Abstract
Autism spectrum disorder (ASD) is characterized by persistent deficits within two core symptom domains: social communication and restricted, repetitive behaviors. Although numerous studies have reported psychopharmacological treatment outcomes for the core symptom domains of ASD, there are not enough studies on fundamental treatments based on the etiological pathology of ASD. Studies on candidate medications related to the pathogenesis of ASD, such as naltrexone and secretin, were conducted, but the results were inconclusive. Oxytocin has been identified as having an important role in maternal behavior and attachment, and it has been recognized as a key factor in the social developmental deficit seen in ASD. Genetic studies have also identified associations between ASD and the oxytocin pathway. As ASD has its onset in infancy, parents are willing to try even experimental or unapproved treatments in an effort to avoid missing the critical period for diagnosis and treatment, which can place their child in an irreversible state. While therapeutic application of oxytocin for ASD is in its early stages, we have concluded that oxytocin would be a promising therapeutic substance via a thorough literature review focusing on the following: the relationship between oxytocin and sociality; single nucleotide polymorphisms as a biological marker of ASD; and validity verification of oxytocin treatment in humans. We also reviewed materials related to the mechanism of oxytocin action that may support its potential application in treating ASD.
Keywords: Autism spectrum disorder, Oxytocin, Sociality, Gene, Behavior, Intranasal
INTRODUCTION
Why use oxytocin for autism spectrum disorder treatment?
After Leo Kanner published on children exhibiting "autistic disturbances of affective contact" in 1943 [1], pervasive developmental disorder was registered in the DSM-III in 1980 [2]. Although the terminology was changed to autism spectrum disorder (ASD) in the DSM-5 in 2013 [3], the representative symptoms still remain as 'restricted and repetitive patterns of behavior, interests or activities,' along with 'abnormalities of individuals to engage in reciprocal social interactions and communication' [4,5]. Treatment of autism is focused on improving behavioral problems and promoting sociability. We currently lack a clearly identified pathogenesis of ASD and various treatments are based on mechanisms assumed to be related to the symptoms. Though Risperidone and Aripiprazole has approved by United States Food and Drug Administration (FDA) for autism, but it was only for the treatment of irritability. There are no approved drugs for the treatment of core symptoms of ASD [6]. Since treatments are not based on any precise pathogenesis, treatments are developed through repeated trial and error. Nevertheless, some parents of children with ASD strive to help their kids even with not-evidence-based materials or medicine [7].
Since the early 1990s, naltrexone administration has been considered a promising treatment for ASD, based on the hypothesis that autism is related to hypersecretion of brain opioids [8] and on evidence that naltrexone decreases self-mutilating behavior in patients with autism [9]. Elchaar et al. reported that naltrexone was effective against self-injurious behavior [10]; however, other studies reported that it was ineffective for reducing self-injurious behavior and for other autism symptoms [11,12,13]. Following a systematic review, Roy et al. concluded that naltrexone was effective for treating hyperactivity and restlessness in autism, but there was no significant evidence supporting its treatment effects on the core features of autism [14]. In 1998, a promising report stated that cognitive and communicative symptoms in three children with ASD were ameliorated via intravenous secretin injection during endoscopic evaluation for gastrointestinal problems [15]. Data from pursuant research suggested that these effects were mediated by secretin activating the metabolic turnover of dopamine in the central nervous system via tetrahydrobiopterin, resulting in the improvement of ASD symptoms [16]. However, a recent meta-analysis concluded that there was no evidence that intravenous secretin improved the main symptoms in ASD [17]. Therefore, currently, secretin should not be recommended as a treatment for ASD.
In 1979, Pedersen and Prange first identified that intracerebroventricular oxytocin administration to nulliparous, ovariectomized female rats primed with estradiol benzoate induced the onset of maternal behavior [18]. Insel et al. proposed that oxytocin influences the infant response to maternal separation, based on evidence demonstrating that a rat pup, after receiving an intracerebroventricular oxytocin injection, emitted fewer ultrasonic vocalizations, which generate maternal retrieval [19]. In other words, the study emphasized that exogenous oxytocin has prosocial effects, and endogenous oxytocin is crucial for social interaction [18]. The administration of central oxytocin antagonists produced socio-sexual contact avoidance, and the development of an autism or schizoid personality model in humans through oxytocin blockade became a possibility [20,21]. Aberrant regulation of oxytocin signaling has been shown to play a role in the development of high-functioning ASD [20,21]. Since oxytocin has been linked to infant-mother attachment, maternal care, and pair bond formation in animal studies with rats and sheep, interest in the relationship between oxytocin and ASD has been greatly amplified [22,23,24]. Even in genetic linkage studies, the oxytocin signaling pathway has been found to be related to the etiology of autism [25]. Clinical trials for oxytocin's beneficial effects on ASD have been increasing and oxytocin treatment for particular aspects of ASD have proven to be effective [26,27].
Although there have been an increasing number of studies supporting the theory that the oxytocin signaling pathway is related to the pathogenesis and treatment of ASD, they have not been able to fully explain the heterogeneity and multifactorial nature of ASD. Some propose that finding groups of patients with ASD having oxytocin receptor sequences in response to oxytocin treatment must precede recommendation of oxytocin treatment or diagnosis of ASD, because oxytocin receptor genotype strongly associate with social cognition but not ASD diagnosis [28]. There were numerous reports of promising ASD treatment substances to turn out to be not effective in the end. In order to avoid such trials and errors, we reviewed the possibility of oxytocin as an etiological treatment for ASD in various perspectives.
