Abstract
Serpentine receptors with G-protein coupled receptor like seven transmembrane (7 TM) topology are identified in Plasmodium. A class of 7 TM receptors known as purinergic receptors binds to purines such as ADP, ATP and UTP and mediates important physiological functions including regulation of calcium signaling. Here we performed in silico analysis of Plasmodium falciparum serpentine receptors and found that one of the P. falciparum serpentine receptors, PfSR12 possess nucleotide binding consensus P-loop sequence in addition to seven transmembrane domains. The presence of conserved seven transmembrane domains and a consensus nucleotide binding sequence (P-loop) suggest that PfSR12 is a putative purinergic receptor. On further analysis using docking programmes we found four active binding residues Asn149, Lys150, Asn151 and Gly152 in P-loop of PfSR12, interact with ATP. This work gives insights into the interactions between putative purinergic receptor PfSR12 and its ligand ATP which can be explored in structure based drug designing against malaria.
Keywords: Serpentine receptor, ATP, Purinergic receptor, Malaria, Plasmodium falciparum
Introduction
Malaria is a major health problem responsible for millions of deaths worldwide. Plasmodium falciparum, the most virulent species of Plasmodium, causes lethal form of malaria in humans. Increasing drug resistance in malaria parasite and lack of effective vaccine advocates the need to explore new drugs and vaccine targets to eliminate the disease. Over past few years, there are several reports implicating the role of secondary messengers like calcium, cyclic adenosine monophosphate (cAMP) and their downstream effectors (Merckx et al. 2008; Ono et al. 2008; Singh et al. 2010) at different stages of life cycle of Plasmodium. Recent studies suggested the role of intracellular calcium in the regulation of apical organelles secretion at the time of invasion in P. falciparum (Singh et al. 2010). However, the upstream signaling that leads to increase in calcium levels in parasite remains to be elucidated. Thus deciphering novel signaling pathways in Plasmodium is an important step towards developing new strategies to fight against malaria.
Nucleosides and nucleotides like adenosine, Adenosine di-phosphate (ADP), Adenosine tri-phosphate (ATP) and guanosine tri-phosphate (GTP) are well studied signaling molecules that bind to purinergic receptors and mediate several biological processes in eukaryotes (Burnstock and Verkhratsky 2009). In response to various stimuli, erythrocytes are known to release ATP in adequate amounts which can activate purinergic receptors (Sprague et al. 2001). The role of extracellular ATP in erythrocyte invasion by Plasmodium via purinergic receptors has been postulated recently (Levano-Garcia et al. 2010). Inhibitors of purinergic receptors impair in vitro growth of P. falciparum further supporting the functional significance of purinergic signaling in blood stages of Plasmodium (Tanneur et al. 2006). The activation of purinergic receptors results in rise of cytosolic calcium and cAMP accumulation in different organisms (Burnstock 2009). Together, these studies suggest the role of purinergic receptors in regulation of intracellular calcium and cAMP levels that affects growth of Plasmodium.
Purinergic receptors are divided into two major classes: P1 receptors activated by adenosine and P2 receptors activated by ATP and other nucleotides. Further P2 receptors are classified as: ion gated P2X receptors and G-protein coupled P2Y receptors (Burnstock 2004, 2007). P2Y families of receptors possess seven tramsmembrane topology (heptahelical) with the NH2 terminus facing the extracellular environment, and the COOH terminus on the cytoplasmic face of the plasma membrane (Di Virgilio et al. 2001). These heptahelical transmembrane proteins signal through heterotrimeric G proteins which either activate phopholipase C (PLC) or affects adenyl cyclase (AC) (Di Virgilio et al. 2001). However, it has also been shown that the heptahelical receptors can also mediate signaling independent of G proteins (Hall et al. 1999). In response to binding of a nucleotide, purinergic receptors P1 or P2 are capable of transducing signals and can regulate many physiological functions in eukaryotes via activating downstream calcium signaling pathways (Vassort 2001). The binding of nucleotides i.e. ATP with Walker A motif or P-loop has been reported in various proteins like ATP synthase, myosin, kinases (Ramakrishnan et al. 2002).
