total of five times, and the sequential passages were denoted as generations 1–5. After subculturing, generations 1 and 5 of transformant L#61 were cultured in Kirk liquid medium (10-mL/flask in three 100-mL flasks) and their laccase activities were measured as described above. The laccase activity of each of the two tested generations was calculated as the mean ± standard deviation of the values from each trio of cultures.
Determination of ligninolytic properties and ethanol production from wood
Erlenmeyer flasks (50-mL) containing 0.5 g extractive-free Japanese cedar wood meal (80–100 mesh) and 1.25 mL distilled water each were inoculated with mycelial discs of wild-type G. trabeum KU-41(WT) or transformant L#61 and the resulting cultures were incubated at 30 °C for 28 days. Weight loss, Klason lignin content, and acid-soluble lignin content in the fungal-treated wood meal were determined after the incubation period, as described previously (Hirai et al. 1994). Total lignin content was calculated as sum of Klason lignin and acid-soluble lignin contents.
Following the 28-day incubation, each wood meal culture was supplemented by the addition of 10 mL of basal liquid medium without saccharides (10 g L−1 yeast extract, 10 g L−1 KH2SO4, 2 g L−1 (NH4)2SO4, and 0.5 g L−1 MgSO4-7H2O, pH 4.5) and then homogenized using a Polytron PT1200E. The cultures were further incubated with shaking (150 rpm) at 30 °C for 3, 6, 9, and 12 days in semi-aerobic conditions (obtained by sealing each culture flask with a silicon plug stopper). After fermentation, the wood meal cultures were centrifuged (10,000×g, 5 min). Ethanol levels in the resulting supernatants were measured by high-performance liquid chromatography (HPLC) using a Shodex SH1821 column (8.0 mm × 300 mm, Showa Denko K.K., Tokyo, Japan) at 75 °C with 0.5 mM H2SO4 as the mobile phase at a flow rate of 0.6 mL min−1. Detection was performed using an online Refractive Index Detector.
Results
Construction of a genetic transformation system for KU-41
We varied experimental conditions to investigate the appropriate conditions for preparing protoplasts of our strain. A sufficient number of protoplasts (0.2 × 108 protoplasts per gram of wet mycelium, 4 % cell viability) were obtained from the 4-day mycelial cultures. The marker plasmid pAH was constructed to express the hygromycin resistance-encoding gene (hpt) under the control of a constitutive promoter (for a gene encoding a cytosolic actin) (Fig. 1a). Genetic transformation of KU-41 protoplasts with 10 µg of the marker plasmid pAH yielded 26–35 transformants; no clones were obtained in the absence of pAH (Table 1). Direct PCR of clones transformed with pAH confirmed that all hygromycin-resistant clones contained the hpt gene (Fig. 2a).
Table 1.
Transformed clones regenerated in uracil-free regenerating medium
| Transformed plasmids | Exp. 1 | Exp. 2 | |
|---|---|---|---|
| pAH | 20 μg | 75 | 77 |
| pAH | 10 μg | 26 | 35 |
| pAH | 5 μg | 20 | 24 |
| pAH | 2.5 μg | 10 | 17 |
| pAH + pGL | 10 + 30 μg | 100 | 106 |
| Control | – | 0 | 0 |
Transformation experiments were performed twice (experiment 1 and experiment 2)
Exp experiment
Fig. 2.