NEUROSCIENTIFIC BACKGROUND OF SOCIALITY IN ANIMALS AND HUMANS
Prairie voles develop long-term monogamous relationships with their mates and both sexes provide parental care [29]. These characteristics make the prairie vole a useful model for studying affiliative behavior, especially pair bonding. On the other hand, montane voles exhibit polygamy, and are appropriate for comparison studies with prairie voles. In a study comparing prairie voles and montane voles, there was no significant difference in the expression of oxytocin between the species. However, there was a difference in the distribution of regional oxytocin receptors, which has been suggested to mediate pair bond formation [29]. In prairie voles, oxytocin receptors are highly expressed in the nucleus accumbens, prelimbic cortex, lateral amygdala, and midline thalamic nuclei. In contrast, oxytocin receptors in montane voles are expressed in the lateral septum and cortical nucleus of amygdala. Although gaps in knowledge remain, it is assumed that oxytocin is released in prairie voles during mating and it acts on the limbic sites, producing selective reinforcement of the mate. Furthermore, infusion of oxytocin into the cerebral ventricles facilitates partner preference in female prairie voles [30]. Though partner preference may differ from the complicated process of pair bonding, formation of a preference may be one of the earliest behavioral events in selective, enduring attachment. The formation of partner preference is blocked in the mating female prairie vole by infusion of oxytocin receptor antagonist into the prelimbic cortex and nucleus accumbens [30,31]. This result suggests that these brain regions may be important for pair bond formation, although as yet, no common physiological mechanisms for pair bond formation have been identified between animals and humans. Pair bond formation is also a necessary component of human sexuality. In a healthy human population, nipple stimulation facilitates oxytocin release [32] and plasma oxytocin levels are increased during orgasm in both women and men [33,34]. If oxytocin induces social attachment, sexual activity may reinforce sexual bonding [35]. In an experiment with healthy male students who had demonstrated insecure attachment patterns, administration of intranasal oxytocin produced an increase in secure attachment experience [36].
Evidence indicates that oxytocin is not only associated with pair bond formation, but also with social cognition. Since rats are born functionally blind and deaf, nipple attachment by olfactory learning and response to maternal odor is important for survival [37]. Endogenous oxytocin promotes associative learning of maternal odor in young rats. On the other hand, if the rats are pretreated with oxytocin antagonists, the odor-mother conditioning is blocked. This suggests that oxytocin is an important neuropeptide for young rats to form associations, especially with their mothers. In adult rats, onset of maternal behavior was facilitated with intracerebroventricular administration of oxytocin [38]. Onset of maternal behavior in rats includes overcoming natural avoidance of neonates, which can be induced by neonatal odors. It appears, therefore, that in rats, oxytocin induces maternal care by blunting olfactory processing in associated brain areas [39]. Oxytocin released during birth seems to reduce the firing rate of the mitral and granule cells in the olfactory bulb, and thus may facilitate approach behavior [40]. Oxytocin knockout mice suffer deficits in social recognition memory, despite normal olfactory bulb function and spatial learning abilities [41]. Social recognition was restored following intraventricular oxytocin administration to the medial nucleus of the amygdala [42]. This suggests that oxytocin receptor activation in the medial amygdala is necessary for social recognition in mouse. Adult oxytocin receptor knockout mice demonstrate deficits in social discrimination and exhibit more aggressive behavior than wild type mice [43]. Oxytocin receptor gene null mutant mice have two additional characteristics: 'resistance to change in a learned pattern of behavior,' corresponding to ASD's 'restricted interests and repetitive behavior'; 'increased susceptibility to seizures', which is frequently comorbid with ASD. However, administering intracerebroventricular oxytocin restores social exploration and social recognition, along with aggressive behavior [43]. There is an increasing body of literature on humans suggesting that oxytocin reinforces social memory by playing an important role selectively in facial processing and social communication. Through primarily employing intranasal administration, the following effects were reported: improvements in mental status inference ability [44]; increase in eye gaze number and duration for neutral human faces [45]; improvements in recognition memory for faces [46]. While a few authors reported enhancements in recognition of prosocial facial expression [47,48], other studies reported enhancement of neutral, angry, and fearful face recognitions [49,50,51].
In studies employing peripheral oxytocin injection in rats, prosocial behaviors such as increased adjacent lying (side-by-side contact) and decreased anogenital sniffing were observed [52]. Two specific mouse strains (BALB/cByJ, C58/J) with deficits in sociability showed an increase in social approach activities after intraperitoneal administration of oxytocin, based on performance in the three-chambered choice test [53]. The amygdala has been highlighted as one of the core areas thought to mediate the prosocial effects of exogenous oxytocin. In a double blind study with 15 men, each performed visual matching tasks for different fear-inducing visual stimuli after intranasal administration of oxytocin (27 IU) or placebo. Comparing the activity of the amygdala before and after the task with functional magnetic resonance imaging (fMRI), the authors observed reduced activity of the amygdala during viewing of aversive, fear-inducing visual stimuli along with decreased functional coupling of the amygdala regions mediating autonomic and behavioral aspects of fear and the brain stem [54]. These findings suggest that the prosocial effect of oxytocin is the result of an anxiolytic effect, which may include an adjustment in amygdala responsivity. Additionally, some groups have reported attenuation of aversive conditioning of fear-conditioned face stimuli following intranasal oxytocin (32 IU) administration [55]. These results were interpreted as resulting from decreased activity in the right amygdala and right fusiform face area during aversive conditioning. In a recent high-resolution fMRI study, subjects who received intranasal oxytocin (24 IU) frequently gazed at the eye region despite instructions to gaze at the mouth region [56]. Such fixation changes are correlated with increased right posterior amygdala activity and are coupled with increased activity of the superior colliculi. Intranasal oxytocin administration attenuated activity in the lateral and dorsal regions of the anterior amygdala in response to fearful faces, and increased activity in response to happy faces.
