Abstract
Objective To evaluate the effectiveness of replacing dietary saturated fat with omega 6 linoleic acid, for the secondary prevention of coronary heart disease and death.
Design Evaluation of recovered data from the Sydney Diet Heart Study, a single blinded, parallel group, randomized controlled trial conducted in 1966-73; and an updated meta-analysis including these previously missing data.
Setting Ambulatory, coronary care clinic in Sydney, Australia.
Participants 458 men aged 30-59 years with a recent coronary event.
Interventions Replacement of dietary saturated fats (from animal fats, common margarines, and shortenings) with omega 6 linoleic acid (from safflower oil and safflower oil polyunsaturated margarine). Controls received no specific dietary instruction or study foods. All non-dietary aspects were designed to be equivalent in both groups.
Outcome measures All cause mortality (primary outcome), cardiovascular mortality, and mortality from coronary heart disease (secondary outcomes). We used an intention to treat, survival analysis approach to compare mortality outcomes by group.
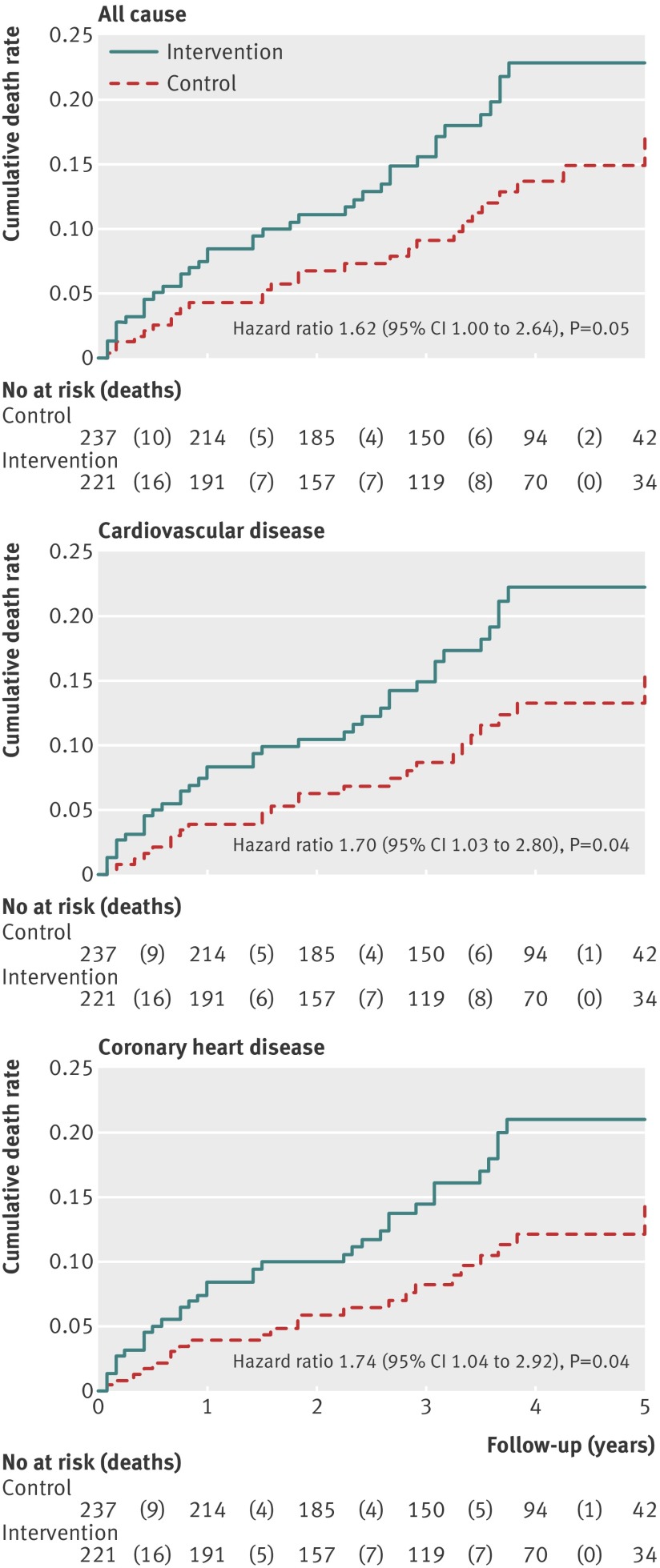
Results The intervention group (n=221) had higher rates of death than controls (n=237) (all cause 17.6% v 11.8%, hazard ratio 1.62 (95% confidence interval 1.00 to 2.64), P=0.05; cardiovascular disease 17.2% v 11.0%, 1.70 (1.03 to 2.80), P=0.04; coronary heart disease 16.3% v 10.1%, 1.74 (1.04 to 2.92), P=0.04). Inclusion of these recovered data in an updated meta-analysis of linoleic acid intervention trials showed non-significant trends toward increased risks of death from coronary heart disease (hazard ratio 1.33 (0.99 to 1.79); P=0.06) and cardiovascular disease (1.27 (0.98 to 1.65); P=0.07).