In Plasmodium, four heptahelical or serpentine receptors (SRs) namely SR1, SR10, SR12 and SR25 (Madeira et al. 2008) are reported indicating the presence of membrane receptor signaling in Plasmodium. Out of the four known serpentine receptors, by computational analysis we found that P. falciparum serpentine receptor 12 (PfSR12) contains consensus P-loop motif which could act as binding pocket for nucleotides like ATP. The presence of consensus nucleotide binding sequence (P-loop) in PfSR12 suggests its role as purinergic receptor which might regulate downstream calcium signaling in parasite during the course of infection. Computational structural analysis of this receptor and mapping its putative ligands will provide insights in understanding of receptor signaling in malaria parasite.
In the present study, we performed in silico characterization of serpentine receptor 12 (SR12) of P. falciparum and mapped consensus nucleotide binding motif (P-loop) in its protein sequence. Our study strengthens the presence of purinergic signaling in Plasmodium and explores the possibility of designing new therapeutic targets for treatment of malaria.
Methods and materials
The sequences of Plasmodium serpentine receptors SR1, SR10, SR12 and SR25 were retrieved from PlasmoDB database (PF3D7_1131100,PF3D7_1215900, PF3D7_0422800 and PF3D7_0713400 respectively) and the chemical structure of the Adenosine triphosphate (ATP) was obtained from online PuBChem Database (CID: 5957). The pdb format file of ATP was obtained from http://www.chm.bris.ac.uk/motm/atp/atp_mol.htm.
Bioinformatics studies
The protein sequences encoding Plasmodium falciparum serpentine receptors (PfSRs) were analyzed using normal SMART (Simple Modular Architecture Research Tool) programme (Letunic et al. 2004) and PROSITE-EXPASY (Sigrist et al. 2010) to search for a conserved domain/motif. The domain architecture of PfSR1, PfSR10 and PfSR25 serpentine receptors highlighting signal sequence and transmembrane domains was constructed using MYDOMAIN tool in PROSITE-EXPASY while all three have the canonical 7-transmembrane domains, only PfSR12 receptor showed the consensus sequence of P-loop motif in addition to other domains (Saraste et al. 1990; Walker et al. 1982). PSIPRED server (McGuffin et al. 2000) was used for the prediction of secondary structure of serpentine receptor PfSR12. The hydropathic nature of serpentine receptor PfSR12 and validation of seven transmembrane topology was done using Kyte-Doolittle hydropathy plots (Kyte and Doolittle 1982) and TMHMM server (Krogh et al. 2001).
3D Structure of P. falciparum serpentine receptors
Tertiary protein structure model of PfSR12 was generated using I-TASSER. I-TASSER (Iterative Threading ASSEmbly Refinement) is a bioinformatics tool for generating three dimensional proteins structure and function prediction (Zhang 2009). Out of the five models generated by online server, the final model was selected on the basis of highest C-score and TM value. The stability of the final selected structure was analyzed by Ramachandran plot (Ramachandran et al. 1963) using online service RAMAPAGE and validated by PROCHECK server (Laskowski et al. 1996).
Docking study
To gain better insights into the interaction of ATP with P-loop residues of PfSR12, we performed in silico docking using automated docking program AutoDock (v.4.0) (Goodsell et al. 1996; Morris et al. 2009). The ligand and receptor pdb files were prepared for docking by addition of hydrogen atoms and computing charges. Following the ligand and receptor preparation Auto-Grid module was used for grid map calculations. The grid with a volume 32 × 40 × 36 Å with a spacing of 0.375 Å centered on the receptor binding site. Then the docking calculations were performed using the genetic algorithm (GA) procedure with default parameters.