GENETIC BACKGROUND IN ANIMALS AND HUMANS
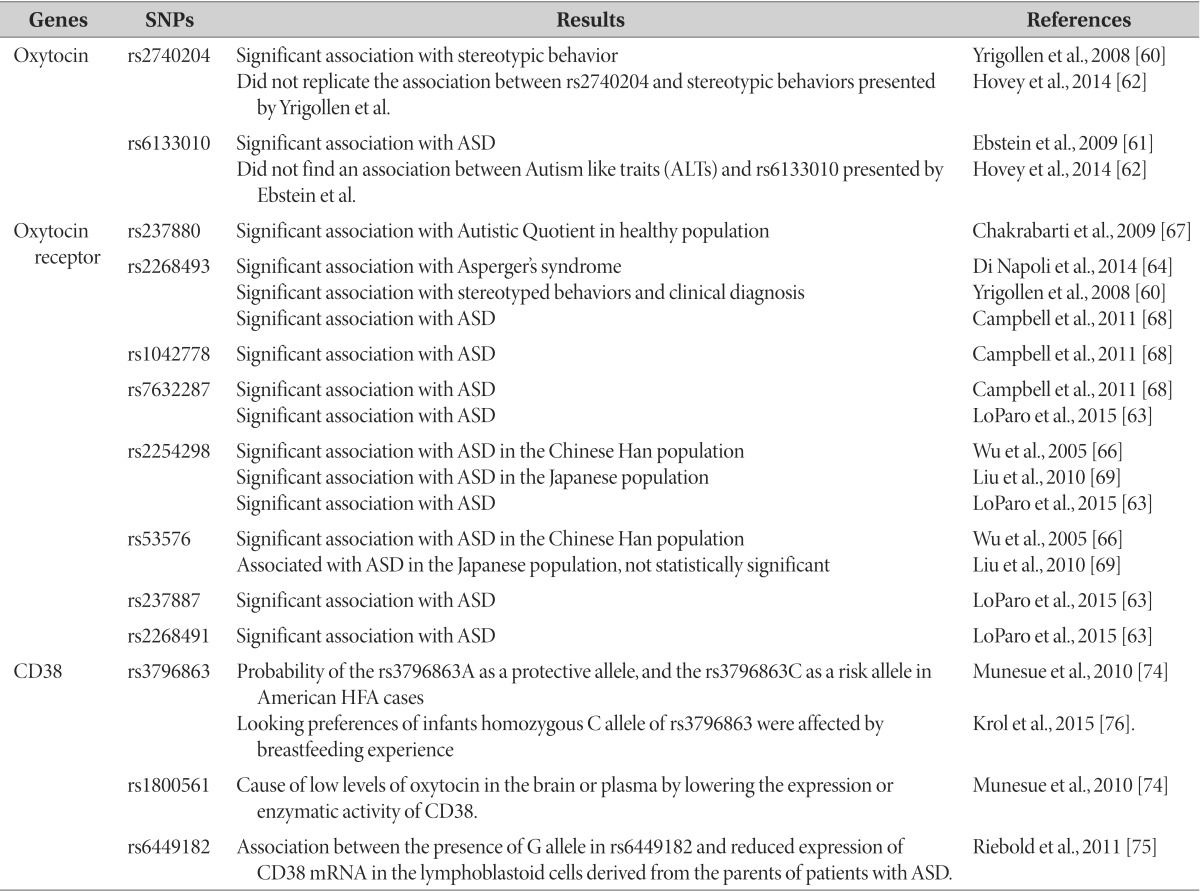
Based on the fact that children with autism have lower average levels of blood oxytocin [57] and higher oxytocin precursor levels [58] in comparison with typically developing age-matched children, early genetic studies were designed to attempt to find genetic evidence for such differences. However, correlation between peripheral oxytocin levels and ASD psychopathology is uncertain [59]. Although single nucleotide polymorphism (SNP) correlation studies on behavioral problems and oxytocin are in progress, results vary across research groups. Yrigollen et al. reported a correlation between rs2740204 of the oxytocin genes and stereotyped behaviors [60], and Ebstein et al. reported a correlation between rs6133010 and ASD [61]. However, Hovey et al. could not replicate such correlations [62].
In addition to blood oxytocin concentration, there is also increasing evidence that oxytocin receptor genotype is strongly associated with ASD, more with social cognition than with ASD diagnosis itself [63] (Table 1). Since the physiological effect of oxytocin is mediated by the oxytocin receptor, many studies have identified the oxytocin receptor as the modifier of social cognition and behavior. Several studies indicated an association between genetic variations in the oxytocin receptor gene and ASD or related phenotypes in the Caucasian population [64,65,66]: a nominal association between SNP rs237880 in the oxytocin receptor gene and autistic traits [67]; a significant association between SNP rs2268493 in the oxytocin receptor gene and Asperger syndrome [64]; an association between rs2268493 and affiliative behavior in ASD [60]. Campbell et al. [68] identified three SNPs (rs2268493, rs1042778, rs7632287) nominally associated with autism. Several studies have indicated linkages in Asian populations: two SNPs (rs2254298, rs53576) in the Chinese Han populations [66] and in the Japanese populations [69]. In a recent meta-analysis, 4 SNPs, rs7632287, rs237887, rs2268491, rs2254298, were correlated with ASD, and there was a significant correlation between the oxytocin receptor gene and ASD in gene-based tests for association [63].