Conclusions Advice to substitute polyunsaturated fats for saturated fats is a key component of worldwide dietary guidelines for coronary heart disease risk reduction. However, clinical benefits of the most abundant polyunsaturated fatty acid, omega 6 linoleic acid, have not been established. In this cohort, substituting dietary linoleic acid in place of saturated fats increased the rates of death from all causes, coronary heart disease, and cardiovascular disease. An updated meta-analysis of linoleic acid intervention trials showed no evidence of cardiovascular benefit. These findings could have important implications for worldwide dietary advice to substitute omega 6 linoleic acid, or polyunsaturated fats in general, for saturated fats.
Trial registration Clinical trials NCT01621087.
Introduction
Advice to substitute vegetable oils rich in polyunsaturated fatty acids (PUFAs) for animal fats rich in saturated fatty acids (SFAs) has been a cornerstone of worldwide dietary guidelines for the past half century.1 When this advice originated in the 1960s, PUFAs were regarded as a uniform molecular category with one relevant biological mechanism—the reduction in blood cholesterol.1 2 Omega 6 (n-6) linoleic acid (LA) was the best known dietary PUFA at the time. Therefore, the terms “PUFA” and “LA” were often used interchangeably when interpreting clinical trial results and delivering dietary advice.
Since that time, there has been increased recognition that the general category of PUFAs comprises multiple species of omega 3 (n-3) and n-6 PUFAs, each with unique biochemical properties and perhaps divergent clinical cardiovascular effects. Favorable biological actions of n-3 eicosapentaenoic acid and docosahexaenoic acid (and to a lesser extent, n-3 α linolenic acid) have been extensively described.3 Clinical cardiovascular benefits of eicosapentaenoic acid and docosahexaenoic acid have also been reported in several4 5 but not all6 7 randomized controlled trials.3 8
However, there is currently no clinical trial evidence indicating that replacing SFAs with n-6 LA, without a concurrent increase in n-3 PUFAs, lowers the risk of cardiovascular disease or death. Thus, benefits attributed to PUFAs as a general category might be due to n-3 PUFAs specifically, particularly eicosapentaenoic acid and docosahexaenoic acid. Such benefits are not necessarily generalizable to n-6 LA or other PUFA species. Since n-6 LA is the most abundant dietary PUFA, and edible oil sources with markedly different contents of fatty acids are commercially available (table 1),9 it is important to ascertain the benefits and risks specific to n-6 LA.
Table 1.
Content of n-6 LA and n-3 α LNA in commercially available edible oils
| Cooking oil | LA (g per 100 g of cooking oil) | α LNA (g per 100 g of cooking oil) |
|---|---|---|
| Vegetable oil* | Depends on specific oil | Depends on specific oil |
| Safflower† | 74.6 | 0.0 |
| Sunflower† | 65.7 | 0.0 |
| Cottonseed | 51.5 | 0.0 |
| Corn | 53.5 | 0.2 |
| Soybean | 50.3 | 7.0 |
| Canola | 18.6 | 9.1 |
| Olive | 9.8 | 0.8 |
| Butter oil | 2.3 | 1.4 |
| Coconut | 1.8 | 0.0 |
n-6 LA=omega 6 linoleic acid; n-3 α LNA=omega 3 α linolenic acid. Fatty acid contents of oils vary to some extent by season, latitude, and other conditions. USDA National Nutrient Database numbers: safflower 04510, sunflower 04510, cottonseed 04502, corn 04518, soybean 04669, canola 04582, olive 04053, butter 01003, coconut 04047.9
*Food items labeled “vegetable oil” may contain one or more of the above oils.
†Varieties of safflower and sunflower oils with lower LA content are commercially available.
The Sydney Diet Heart Study (SDHS),10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 a randomized controlled trial conducted from 1966 to 1973, provides a unique opportunity to evaluate the cardiovascular effects of replacing SFA with n-6 LA from safflower oil. Safflower oil is a concentrated source of LA (about 75 g LA per 100 g serving of oil9; table 1) containing no other reported PUFAs. Increased all cause mortality in the safflower oil group was reported in 1978,10 although deaths due to cardiovascular disease and coronary heart disease were not reported by group. Clinical outcomes for cardiovascular disease and coronary heart disease have been considered to be more relevant than all cause mortality when evaluating the evidence base25 and formulating dietary guidelines.26 Therefore, previous meta-analyses of PUFA intervention trials and risk of cardiovascular disease25 27 28 have been incomplete because they were not able to include these missing data from the SDHS.
