Abstract
Plasmodium falciparum perforin like proteins (PfPLPs) are an important arsenal for the entry and exit of malaria parasites. These proteins bind and oligomerize on the membrane in calcium dependent manner and form an open pore. The calcium dependent pore forming activity of PLPs is usually conferred by their C2 like C-terminal domain. Here, we have tried to elucidate the calcium binding residues in the C-terminal domain of PfPLP1, a member of P. falciparum PLPs, playing a crucial role in calcium dependent egress of blood stage parasites. Through our in silico study, we have found that the C-terminal domain of all PfPLPs is rich in β-pleated sheets and is structurally similar to C2 domain of human perforin. Furthermore, homology search based on 3-D structure of PfPLP1 confirmed that it is structurally homologous to the calcium binding C2 domain of many proteins. On further elucidation of the calcium-binding pocket of the C2 like domain of PfPLP1 showed that it binds to two calcium molecules. The calcium-binding pocket could be a target of novel chemotherapeutics for studying functional role of PfPLPs in parasite biology as well as for limiting blood stage growth of malaria parasite.
Keywords: Perforin, Plasmodium, Malaria, Calcium, Perforin like protein (PLP), C2 domain
Introduction
Malaria, a parasitic killer, causes more than 1 million human deaths each year. It is caused by five Plasmodium spp., P. falciparum being the deadliest one. The life cycle of Plasmodium is divided into two hosts: Mosquito or primary host where the sexual reproduction occurs and human beings or secondary hosts where asexual reproduction takes place. All the pathophysiological symptoms of malaria are attributed to the blood stage infection of malaria parasite, where P. falciparum merozoites invades human RBCs, multiply within them and exit out to re-invade fresh RBCs (Cowman et al. 2012). Being important for continuation of parasite life cycle, these steps are usually targeted for therapeutic interventions.
Ca2+ plays a pivotal role in the physiology of malaria parasite (Agarwal et al. 2013; Garg et al. 2013; Singh et al. 2010). It regulates invasion and egress of malaria parasites through various molecular players, perforin like proteins (PLPs) being one of them (Agarwal et al. 2013; Ecker et al. 2007; Garg et al. 2013; Ishino et al. 2005; Kaiser et al. 2004; Kadota et al. 2004; Singh et al. 2010; Wirth et al. 2014). These proteins help in transversal of malaria parasite from one cell to the other in liver and mosquito stages (Ecker et al. 2007; Ishino et al. 2005; Kadota et al. 2004). Recently, they are shown to play a crucial role in the egress of malaria parasites in the blood stage and gametocyte stages (Garg et al. 2013; Wirth et al. 2014). Being proteins of multistage functional relevance, these are usually an interest of the researchers to develop them as a target for intervention of parasite growth at various stages of life cycle.
Plasmodium falciparum PLPs (PfPLPs) are multi domain proteins with an N-terminal signal sequence, a central MACPF domain and a C-terminal domain (Garg et al. 2013). PfPLP1 and PfPLP2 are expressed in blood stage and exhibit calcium dependent lytic activity dependent activity (Garg et al. 2013; Wirth et al. 2014). The lytic activity of these PfPLPs is due to a structurally conserved, pore forming central MACPF domain. However, the domain of PfPLPs that confers calcium dependence to their lytic activity was not elucidated. In this work, we tried to elucidate the calcium-binding domain of PfPLP1, a PLP playing a crucial role in egress of merozoites from host RBC. We showed that the C-terminal domain of PfPLP1 is rich in β-pleated sheets and shares structural homology with C2 domains of various proteins such as human perforin. The C2 domains of various proteins are well characterized as calcium sensing molecules and provide calcium dependent lipid binding (Davletov and Sudhof 1993). By extensive bio-informatic analyses, we found the calcium binding residues of C-terminal domain of PfPLP1 which can be further characterized biochemically.