Table 1. Promising genes for investigating the mechanism of autism spectrum disorder.
ASD: autism spectrum disorder; HFA: high functioning autism; SNP: single nucleotide polymorphism.
Methylation of the oxytocin receptor gene, which decreases its expression, has been proposed as one of the etiologies of autism [70]. Postmortem analysis of a separate subgroup of brains of individuals with autism revealed that the oxytocin receptor gene was significantly methylated and oxytocin mRNA was reduced in some samples when compared with that in the control brain samples in the temporal cortex [70]. These findings suggest that the decrease of the oxytocin receptor gene expression is related to some subgroups of ASD. The methylation of the oxytocin receptor gene was reported to increase the activity in the temporal parietal junction, which is known to participate in social perception [71]. In other words, methylation of the oxytocin receptor gene affects brain activity, and thus, may affect the social perception of an individual.
CD38 is a transmembrane antigen that has been studied as a negative prognostic marker for chronic lymphocytic leukemia [72]. CD38 participates in the oxytocin secretion in the brain and affects maternal nurturing and social behavior [73]. Plasma levels of oxytocin are strongly reduced in CD38 knockout mice (CD38-/-mice) and subcutaneous oxytocin injection or lentiviralvector-mediated delivery of human CD38 into the hypothalamus rescued social memory and maternal care in these mice [73]. In an association study on humans in the U.S., rs6449197 and rs3796863, which are SNPs of CD38, were correlated with high functioning autism (Table 1) [74]. ASD probands, with a mutant allele (W140) of rs1800561 SNP (R140W), had significantly lower plasma oxytocin levels than those without the mutant allele [74]. CD38 expression in lymphoblastoid cells derived from patients with ASD was reduced [75]. Transition of rs6449182 SNP was correlated with CD38 mRNA expression levels, which were in turn significantly correlated with social skill, and communication subscores on the Vineland Adaptive Behavior Scales in patients with ASD [75]. According to the attention capacity to emotional information conveyed by the eyes, the attention of human babies to angry and happy eyes varies as a function of exclusive breastfeeding experience and genetic variation in CD38 [76]. Extended duration of breastfeeding resulted in enhanced looking preference to happy eyes and decreased looking preference to angry eyes. While looking preferences of infants with CA/AA genotype were not influenced by breastfeeding exposure, infants who were homozygous for the C allele of rs3796863 were affected by feeding experience [76].
PSYCHOPHARMACOLOGIC BACKGROUND OF SIGNIFICANT AND NEGATIVE HUMAN STUDIES
Application route of oxytocin
In clinical trials with oxytocin administration targeting a diversity of psychiatric disorders (including ASD), the routes of oxytocin administration are oral, intravenous, and intranasal [77] (Table 2). Oral administration is not suitable due to the extensive metabolism of oxytocin by the liver and gastrointestinal tract. Through intravenous administration, only a small amount is able to pass the blood-brain barrier, and there is a latent possibility of uterus contraction side effects in females [77]. Moreover, since it is an invasive method, applications for community treatment are limited. The intranasal route has several advantages, such as bypassing the bloodstream and directly accessing the cerebrospinal fluid (CSF) within 30 min, achieving effective concentrations of neuropeptides in the brain without systemic side effects [78], no requirement for specific skill, and ease of application at home [79].
Table 2. Results of randomized placebo controlled trials with oxytocin in autism spectrum disorder grouped by administration route.
AS: Asperger's syndrome; ASD: autism spectrum disorder; HFA: high functioning autism.
Studies using oxytocin with clinical significance
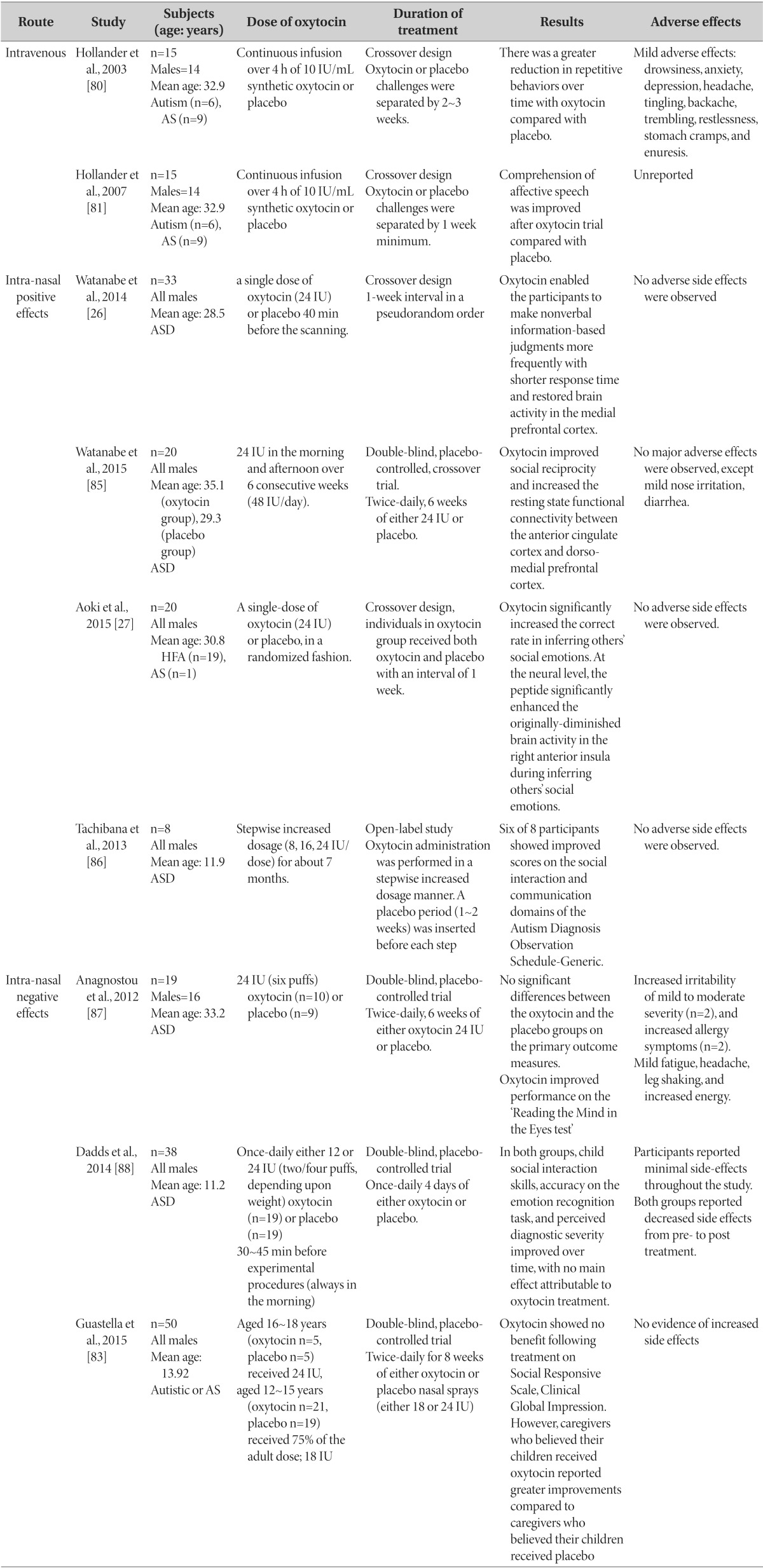