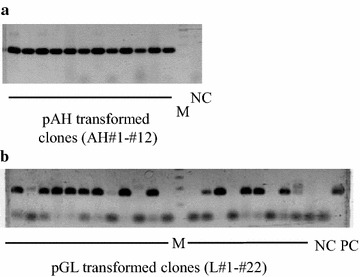
Direct PCR analysis of genomic DNA recovered from individual transformants. Supernatants of homogenized mycelium of the transformants were used in PCR amplification with primer pair hptF1-hptR1 (a) or gpdF12-lccR12 (b). “M” indicates a 100 bp ladder size marker. “NC” indicates negative control (reactions with no PCR template). “PC” indicates positive control using pGL as PCR template. The PCR products were electrophoresed in 2 % agar and stained with SYBR Green (Takara)
Forced expression of the endogenous gene Gtlcc3
To confirm transgene expression in KU-41, the co-transforming plasmid pGL was constructed (Fig. 1b). pGL was designed to express an endogenous (G. trabeum) laccase candidate gene (Gtlcc3) under control of the Gtgpd promoter (Additional file 1: Figs. S4, S5). [Gtlcc3 represents one of 4 putative laccase genes (Gtlcc1–4) identified in the G. trabeum genome database (http://genome.jgi-psf.org/Glotr1_1/Glotr1_1.home.html; protein ID: 127593)]. A total of 72 hygromycin-resistant clones were characterized following co-transformation with pAH and pGL; direct PCR revealed that 44 of the 72 clones harbored pGL (Fig. 2b; Table 1). All 72 clones were inoculated on semi-solid medium containing ABTS. After 2 days, green haloes were visible around 30 of the 44 pGL-positive clones. Haloes were not observed around any of the negative control strains, including the KU-41 parent; 17 hygromycin-resistant strains recovered following transformation with pAH alone; and the separate G. trabeum ATCC 11539 strain. The production of laccase in Kirk liquid medium also was evaluated (Fig. 3). Co-transformant L#61 exhibited 0.73 nkat mL−1 laccase activity. Although the laccase activity of co-transformant L#61 was lower than that of typical white-rot fungus T. versicolor, the activity was similar to that of P. coccineus (Fig. 3b).
Fig. 3.
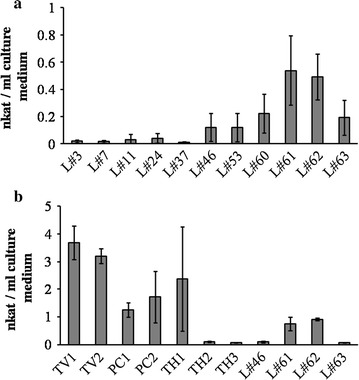
Laccase activities of co-transformants. a Laccase activities produced in liquid culture medium by individual transformants. b Comparison of laccase producing abilities between the co-transformants and typical laccase producing white-rot basidiomycetes. TV1: Trametes versicolor NBRC4937, TV2: T. versicolor NBRC30340, PC1: Pycnoporus coccineus NBRC6489, PC2: P. coccineus NBRC9495, TH1: Trametes hirsuta NBRC7038, TH2: T. hirsuta NBRC4917, TH3: T. hirsuta NBRC6477. All values are presented as mean ± standard deviation (n = 3)
Co-transformant L#61 was repeatedly subcultured on semi-solid CYM medium without hygromycin to evaluate the genetic stability of the laccase construct in the clones co-transformed with pAH and pGL. Laccase production by the L#61 co-transformant did not appear to decrease even after 5 cycles of subculturing on hygromycin-free semisolid medium (Fig. 4; Additional file 1: Fig. S6).
Fig. 4.
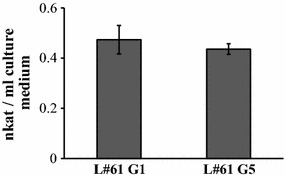
Assessment of genetic stability of transformants in the absence of selective pressure. G1 and G5 indicate generations 1 and 5, respectively. All values are presented as mean ± standard deviation (n = 3)
Degradation of lignin in Japanese cedar wood meal by co-transformant L#61
We tested whether the L#61 co-transformant was capable of degrading lignin in Japanese cedar wood meal. The co-transformant L#61 exhibited significantly elevated ligninolytic activity (2.7 %) compared to the parent (0.8 %), as shown in Fig. 5. L#61 indicates values that were determined by the student’s t test to be significantly different compared to wild type. In a parallel assay, the white-rot fungus P. chrysosporium also hardly degraded Japanese cedar wood meal (data not shown).
Fig. 5.
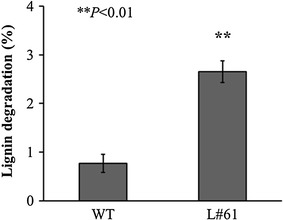
Lignin degradation rate in Japanese cedar wood meals by the parent G. trabeum KU-41 (WT) and the co-transformant L#61. All values are presented as mean ± standard deviation (n = 3). Asterisks indicate values that were determined by the Student’s t test to be significantly different compared to wild type (**P < 0.01)
After a 28-day pretreatment period, the WT or transformant L#61 wood meal cultures were transferred to a basal liquid medium and incubated under semi-aerobic conditions to assess ethanol production. After 12 days of semi-aerobic incubation, co-transformant L#61 exhibited a significant increase (of 45 %) in ethanol production compared to WT (Fig. 6).