Results
In silico structure prediction of C-terminal of PfPLPs
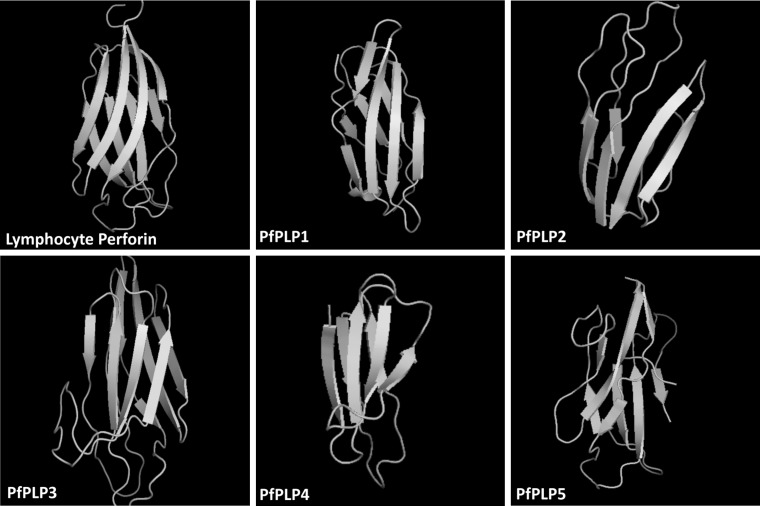
To elucidate the role of c-terminal domain of PfPLPs, we tried to find out their sequence homology with known calcium binding proteins. Since, the sequence based homology of this domain was almost nil, we analysed their homology based on their 3-D structure. 3-D structures of PfPLPs were found to be rich in β-pleated sheets, organized very similarly to C2 domain of human perforin (Fig. 1). The TM-score of structural similarity of PfPLPs with human perforin was found to be more than 0.5 suggestive of similar folds in these structures.
Fig. 1.
Tertiary structure conservation of C-terminal domain of P. falciparum perforin like proteins (PfPLPs) and lymphocyte perforin. C-termini of PfPLPs are rich in β-pleated sheets as found in lymphocyte perforin
Structural homology of PfPLP1 with known C2 domain containing proteins
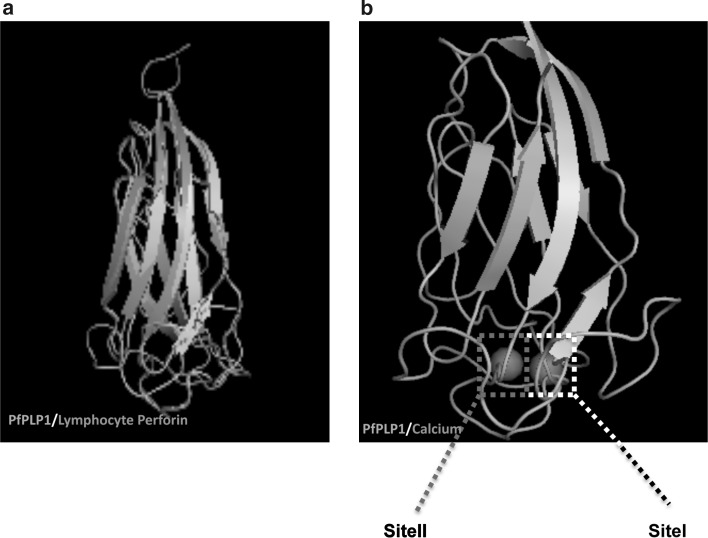
PfPLP1 is one of the most important molecule for blood stage egress and is best characterized for calcium dependent membrane binding. We analyzed its structural homology with known C2 domain containing proteins to understand the structural basis for its calcium interaction. Using TM-align algorithm we found PfPLP1 shares significant structural homology with known C2 domain containing proteins, TM-Score being more than 0.5, as shown in Table 1. Structural alignment of PfPLP1 with lymphocyte perforin showed structures exactly overlaying each other (Fig. 2a). Based on the structural similarity of PfPLP1 with known C2 domain containing proteins, we tried to identify calcium binding site in C-terminal domain of PfPLP1. We found two calcium atoms bound to lower part of the C-terminal of PfPLP1. We labelled these sites as site-I and site-II as shown in the Fig. 2b.
Table 1.