As studies of animal behavior imply that oxytocin is related to one of autism's core features, 'repetitive behavior' [80]. Similar results were replicated in humans. In the first human study on oxytocin infusion effects on autism core behavior, 15 patients with autism and Asperger's syndrome were administered intravenous oxytocin and showed significantly decreased repetitive behavior (Table 2) [80]. In another study by the same authors, intravenous injection of oxytocin for 4 h promoted social learning in patients with ASD [81]. In the first study on adolescent subjects with ASD, 16 participants showed significant improvement in the 'Reading the Mind in the Eyes' task after administration of intranasal oxytocin (24 IU) [82]. Later results from the same research group indicated that, although there was no significant difference between groups, oxytocin nasal spray may improve emotion recognition, in which impairment is one of the core features of ASD [83]. In a study on children with autism and Asperger's syndrome, the subjects were administered intranasal oxytocin twice a day (0.4 IU/kg/dose) for 12 weeks. The results showed improvement in not only repetitive behavior, but also facial recognition and social cognition [84]. In a study on 20 men with ASD, a single-dose of intranasal oxytocin (24 IU) improved the 'deficit in inferring others' social emotion' and restored decreases in right anterior insula activity [27]. In a study on 40 high-functioning men with ASD, a single-dose intranasal administration of oxytocin (24 IU) improved the frequency and response time of nonverbal information-based judgments and also restored activity levels of the medial prefrontal cortex, [26]. To investigate the long-term effect of oxytocin administration, Watanabe et al. [85] performed a randomized controlled trial (RCT), including 6 weeks of intranasal administration of oxytocin (48 IU/day), to 20 high-functioning adult men with ASD. The trial showed improved social reciprocity and enhanced task-independent resting-state functional connectivity between the anterior cingulate cortex and the dorso-medial prefrontal cortex [85]. Unlike previous short-term studies, Tachibana et al. administrated intranasal oxytocin for 7 months to 8 adolescent boys with outcomes presenting improvement in communication and social interaction [86]. Considering that poor social interaction and repetitive behaviors are core symptom domains of autism, the study results imply that oxytocin may be a potential treatment option for ASD.
Studies using oxytocin with negative findings
Sometimes studies fail to reproduce treatment effects of oxytocin reported in previous work. In a study on 19 adult patients with ASD, intranasal oxytocin (24 IU) was administered for 6 weeks, and although social cognition and low order repetitive behaviors (stereotype, self-injury) improved, there was no effect on higher order repetitive behaviors (ritualistic, sameness) [87]. This study is of significant importance as it is the first study on the daily administration of oxytocin. However, its implications may be limited by the small number of subjects and the short duration of administration [87]. In a study on 38 male youth patients, nasal administration (24 or 12 IU) was done for 5 days but results showed no difference from the placebo group in terms of emotion recognition and social interaction skills, among others [88]. The authors suggested that there might be patient subgroups that may not benefit from exogenous oxytocin and that further research is needed. In a RCT of 50 adolescent men with autistic or Asperger's syndrome receiving oxytocin nasal spray (18 or 24 IU) twice-daily for 8 weeks, the oxytocin group did not show improvements in social cognition following treatment as rated by caregivers and clinicians [83]. While the results did not suggest any benefits of oxytocin for autistic symptoms, further research is needed to explore earlier age interventions and package program involving a combination of pharmacological treatment and social-learning exercises.