Fig. 6.
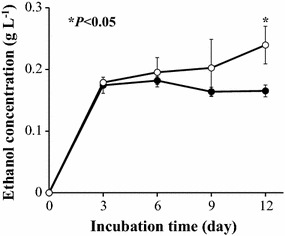
Ethanol production from Japanese cedar wood meal after 28 days of pre-treatment with the parent G. trabeum KU-41 (closed circles) or the co-transformant L#61 (open circles). All values are presented as mean ± standard deviation (n = 3). Asterisks indicate values that were determined by the Student’s t test to be significantly different compared to the parent (*P < 0.05)
Discussion
In the present study, we investigated the appropriate conditions for preparing protoplasts of the KU-41 strain, because preparation of a sufficient number of protoplasts is essential for construction of a genetic transformation system. Among various parameters (e.g., the age of the mycelium, concentration of the osmotic stabilizer in the enzyme solution, and the composition of the regeneration medium), mycelial age showed the strongest effects on protoplast isolation (data not shown). Older mycelium produced a viscous substance that hindered the enzyme treatment. In addition, because some reports have indicated that native introns (i.e., introns derived from the host species) were essential for the expression of exogenous genes in some basidiomycetes (Lugones et al. 1999; Ma et al. 2001; Burns et al. 2005; Yamazaki et al. 2006; Yamagishi et al. 2013), the first and second introns were retained as part of the actin promoter in pAH. However, the necessity of the introns was not confirmed in this study. Genetic transformation of KU-41 protoplasts with the marker plasmid pAH was successful, thus demonstrating our construction of a genetic transformation system for the KU-41 strain of the brown-rot fungus G. trabeum. The endogenous laccase candidate gene was fused with the G. trabeumgpd promoter and co-transformed with marker plasmid pAH. We obtained 44 co-transformants, and identified co-transformant L#61, which showed the highest laccase activity among all of the screened transformants. Our results indicated that forced expression of the introduced endogenous gene was successful and confirmed that Gtlcc3 indeed encodes a laccase activity. Although the laccase activity of co-transformant L#61 is lower than that of typical white-rot fungus T. versicolor, the activity was similar to that of P. coccineus. Transformants with elevated laccase production might be generated by modifying the pGL promoter region and/or by screening among a larger number of co-transformants. Given that pGL does not contain a known origin of replication for G. trabeum and that the transformed plasmids were stably retained in the absence of selective pressure, we infer that the transformed plasmids were integrated into the chromosomes of the transformants.
Our primary concern was whether the co-transformant L#61 could degrade lignin; the parent G. trabeum, like all brown-rot fungi, does not exhibit appreciable lignin degradation activity (Gao et al. 2012). We assayed the ability of co-transformant L#61 to degrade lignin using wood meal derived from Japanese cedar. Laccase mediates one-electron oxidation of phenolic substrates to form many degradation products via various pathways, but the enzyme can not oxidize non-phenolic substrate (Kawai et al. 1989). Thus, co-transformant L#61 showed low delignification activity, because this fungus does not have the delignification systems for non-phenolic structure like white-rot fungi. Additionally, hardwood tree species contain both guaiacyl and syringyl lignin but softwood tree species contain only guaiacyl lignin. Although white rot fungi preferably degrade syringyl units (Dong et al. 2013), they are not contained in Japanese cedar wood (a softwood). Thus, it is suggested that P. chrysosporium hardly degraded Japanese cedar wood meal. From the above, it is estimated that co-transformant L#61 was only able to oxidize phenolic residues that were presented at surface of lignin molecules in Japanese cedar wood.
Our results indicated that KU-41 was invested with ligninolytic activity upon the expression of Gtlcc3; delignification improved the direct yield of ethanol following fermentation of the wood meal. Autoclave sterilization, which could cause an effect similar to steam explosion pretreatment frequently used as pre-treatment for lignocellulosic substrates, was performed before fungal inoculation. Therefore, it is likely that the wood structure was changed into the structure that has better accessibility for fungal hydrolases to cellulose by the sterilization. In addition, due to relatively high xylanase activity, G. trabeum could make wood to accessible structure to cellulose. (Kim et al. 2014). From these reason, G. trabeum wild type might have been able to hydrolyze cellulose effectively and produce ethanol, in this experiment. Moreover, it is generally considered that both lignin and hemicellulose limit the accessibility of cellulase. And hemicellulose and lignin are chemically bind. Therefore, we estimated that recombinant laccase assists the hemicellulose removal due to polysaccharide hydrolases as a result of lignin surface oxidation, and that the improvement of lignin and hemicellulose removal leads to higher ethanol productivity of co-transformant L#61.