3-D blast hits of C-terminal domain of PfPLP1
| PDB ID | Name of protein | TM-score | RMSD | IDEN | Cov. | |
|---|---|---|---|---|---|---|
| 1 | 3nsjA | Lymphocyte perforin | 0.677 | 1.35 | 0.073 | 0.719 |
| 2 | 1rlw | C2 domain of calcium-phospholipid binding domain from cytosolic phospholipase A2 | 0.621 | 2.03 | 0.083 | 0.712 |
| 3 | 3w56A | Structure of a C2 domain | 0.61 | 1.68 | 0.078 | 0.673 |
| 4 | 4npjA | Structure and ca(2+)-binding properties of the tandem c2 domains of e-syt2. Extended-Synaptotagmin 2, C2A- and C2B-domains | 0.608 | 2.31 | 0.064 | 0.712 |
| 5 | 3kwuA | Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis | 0.604 | 2.19 | 0.083 | 0.706 |
| 6 | 1djiB4 | A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-delta1 | 0.604 | 2.3 | 0.1 | 0.712 |
| 7 | 1djxB | C2 domain of Structural mapping of the catalytic mechanism for a mammalian phosphoinositide-specific phospholipase C | 0.604 | 2.31 | 0.091 | 0.712 |
| 8 | 3kwtA | Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis | 0.598 | 2.17 | 0.085 | 0.693 |
| 9 | 2ep6A | Solution structure of the second C2 domain from human MCTP2 protein | 0.598 | 2.3 | 0.065 | 0.706 |
| 10 | 4ihbA | X-RAY Structure of the canonical C2A domain from human dysferlin | 0.596 | 2.4 | 0.074 | 0.706 |
| 11 | 2b3rB | Crystal structure of the C2 domain of class II phosphatidylinositide 3-kinase C2 | 0.541 | 2.68 | 0.078 | 0.66 |
| 12 | 1djgA | A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-delta1 | 0.602 | 2.47 | 0.1 | 0.719 |
| 13 | 1w15A | Rat synaptotagmin 4 C2B domain in the presence of calcium | 0.518 | 2.43 | 0.073 | 0.614 |
| 14 | 2uzpA | Crystal structure of the C2 domain of human protein kinase C gamma | 0.528 | 2.75 | 0.083 | 0.647 |
TM-score: TM-score is an algorithm to calculate the structural similarity of two protein models. TM-score has the value in (0,1]. Based on statistics, a TM-score <0.17 corresponds to a random similarity and a TM-score >0.5 generally corresponds to the same fold; RMSD: The root-mean-square deviation (RMSD) is the measure of the average distance between the atoms (usually the backbone atoms) of superimposed proteins; IDEN: IDEN is the percentage sequence identity in the structurally aligned region; Cov.: Cov. represents the coverage of global structural alignment and is equal to the number of structurally aligned residues divided by length of the query protein
Fig. 2.