Results and discussion
Domain organization of serpentine receptor SR12
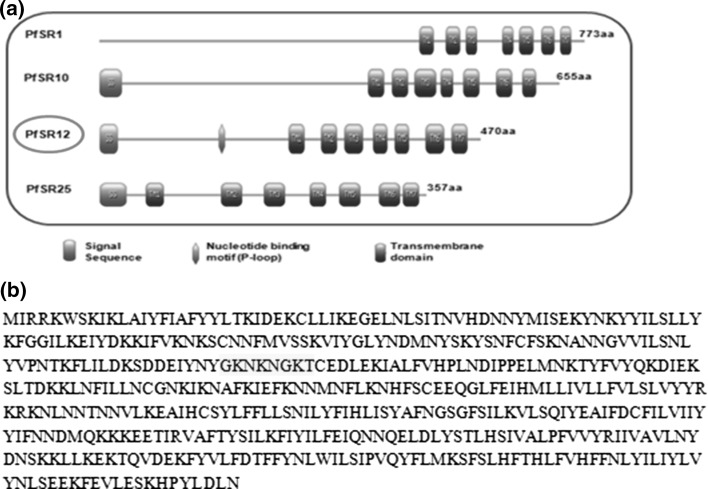
We analyzed the domain architecture of putative serpentine receptors present in PlasmoDB database (Fig. 1a). All serpentine receptors contain canonical 7-transmembrane domains at the C-terminus however one of these receptors, PfSR12 contains P-loop (Walker A) motif at the N-terminus that is characteristic of nucleotide binding proteins (Fig. 1b). P-loop is a phosphate binding loop having [GXXXXGKT/S] consensus sequence and is present in most of the nucleotide binding proteins like kinases, phosphatases (Saraste et al. 1990; Walker et al. 1982). SMART programme also predicted Rhodopsin like GPCR domain at C-terminus of PfSR12 protein (data not shown) strongly suggesting its function as GPCR like purinergic receptor in Plasmodium.
Fig. 1.
Schematic representation of P. falciparum serpentine receptors (PfSRs): a Domain organization of serpentine receptors representing conserved seven transmembrane domains (TM; blue) and signal peptide (SS; orange). P. falciparum serpentine receptor 12 (PfSR12) (highlighted in red) also shows putative nucleotide binding P-loop motif (green). b Deduced amino acid sequence of PfSR12 (PF3D7_0422800) with P-loop sequence: GKNKNGKT (highlighted in yellow). (Color figure online)
Secondary structure and topology of P. falciparum serpentine receptor 12 (PfSR12)
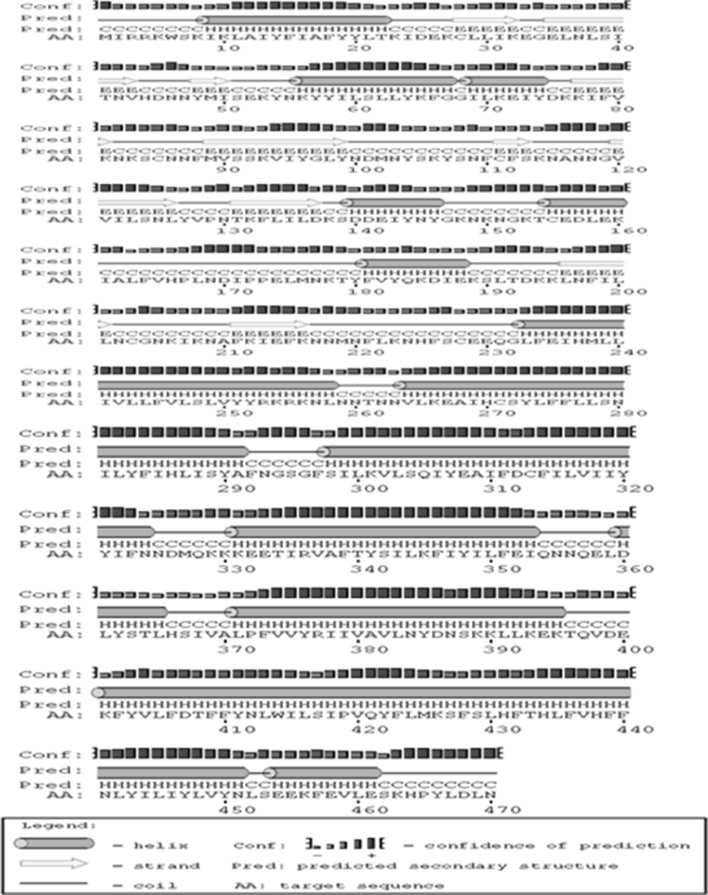
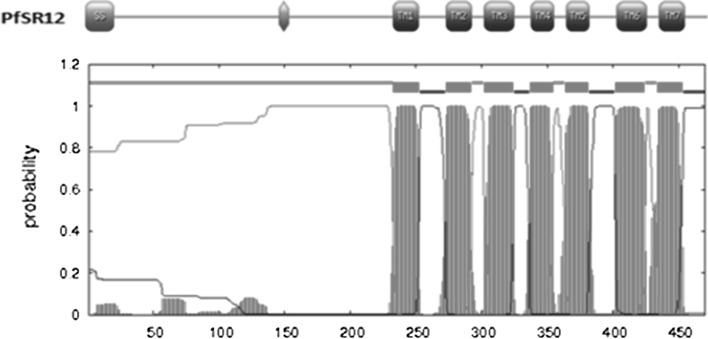
The secondary structure of PfSR12 indicated dominance of helix with occurrence of about 39 % helices and only 16 % beta sheets as shown in Fig. 2. Seven transmembrane protein topology of PfSR12 was confirmed by TMHMM 2.0 prediction program (Fig. 3). It predicts membrane protein topology based on a hidden Markov model with high accuracy (Krogh et al. 2001). Further the presence of hydrophobic transmembrane regions in PfSR12 was validated using Kyte-Doolittle scale where the regions above 1.6 value at window size = 19 are considered as transmembrane regions (Kyte and Doolittle 1982) (data not shown).