Safety of oxytocin treatment
There is some evidence that very young animals may show long-lasting negative consequences in social behavior following direct intraperitoneal injection of large doses of oxytocin [89]. Chronic or repeated intranasal oxytocin treatment in humans might also result in undesired counter-regulatory consequences [90]. A 55-year old patient with obsessive compulsive disorder showed clear improvement in symptoms following intranasal oxytocin treatment for 4 weeks, but concurrently developed severe memory impairments [91]. This case lends support for the amnestic properties of the peptide. When administrating intravenous oxytocin for labor induction or abortion, adverse effects such as reflex tachycardia, seizures, headache, memory impairment, hyponatremia, a syndrome of inappropriate antidiuretic hormone secretion, and anaphylaxis have been reported [92]. Large cohort studies have suggested a very small but significant risk for the future development of autism later in life following augmented childbirth with oxytocin [93]. Although side effects such as irritability, nasal congestion, fatigue, and headache were reported in human studies following intranasal oxytocin administration, there were no statistically significant differences from the placebo group [94]. In a recent report on children with ASD administered long-term nasal oxytocin for more than 6 months, six of eight participants reported positive effects on the quality of reciprocal communication and showed excellent compliance with no side effects [86]. According to the review of previous human data, there are no serious adverse effects with short-term application of oxytocin with 18~40 IU [94], but human data on the outcomes following extended treatment, especially in young children with ASD, are required.
OXYTOCIN-RELATED CANDIDATE AGENTS FOR FUTURE ASD RESEARCH
Since the atypical antipsychotics, risperidone and aripiprazole, are the only FDA-approved medications for ASD, research for new therapeutics based on specific mechanisms and pathways involving etiological factors is urgently necessary [95]. Apart from intranasal oxytocin, molecules or pathways related to oxytocin release can be candidates for such novel research. In animal studies, vasopressin increased prosocial behavior [52], and decreased social interaction was observed in arginine vasopressin receptor 1A (V1aR) knockout mice [96]. In a human study [97], blood measures of arginine vasopressin (AVP) concentrations represented CSF AVP activity in human and were used as a predictor of social functioning in children with ASD. Although AVP has received less attention than oxytocin for a possible role in ASD, AVP levels can be used as a biomarker of ASD, and AVP physiology maybe a promising therapeutic target to improve social cognition in individuals with ASD [97].
Several peptides have been identified that affect endogenous oxytocin levels. Each peptide affects oxytocin release through a different mechanism. Orexin is a peptide produced in the lateral hypothalamus [98]. Adrenaline, noradrenaline, serotonin, and dopamine are known to increase oxytocin levels. After preincubation of rat neurohypophyseal cell cultures with orexin, adrenaline-, histamine- and serotonin-induced increases in oxytocin levels were attenuated. These results indicate that changes in oxytocin secretion induced by the monoaminergic system can be directly influenced by the orexin system [98]. The serotonin system is also involved in oxytocin release [99]. Serotonergic fibers and receptors are located in the oxytocinergic supraoptic nucleus and paraventricular nuclei of the hypothalamus. Serotonergic receptor agonists, including 8-hydroxy-2-(dipropylamino) tetralin (8-OH-DPAT), 1-(2,5-dimethoxy 4-iodophenyl)-2-amino propane hydrochloride (DOI), and buspirone, cause increased release of oxytocin [99]. In addition to increasing levels of oxytocin, buspirone can promote prosocial behaviors. Galanin is a neuropeptide involved in feeding behavior, memory, cognition, gut secretion, and motility [100]. Galanin works on the hypothalamo-neurohypophyseal system and has a modulatory role in oxytocin release.
CD38 transcription is highly sensitive to cytokines and vitamins, including all-trans retinoic acid (ATRA), a known inducer of CD38 [75]. In a study on lymphoblastoid cell lines in patients with ASD and their parents, ATRA exhibited an upmodulatory potential on CD38 mRNA [75]. Although there have been almost no followup studies on ATRA and ASD treatment, there is a possibility that substances affecting CD38 expression, such as ATRA, may be potential therapeutic candidates.
Stimulation of melanocortin 4 receptors (MC4R) on supraoptic neurons activates oxytocinergic neurons and induces central, but not peripheral release of oxytocin in mice [99]. A selective MC4R agonist, Ro27-3225, administered to Cntnap2 knockout mouse restores social behavior [101]. Administration of MC4R agonist, melanotan II (MTII), promotes partner preference development and activates hypothalamic oxytocin neurons [102]. These studies suggest that MC4R agonists may be a possible treatment option to improve social function in individuals with ASD.
CONCLUSION
Considering the complex characteristics of ASD, which are a combination of biologic heterogeneity and phenotypic heterogeneity, developing a single form of medication is extremely difficult. Characteristics of ASD, such as early onset, are one of the inherent challenges limiting clinical research. Since oxytocin is responsible for not only maternal behavior but also core symptom domains of ASD, such as social interaction and repetitive behaviors, association with the pathogenesis of ASD is highly possible. Various animal and human studies are in progress regarding the potential disruption of oxytocin function or secretion leading to the etiology of ASD. Along with studies on oxytocin-related genes, numerous research studies on the oxytocin receptor gene and CD38 gene are being conducted. Through clinical trials applying oxytocin in patients with ASD, future clinical applications are promising. Various studies on novel substances and pathways that affect oxytocin secretion and related gene translation are also in progress. For oxytocin treatment in patients with ASD, standardization of administration route, dosage, and duration of treatment need to be optimized in longterm, large sample-size studies. Even though there are evidences that support the effect of oxytocin on promoting prosocial behaviors, but some results suggest negative findings and also oxytocin is not effective in all core symptom domains of ASD. Therefore, further research about which subgroup is responsive to oxytocin should be carried out.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A120029).