Structural homology with lymphocyte perforin and prediction of two calcium binding pockets in the PfPLP1 C-terminal domain. a C-terminal domain of PfPLP1 was found to be structurally homologous to the C2 domain of lymphocyte perforin and were showing decent structural alignment with each other. b Using COACH software, two calcium binding sites were found in the C-terminal domain of PfPLP1
Identification of calcium-binding residues in C-terminal of PfPLP1
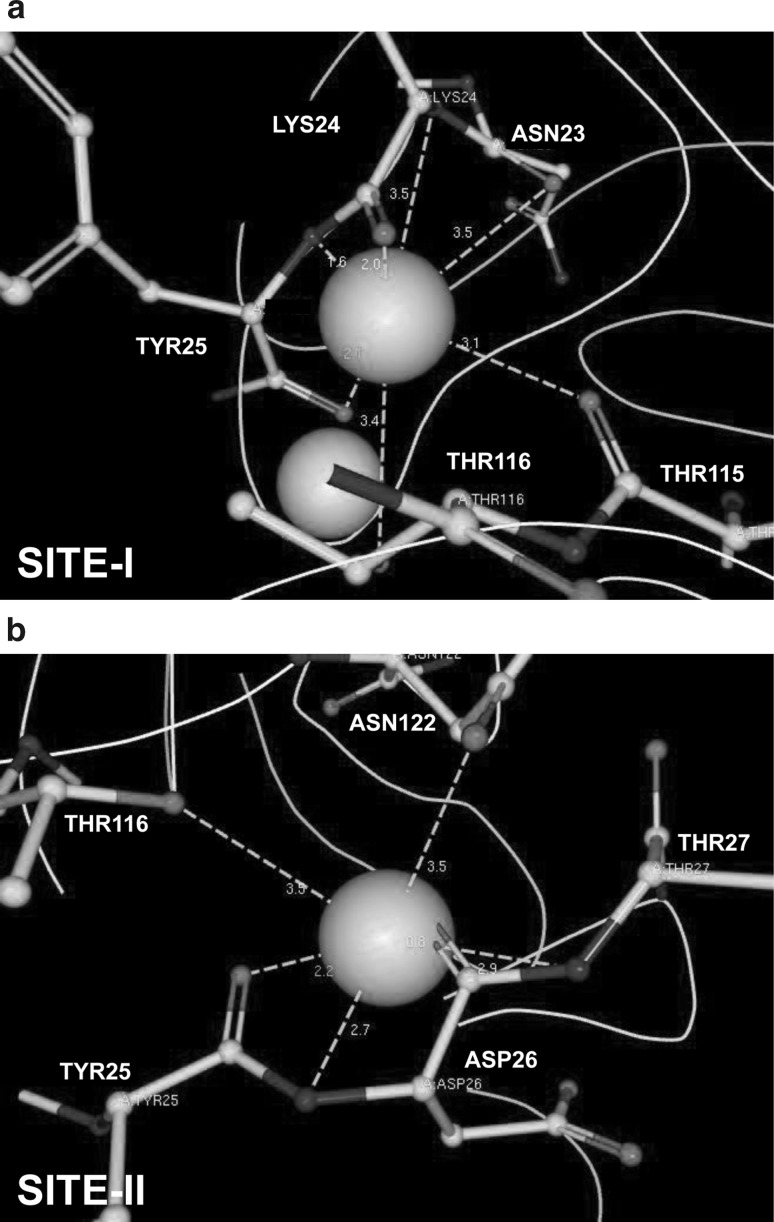
Ligand explorer was employed for visualizing the interaction of docked calcium with PfPLP1. We found that calcium at site-I was interacting with five amino acids: Asp23, Lys24, Tyr25, Thr115 and Thr116 whereas calcium at site-II was interacting with Tyr25, Asp26, Thr27, Thr116 and Asn122 (Fig. 3; Table 2). Calcium was principally co-ordinated by the negatively charged oxygen or the nitrogen atoms of these amino acids. The distance between bound calcium and corresponding amino acid varied from 0.8 to 3.5A° (Table 2).
Fig. 3.
Oxygen and nitrogen atoms of various amino acids coordinate calcium in C-terminal domain of PfPLP1. Asparagine, threonine, tyrosine, lysine and aspartate amino acids at various positions (marked in the figure) coordinate two calcium molecules via metal interactions
Table 2.
Predicted calcium binding amino acids
| Amino acid number: name: coordinating atom |
Distance (A°) |
|---|---|
| Calcium binding site-I | |
| A:23:ASN:O | 3.5 |
| A:24:LYS:N | 3.5 |
| A:24:LYS:O | 2 |
| A:25:TYR:N | 1.6 |
| A:25:TYR:O | 2.1 |
| A:115:THR:O | 3.1 |
| A:116:THR:OG1 | 3.4 |
| Calcium binding site-II | |
| A:25:TYR:O | 2.2 |
| A:26:ASP:N | 2.7 |
| A:26:ASP:O | 0.8 |
| A:27:THR:N | 2.9 |
| A:116:THR:OG1 | 3.5 |
| A:122:ASN:OD1 | 3.5 |
The table is showing the name and number of amino acid that is interacting with the bound calcium. Moreover, the atoms of amino acid, interacting with calcium are also shown. Distance between the calcium and corresponding amino acid is given in A (Angstrom)
Methods
3-D structure prediction of PfPLPs
I-TASSER (http://zhang.bioinformatics.ku.edu/I-TASSER/), a hierarchical pipeline for protein structure prediction, combines the methods of threading, ab initio modeling and structural refinement, was used to predict structures of C-terminal of PfPLPs (Roy et al. 2010, 2011; Zhang 2008). Among the top five models predicted by the server, the one with a high C score (>−2) was chosen for further analyses. Protein structures were superimposed using TM-align (Zhang and Skolnick 2005).