Fig. 2.
Secondary structure prediction of P. falciparum serpentine receptor 12 (PfSR12) by PSIPERD server: Pink, yellow and black colors represent helix, sheets and coils respectively. (Color figure online)
Fig. 3.
Transmembrane topology prediction of P. falciparum serpentine receptor 12 (PfSR12) by TMHMM v.2.0: The probabilities of transmembrane regions of P. falciparum serpentine receptor 12 (PfSR12) as predicted by TMHMM online server. Red, blue, magenta lines represent transmembrane, inside and outside regions respectively. (Color figure online)
3D Modeling and structure validation
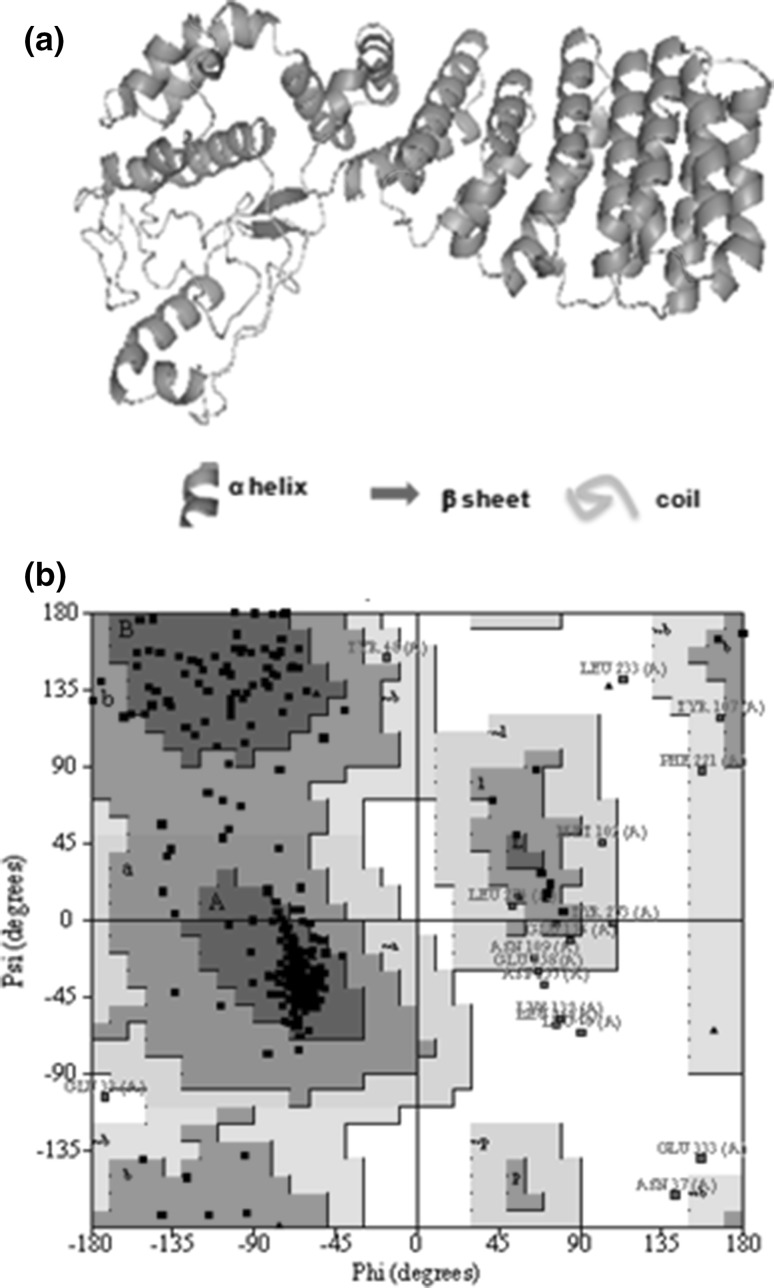
The quality of predicted models by I-TASSER can be assessed by highest C-score (confidence score) and TM score >0.5 (Zhang 2009). On the basis of these parameters, we selected the first model with C-score of −1.59 and TM score 0.52 ± 0.15 (Fig. 4a), which was further used for docking methods. The stereo chemical validation of predicted model of PfSR12 was performed by PROCHECK analysis. Ramachandran plot shows that a total of 96.2 % residues fall in the most favorable region and in additional allowed region (Fig. 4b). The plot also indicates 1.8 % residues are in generously allowed regions and only 2 % residues are in disallowed region of plot. Together these statistic analyses confirmed that the quality of model is acceptable and reliable.