References
- 1.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 2.American Psychiatric Association; Task Force on Nomenclature and Statistics; American Psychiatric Association; Committee on Nomenclature and Statistics. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington DC: American Psychiatric Association; 1980. [Google Scholar]
- 3.American Psychiatric Association; American Psychiatric Association; DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 4.Hu VW. Frontiers in autism research: new horizons for diagnosis and treatment. Hackensack, NJ: World Scientific Publishing Company; 2014. [Google Scholar]
- 5.Preti A, Melis M, Siddi S, Vellante M, Doneddu G, Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J Child Adolesc Psychopharmacol. 2014;24:54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- 6.Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45:185–192. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 7.Giovagnoli G, Postorino V, Fatta LM, Sanges V, De Peppo L, Vassena L, Rose PD, Vicari S, Mazzone L. Behavioral and emotional profile and parental stress in preschool children with autism spectrum disorder. Res Dev Disabil. 2015;45-46:411–421. doi: 10.1016/j.ridd.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Sahley TL, Panksepp J. Brain opioids and autism: an updated analysis of possible linkages. J Autism Dev Disord. 1987;17:201–216. doi: 10.1007/BF01495056. [DOI] [PubMed] [Google Scholar]
- 9.Campbell M, Anderson LT, Small AM, Adams P, Gonzalez NM, Ernst M. Naltrexone in autistic children: behavioral symptoms and attentional learning. J Am Acad Child Adolesc Psychiatry. 1993;32:1283–1291. doi: 10.1097/00004583-199311000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Elchaar GM, Maisch NM, Augusto LM, Wehring HJ. Efficacy and safety of naltrexone use in pediatric patients with autistic disorder. Ann Pharmacother. 2006;40:1086–1095. doi: 10.1345/aph.1G499. [DOI] [PubMed] [Google Scholar]
- 11.Willemsen-Swinkels SH, Buitelaar JK, Nijhof GJ, van England H. Failure of naltrexone hydrochloride to reduce self-injurious and autistic behavior in mentally retarded adults. Double-blind placebo-controlled studies. Arch Gen Psychiatry. 1995;52:766–773. doi: 10.1001/archpsyc.1995.03950210060011. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HM, Kolmen BK, Gonzaga AM. Naltrexone and communication skills in young children with autism. J Am Acad Child Adolesc Psychiatry. 1999;38:587–593. doi: 10.1097/00004583-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Symons FJ, Thompson A, Rodriguez MC. Self-injurious behavior and the efficacy of naltrexone treatment: a quantitative synthesis. Ment Retard Dev Disabil Res Rev. 2004;10:193–200. doi: 10.1002/mrdd.20031. [DOI] [PubMed] [Google Scholar]
- 14.Roy A, Roy M, Deb S, Unwin G, Roy A. Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: a systematic review. J Intellect Disabil Res. 2015;59:293–306. doi: 10.1111/jir.12122. [DOI] [PubMed] [Google Scholar]
- 15.Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J Assoc Acad Minor Phys. 1998;9:9–15. [PubMed] [Google Scholar]
- 16.Toda Y, Mori K, Hashimoto T, Miyazaki M, Nozaki S, Watanabe Y, Kuroda Y, Kagami S. Administration of secretin for autism alters dopamine metabolism in the central nervous system. Brain Dev. 2006;28:99–103. doi: 10.1016/j.braindev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012;4:CD003495. doi: 10.1002/14651858.CD003495.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 20.Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse L, Fein D, Modahl C. Neurofunctional mechanisms in autism. Psychol Rev. 1996;103:457–489. doi: 10.1037/0033-295x.103.3.457. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 23.Insel TR, O'Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 24.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 25.Shao Y, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA, Bass MP, McClain C, von Wendt L, Vance JM, Abramson RH, Wright HH, Ashley-Koch A, Gilbert JR, DeLong RG, Cuccaro ML, Pericak-Vance MA. Genomic screen and follow-up analysis for autistic disorder. Am J Med Genet. 2002;114:99–105. doi: 10.1002/ajmg.10153. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Aoki Y, Takao H, Kawakubo Y, Kamio Y, Kato N, Miyashita Y, Kasai K, Yamasue H. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry. 2014;71:166–175. doi: 10.1001/jamapsychiatry.2013.3181. [DOI] [PubMed] [Google Scholar]
- 27.Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain. 2014;137:3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- 28.Young LJ, Barrett CE. Neuroscience. Can oxytocin treat autism? Science. 2015;347:825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 31.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 32.Christensson K, Nilsson BA, Stock S, Matthiesen AS, Uvnäs-Moberg K. Effect of nipple stimulation on uterine activity and on plasma levels of oxytocin in full term, healthy, pregnant women. Acta Obstet Gynecol Scand. 1989;68:205–210. doi: 10.3109/00016348909020990. [DOI] [PubMed] [Google Scholar]
- 33.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy MM, Altemus M. Central nervous system actions of oxytocin and modulation of behavior in humans. Mol Med Today. 1997;3:269–275. doi: 10.1016/S1357-4310(97)01058-7. [DOI] [PubMed] [Google Scholar]