In conclusion, the homologous overexpression of a putative laccase-encoding gene provided G. trabeum with ligninolytic activity against Japanese cedar wood. To our knowledge, this is the first report that the molecular construction of a lignin-degrading brown-rot fungus. We expect that our transformation system and the resulting strain constructs will facilitate molecular analysis of the lignocellulose biodegrading system of G. trabeum and other brown-rot fungi.
Authors’ contributions
MA, KY, JW, and TM participated in the experimental procedures of this study and drafting of the manuscript. KT, TM, IK, RK and HK involved in the data analysis and interpreted the results. HH participated in the design this study, data analysis, interpretation of results and drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported in part by a project, titled “Research and Development of Catalytic Process for Efficient Conversion of Cellulosic Biomass into Biofuels and Chemicals”, which was funded through the Special Funds for Education and Research (No. 21248023); and by a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 24248030).
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s13568-015-0173-9 List of strains used in this study. Table S2. Oligonucleotides used as primers in this study. Fig. S1. Procedure for cloning Gloeophyllum trabeum actin-encoding genomic DNA. Fig. S2. Procedure for cloning Gloeophyllum trabeum glyceraldehyde-3-phosphate dehydrogenase-encoding genomic DNA. Fig. S3. Construction of marker plasmid pAH. Fig. S4. Addition of an AscI site in pGtgpd. Fig. S5. Construction of pGL. Fig. S6. Zymogram activity of the purified laccase enzyme with native PAGE.
Contributor Information
Misa Arimoto, Email: misa_ch_2_29@yahoo.co.jp.
Kenji Yamagishi, Email: yamagisi@affrc.go.jp.
Jianqiao Wang, Email: o_wangjianqiao@yahoo.co.jp.
Kanade Tanaka, Email: kan.tanaka@teijin.co.jp.
Takanori Miyoshi, Email: ta.miyoshi@teijin.co.jp.
Ichiro Kamei, Email: kamei@cc.miyazaki-u.ac.jp.
Ryuichiro Kondo, Email: ryukondo@agr.kyushu-u.ac.jp.
Toshio Mori, Email: mori.toshio@shizuoka.ac.jp.
Hirokazu Kawagishi, Email: kawagishi.hirokazu@shizuoka.ac.jp.
Hirofumi Hirai, Phone: +81 54 238 4853, Email: hirai.hirofumi@shizuoka.ac.jp.
References
- Arantes V, Jellison J, Goodell B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl Microbiol Biotechnol. 2012;94:323–338. doi: 10.1007/s00253-012-3954-y. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,29-azinobis(3-ethylbenzthiazoline- 6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C, Gregory KE, Kirby M, Cheung MK, Riquelme M, Elliott TJ, Challen MP, Bailey A, Foster GD. Efficient GFP expression in the mushrooms Agaricus bisporus and Coprinus cinereus requires introns. Fungal Genet Biol. 2005;42:191–199. doi: 10.1016/j.fgb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Yang JS, Zhu N, Wang ET, Yuan HL. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour Technol. 2013;131:443–451. doi: 10.1016/j.biortech.2012.12.182. [DOI] [PubMed] [Google Scholar]
- Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336:1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- Gao Z, Mori T, Kondo R. The pretreatment of corn stover with Gloeophyllum trabeum KU-41 for enzymatic hydrolysis. Biotechnol Biofuels. 2012;5:28. doi: 10.1186/1754-6834-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol. 1997;53:133–162. doi: 10.1016/S0168-1656(97)01681-7. [DOI] [Google Scholar]
- Hirai H, Kondo R, Sakai K. Screening of lignin degrading fungi and their ligninolytic enzyme activities during biological bleaching of kraft pulp. Mokzai Gakkaishi. 1994;40:980–986. [Google Scholar]
- Hyde SM, Wood PM. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology. 1997;143:259–266. doi: 10.1099/00221287-143-1-259. [DOI] [PubMed] [Google Scholar]