Fig. 4.
Molecular modeling and Structure validation: a 3D structure of P. falciparum serpentine receptor 12 (PfSR12): Ribbon molecular model generated by providing inputs of amino acid sequence of P. falciparum serpentine receptor 12 (PfSR12) in I-TASSER program. b Ramachandran plot analysis of P. falciparum serpentine receptor 12 (PfSR12): The plot statistics of 3D model of PfSR12 was calculated with the PROCHECK server
Molecular docking
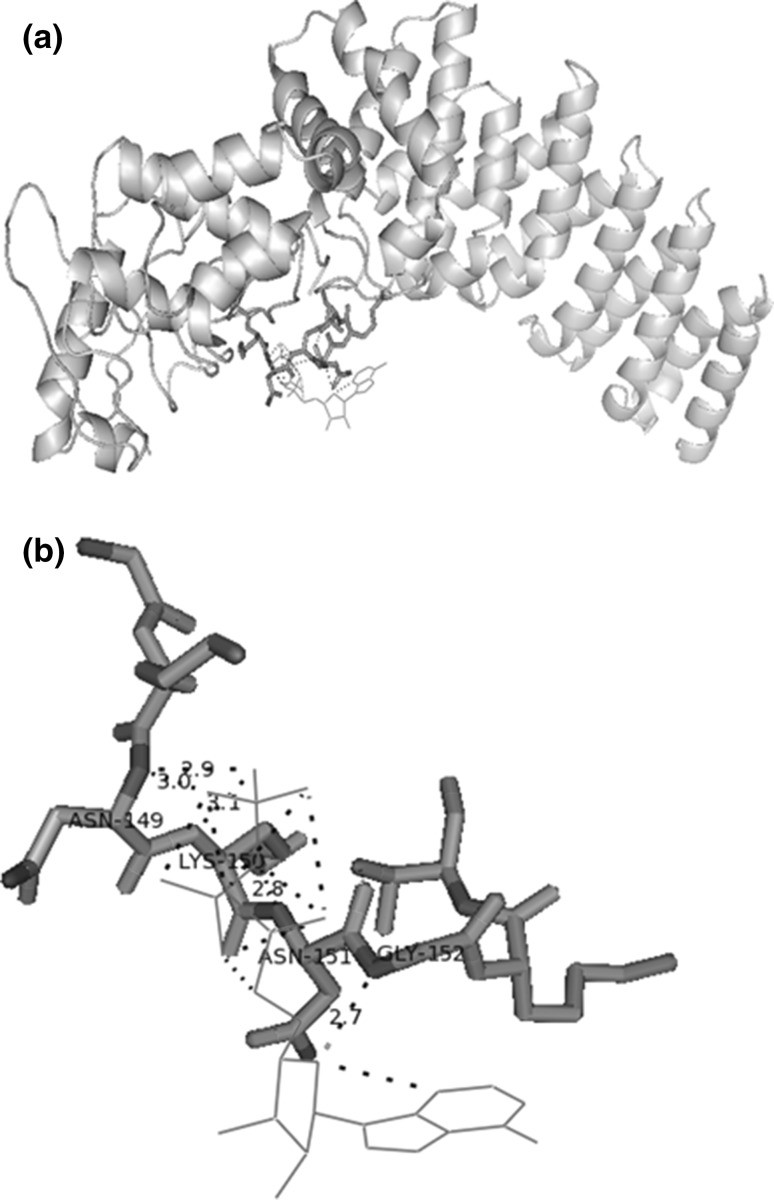
Docking studies using automated AutoDock programme (v.4.0) showed the close proximity of the nucleotide binding site residues in PfSR12 and ATP ligand (Fig. 5a). We found that four residues of P-loop of PfSR12 i.e. Asn149, Lys150, Asn151 and Gly152 are important for binding to ATP. Of these binding residues, Lys150, Asn151 and Gly152 are conserved among other Plasmodium sp. suggestive of the presence of conserved ATP signaling via serpentine receptors in Plasmodium. These results were also confirmed using Z-Dock server online (Chen et al. 2003; Pierce et al. 2014) (data not shown). The identification of ATP binding site could be exploited for development of novel synthetic compounds.