- 35.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 36.Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, O'Connor MF, Gündel H. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2009;34:1417–1422. doi: 10.1016/j.psyneuen.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav Neurosci. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 39.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psychol. 1974;86:233–246. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- 40.Yu GZ, Kaba H, Okutani F, Takahashi S, Higuchi T, Seto K. The action of oxytocin originating in the hypothalamic paraventricular nucleus on mitral and granule cells in the rat main olfactory bulb. Neuroscience. 1996;72:1073–1082. doi: 10.1016/0306-4522(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mind-reading" in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209:225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- 49.Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- 50.Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–184. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 58.Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- 59.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 60.Yrigollen CM, Han SS, Kochetkova A, Babitz T, Chang JT, Volkmar FR, Leckman JF, Grigorenko EL. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63:911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Riebold M, Salomon S, Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Ann N Y Acad Sci. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- 62.Hovey D, Zettergren A, Jonsson L, Melke J, Anckarsäter H, Lichtenstein P, Westberg L. Associations between oxytocin-related genes and autistic-like traits. Soc Neurosci. 2014;9:378–386. doi: 10.1080/17470919.2014.897995. [DOI] [PubMed] [Google Scholar]
- 63.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 64.Di Napoli A, Warrier V, Baron-Cohen S, Chakrabarti B. Genetic variation in the oxytocin receptor (OXTR) gene is associated with Asperger Syndrome. Mol Autism. 2014;5:48. doi: 10.1186/2040-2392-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, Banerjee-Basu S, Baron-Cohen S. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2:157–177. doi: 10.1002/aur.80. [DOI] [PubMed] [Google Scholar]
- 68.Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA, Levitt P. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord. 2011;3:101–112. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, Nishida H, Hashimoto O, Nakagami R, Tochigi M, Umekage T, Kano Y, Miyagawa T, Kato N, Tokunaga K, Sasaki T. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- 70.Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci. 2012;6:280. doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deaglio S, Aydin S, Vaisitti T, Bergui L, Malavasi F. CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med. 2008;14:210–218. doi: 10.1016/j.molmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 74.Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, Asaka T, Liu HX, Jin D, Koizumi K, Islam MS, Huang JJ, Ma WJ, Kim UH, Kim SJ, Park K, Kim D, Kikuchi M, Ono Y, Nakatani H, Suda S, Miyachi T, Hirai H, Salmina A, Pichugina YA, Soumarokov AA, Takei N, Mori N, Tsujii M, Sugiyama T, Yagi K, Yamagishi M, Sasaki T, Yamasue H, Kato N, Hashimoto R, Taniike M, Hayashi Y, Hamada J, Suzuki S, Ooi A, Noda M, Kamiyama Y, Kido MA, Lopatina O, Hashii M, Amina S, Malavasi F, Huang EJ, Zhang J, Shimizu N, Yoshikawa T, Matsushima A, Minabe Y, Higashida H. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Riebold M, Mankuta D, Lerer E, Israel S, Zhong S, Nemanov L, Monakhov MV, Levi S, Yirmiya N, Yaari M, Malavasi F, Ebstein RP. All-trans retinoic acid upregulates reduced CD38 transcription in lymphoblastoid cell lines from Autism spectrum disorder. Mol Med. 2011;17:799–806. doi: 10.2119/molmed.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krol KM, Monakhov M, Lai PS, Ebstein RP, Grossmann T. Genetic variation in CD38 and breastfeeding experience interact to impact infants' attention to social eye cues. Proc Natl Acad Sci U S A. 2015;112:E5434–E5442. doi: 10.1073/pnas.1506352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamasue H. Promising evidence and remaining issues regarding the clinical application of oxytocin in autism spectrum disorders. Psychiatry Clin Neurosci. 2015 doi: 10.1111/pcn.12364. (in press) [DOI] [PubMed] [Google Scholar]
- 78.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 79.Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 81.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 82.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 83.Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- 84.Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, Jacob S. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 86.Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, Oishi M, Kimura T, Onaka T, Ozono K, Taniike M. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- 87.Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 89.Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.004. (in press) [DOI] [PubMed] [Google Scholar]
- 91.Ansseau M, Legros JJ, Mormont C, Cerfontaine JL, Papart P, Geenen V, Adam F, Franck G. Intranasal oxytocin in obsessive-compulsive disorder. Psychoneuroendocrinology. 1987;12:231–236. doi: 10.1016/0306-4530(87)90009-6. [DOI] [PubMed] [Google Scholar]
- 92.Cabestrero D, Pérez-Paredes C, Fernández-Cid R, Arribas MA. Bronchospasm and laryngeal stridor as an adverse effect of oxytocin treatment. Crit Care. 2003;7:392. doi: 10.1186/cc2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases. JAMA Pediatr. 2013;167:959–966. doi: 10.1001/jamapediatrics.2013.2904. [DOI] [PubMed] [Google Scholar]
- 94.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 95.Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, Choi DH, Lee J, Han SH, Yang SM, Cheong JH, Shin CY, Bahn GH. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS One. 2014;9:e104927. doi: 10.1371/journal.pone.0104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 97.Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, Jackson LP, Sumiyoshi RD, Howerton CL, Hannah SL, Partap S, Phillips JM, Hardan AY, Parker KJ. Arginine vasopressin is a blood-based biomarker of social functioning in children with autism. PLoS One. 2015;10:e0132224. doi: 10.1371/journal.pone.0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ocskó T, Gálfi M, Radács M, Molnár Z, Kis GK, Rákosi K, Molnár AH, László F, László FA, Varga C. Effects of orexin-monoaminergic interactions on oxytocin secretion in rat neurohypophyseal cell cultures. Regul Pept. 2012;175:43–48. doi: 10.1016/j.regpep.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 99.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Izdebska K, Ciosek J. Galanin influences on vasopressin and oxytocin release: in vitro studies. Neuropeptides. 2010;44:341–348. doi: 10.1016/j.npep.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ. Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology. 2015;40:1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]