- Jaouani A, Guillén F, Penninckx MJ, Martínez AT, Martínez MJ. Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzyme Microb Technol. 2005;36:478–486. doi: 10.1016/j.enzmictec.2004.11.011. [DOI] [Google Scholar]
- Jensen KA, Jr, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol. 2001;67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei I, Hirota Y, Meguro S. Integrated delignification and simultaneous saccharification and fermentation of hard wood by a white-rot fungus, Phlebia sp. MG-60. Bioresour Technol. 2012;126:137–141. doi: 10.1016/j.biortech.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Katagiri N, Tsutsumi Y, Nishida T. Biobleaching of softwood kraft pulp by white-rot fungi and its related enzymes. Mokzai Gakkaishi. 1997;43:678–685. [Google Scholar]
- Kawai S, Umezawa T, Higuchi T. Oxidation of methoxylated benzyl alcohol by Coriolus versicolor in the presence of syringaldehyde. Wood Res. 1989;76:10–16. [Google Scholar]
- Kerem Z, Kenneth AJ, Kenneth EH. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven fenton reaction. FEBS Lett. 1999;446:49–54. doi: 10.1016/S0014-5793(99)00180-5. [DOI] [PubMed] [Google Scholar]
- Kim HM, Lee KH, Kim KH, Lee DS, Nguyen QA, Bae HJ. Efficient function and characterization of GH10 xylanase (Xyl10g) from Gloeophyllum trabeum in lignocellulose degradation. J Biotechnol. 2014;172:38–45. doi: 10.1016/j.jbiotec.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Lugones LG, Scholtmeijer K, Klootwijk R, Wessels JG. Introns are necessary for mRNA accumulation in Schizophyllum commune. Mol Microbiol. 1999;32:681–689. doi: 10.1046/j.1365-2958.1999.01373.x. [DOI] [PubMed] [Google Scholar]
- Lynd LR, van Zyl WH, McBride JE, Laser M. Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol. 2005;16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ma B, Mayfield MB, Gold MH. The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Appl Environ Microbiol. 2001;67:948–955. doi: 10.1128/AEM.67.2.948-955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H. Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol. 2002;60:327–335. doi: 10.1007/s00253-002-1109-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen ML, Shrestha P, Khanal SK, Pometto AL, 3rd, Hans van Leeuwen J. Sequential saccharification of corn fiber and ethanol production by the brown rot fungus Gloeophyllum trabeum. Bioresour Technol. 2010;101:3526–3533. doi: 10.1016/j.biortech.2009.12.115. [DOI] [PubMed] [Google Scholar]
- Tien M, Kirk TK. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161:238–248. doi: 10.1016/0076-6879(88)61025-1. [DOI] [Google Scholar]
- Wu J, Zhang X, Wan J, Ma F, Tang Y, Zhang X. Production of fiberboard using corn stalk pretreated with white-rot fungus Trametes hirsute by hot pressing without adhesive. Bioresour Technol. 2011;102:11258–11261. doi: 10.1016/j.biortech.2011.09.097. [DOI] [PubMed] [Google Scholar]
- Yamagishi K, Kimura T, Oita S, Sugiura T, Hirai H. Transformation by complementation of a uracil auxotroph of the hyper lignin-degrading basidiomycete Phanerochaete sordida YK-624. Appl Microbiol Biotechnol. 2007;76:1079–1091. doi: 10.1007/s00253-007-1093-7. [DOI] [PubMed] [Google Scholar]
- Yamagishi K, Kimura T, Watanabe T. Construction of an endogenous selectable marker gene for the rice straw degrading white rot basidiomycete Cyathus stercoreus. Biosci Biotechnol Biochem. 2013;77:771–775. doi: 10.1271/bbb.120912. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Okajima Y, Kawashima H, Tsukamoto A, Sugiura J, Shishido K. Intron-dependent accumulation of mRNA in Coriolus hirsutus of lignin peroxidase gene the product of which is involved in conversion/degradation of polychlorinated aromatic hydrocarbons. Biosci Biotechnol Biochem. 2006;70:1293–1299. doi: 10.1271/bbb.50471. [DOI] [PubMed] [Google Scholar]