Fig. 5.
Molecular Docking of P. falciparum serpentine receptor 12 (PfSR12) with ATP: a Binding of P-loop residues of PfSR12 (stick structure, yellow and blue) with ATP (line structure, red) b Interaction of P-loop residues; Asn149, Lys150, Asn151 and Gly152 with ATP (red) via polar contacts (Hydrogen bonds). (Color figure online)
Conclusions
Purinergic signaling is very well studied in different systems and it has been exploited as target for chemotherapeutics against various diseases. The present study identifies the P. falciparum serpentine receptor 12 (PfSR12) with 7 TM topology and characteristic P-loop sequence as putative purinergic receptor in Plasmodium. The identification of purinergic receptor PfSR12 enables the possibility of ATP signaling and its role in the growth of malaria parasite. The characterization of ATP binding sites in PfSR12 opens new avenues for development and designing of novel drugs against malaria.
Acknowledgments
Authors would like to thank Shiv Nadar University, Uttar Pradesh for providing the resources to conduct these studies.SS is a recipient of the IYBA Award from Department of Biotechnology (DBT). Sonal Gupta is supported by UGC postdoctoral fellowship. A special thanks to Ms. Chhaya Dhiman,National Institute of Immunology, New Delhi for helping with AutoDock based modeling. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42:3–8. doi: 10.1590/S0100-879X2008005000037. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Li L, Weng Z. ZDOCK: an initial-stage protein-docking algorithm. Proteins. 2003;52:80–87. doi: 10.1002/prot.10389. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit. 1996;9:1–5. doi: 10.1002/(SICI)1099-1352(199601)9:1<1::AID-JMR241>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, et al. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, et al. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Letunic I, et al. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 2004;32:D142–D144. doi: 10.1093/nar/gkh088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levano-Garcia J, et al. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010;6:365–372. doi: 10.1007/s11302-010-9202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira L, et al. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS One. 2008;3:e1889. doi: 10.1371/journal.pone.0001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Merckx A, et al. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathog. 2008;4:e19. doi: 10.1371/journal.ppat.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, et al. Adenylyl cyclase alpha and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog. 2008;4:e1000008. doi: 10.1371/journal.ppat.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BG et al. (2014) ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 30:1771–1773 [DOI] [PMC free article] [PubMed]
- Ramachandran GN, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963;7:95–99. doi: 10.1016/S0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C, Dani VS, Ramasarma T. A conformational analysis of Walker motif A [GXXXXGKT (S)] in nucleotide-binding and other proteins. Protein Eng. 2002;15:783–798. doi: 10.1093/protein/15.10.783. [DOI] [PubMed] [Google Scholar]
- Saraste M, Sibbald PR, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-F. [DOI] [PubMed] [Google Scholar]
- Sigrist CJ, et al. PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res. 2010;38:D161–D166. doi: 10.1093/nar/gkp885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, et al. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, et al. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- Tanneur V, et al. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006;20:133–135. doi: 10.1096/fj.04-3371fje. [DOI] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Walker JE, et al. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER: fully automated protein structure prediction in CASP8. Proteins. 2009;77(Suppl 9):100–113. doi: 10.1002/prot.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]