Calcium binding site prediction
COACH meta-server was used to predict calcium-binding site in the C-terminal of PfPLP1 (Yang et al. 2013a, b). COACH meta-server predicts protein–ligand binding site based on specific structural comparison (TM-SITE) as well as on sequence profile alignment (S-SITE). The results from COACH meta-server were then viewed and analyzed using Ligand Explorer. The cut-off for metal interactions in Ligand Explorer was kept at 3.5A (Moreland et al. 2005).
Discussion
PfPLPs are important molecules, essential for progression of P. falciparum life cycle. The structural information of lymphocyte perforin is known, however, no data is available on the structure of PfPLPs. In this paper, we tried to in silico predict the structure of C-terminal domain of PfPLPs and further identify the calcium-binding site of PfPLP1. We found that C-terminal domain of PfPLP1 is similar calcium binding C2 domain such as perforin, synaptotagmin, dysferlin, etc. Based on this structural homology, we further narrowed down to identify calcium-binding site of C-terminal domain of PfPLP1. We found that two calcium molecules interact with amino acids-Asp23, Lys24, Tyr25, Asp26, Thr27, Thr115, Thr116 and Asn122 via metal interactions. Further biochemical characterization by mutation analysis will confirm the importance of these amino acids in coordinating bound calcium. Previous studies have shown that calcium is essential for the activity of PfPLP1. Therefore, the calcium-binding site of PfPLPs could be a good chemotherapeutic target for development of multistage drugs against malaria.
Footnotes
Swati Garg and Vijeta Sharma have contributed equally to the work.
References
- Agarwal S, Singh MK, Garg S, Chitnis CE, Singh S. Ca2+ mediated exocytosis of subtilisin-like Protease 1: a key step in egress of P. falciparum merozoites. Cell Microbiol. 2013;15:910–921. doi: 10.1111/cmi.12086. [DOI] [PubMed] [Google Scholar]
- Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasites invasion of the human red blood cell. J Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Sudhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2 +/phospholipid binding. J Biol Chem. 1993;268(35):26386–26390. [PubMed] [Google Scholar]
- Ecker A, Pinto SB, Baker KW, Kafatos FC, Sinden RE. Plasmodium berghei: plasmodium perforin-like protein 5 is required for mosquito midgut invasion in Anopheles stephensi. Exp Parasitol. 2007;116:504–508. doi: 10.1016/j.exppara.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Agarwal S, Kumar S, Yazdani SS, Chitnis CE, Singh S. Calcium-dependent permeabilization of erythrocytes by a perforin-like protein during egress of malaria parasites. Nat Commun. 2013;4:1736. doi: 10.1038/ncomms2725. [DOI] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci USA. 2004;101:16310–16315. doi: 10.1073/pnas.0406187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol. 2004;133:15–26. doi: 10.1016/j.molbiopara.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Moreland JL, Gramada A, Buzko OV, Zhang Q, Bourne PE. The molecular biology toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinform. 2005;6:21. doi: 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Xu D, Poisson J, Zhang Y. A protocol for computer-based protein structure and function prediction. J Vis Exp. 2011;57:e3259. doi: 10.3791/3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, Kachman MM, Weißbach T, Zimmerberg J, Dandekar T, Griffiths G, Chitnis CE, Singh S, Fischer R, Pradel G. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol. 2014;16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Roy A, Zhang Y. BioLiP: a semi-manually curated database for biologically relevant ligand-protein interactions. Nucleic Acids Res. 2013;41:D1096–D1103. doi: 10.1093/nar/gks966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Roy A, Zhang Y. Protein-ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment. Bioinformatics. 2013;29:2588–2595. doi: 10.1093/bioinformatics/btt447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Skolnick J. TM-align: a protein structure alignment algorithm based on TM-score. Nucleic Acids Res. 2005;33:2302–2309. doi: 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]