We recovered the original SDHS dataset and used modern statistical methods to compare rates of all cause, cardiovascular, and coronary heart disease mortality by group; and to examine whether longitudinal dietary changes in PUFAs (that is, n-6 LA from safflower oil) or SFAs were associated with mortality outcomes. SDHS data recovery also allowed us to update our previously incomplete meta-analysis published in 2010,28 permitting a comprehensive risk-benefit assessment for n-6 LA including datasets from all known randomized controlled trials evaluating dietary PUFAs for cardiovascular risk reduction.
Methods
SDHS data recovery and validation
We obtained permission from an original study investigator (B Leelarthaepin, coauthor) and approval from the Office of Human Research Protection to recover, analyze, and interpret de-identified SDHS data stored on a 9 track magnetic tape. Part 1 of the web appendix describes the methods used to recover the original SDHS dataset; convert these data into a useable format; and identify, confirm, and verify the recovered data. Only variables that exactly matched published data were included. All matching variables were further verified by an original study investigator (B Leelarthaepin) to ensure accuracy.
Study design and participants
The SDHS was a randomized controlled dietary trial, evaluating the effects of increasing n-6 LA from safflower oil in place of SFA for secondary prevention of coronary heart disease. Eligible patients were men aged 30-59 years admitted to one of four academic teaching hospitals (Prince Henry, Prince of Wales, Sutherland, St George)13 affiliated with the University of New South Wales near Sydney, Australia, for an episode of acute myocardial infarction (86%), or acute coronary insufficiency or angina (14%). Patients were subsequently referred to the Prince Henry Hospital Coronary Clinic for inclusion in the trial. Participants entered the study at least eight weeks (mean 11 weeks) after their acute coronary event, by which time most had resumed normal activity, and many had returned to work.13
Randomization and masking
After provision of informed consent, participants were allocated by a table of random numbers to either the dietary intervention group (n=221) or a control group with no specific dietary instruction (n=237). The number sequence was generated by a research assistant and was concealed until after the medical evaluations and testing at baseline were completed. After the baseline phase, the study dietitians were unmasked to assign patients to their groups and administer the interventions. Medical investigators were initially masked to group assignment, although the success of blinding was not evaluated. Deaths were assigned codes from ICD-7 (international classification of diseases, 7th revision)29 according to death certificates or final hospital admission records.
Procedures
During the baseline phase, a testing battery of clinical, laboratory, and dietary assessments was administered. Levels of serum total cholesterol and triglycerides after fasting (>12 h) were assessed at the first baseline visit and again one week later, by a modification of the methods of Abell and Van Handel,30 31 respectively. Participants recorded their dietary intake in a seven day food log between these two baseline visits. Experienced dietitians assessed daily intake via a combination of this food log and accompanying interview at the second baseline visit.13 Data were converted to mean daily nutrient intake using food composition tables supplemented by laboratory analyzed values on a previously established computer program.10 32
Diet intervention
The intervention group received instructions to increase their PUFA intake to about 15% of food energy, and to reduce their intake of SFA and dietary cholesterol to less than 10% of food energy and 300 mg per day, respectively.10 To achieve these targets, intervention participants were provided with liquid safflower oil and safflower oil polyunsaturated margarine (“Miracle” brand, Marrickville Margarine). Liquid safflower oil was substituted for animal fats, common margarines and shortenings in cooking oils, salad dressings, baked goods, and other products, and was also taken as a supplement. Safflower oil polyunsaturated margarine was used in place of butter and common margarines. Safflower oil is a concentrated source of n-6 LA (table 1)9 and contains no other reported PUFAs. Therefore, the intervention oil selectively increased n-6 LA without a concurrent increase in n-3 PUFAs; this LA selective PUFA intervention will be referred to as the LA intervention.
Control
The control group received no specific dietary instruction. However, some participants began substituting polyunsaturated margarine for butter after their coronary event.33 Because the research team made no effort to alter the PUFA or SFA content of control diets, such dietary changes were allowed to continue. All non-dietary aspects of the study were designed to be equivalent in both groups, with all participants receiving standard medical care available at the time for secondary prevention of coronary heart disease and other medical conditions. This treatment included equivalent advice for all active smokers to quit and all overweight patients to lose weight.
For follow-up, participants returned for testing three times in the first year after entry, and every six months thereafter (web appendix, part 4). At each visit, a clinical assessment was performed and fasting levels of cholesterol and triglycerides were assessed. Between each study visit, participants recorded their dietary intake in a seven day food log. At each follow-up visit, mean daily nutrient intakes were assessed by a combination of the food log and dietitian interview, similar to the baseline phase. The full food log was repeated if doubts arose about accuracy,32 although the specific criteria used to assess accuracy were not recovered. Duration of diet exposure and ICD-7 codes were recorded for all study deaths.
Statistical analysis for the SDHS
The published objective of the SDHS was to evaluate whether increasing PUFAs in place of SFAs in men with “premature coronary heart disease might so improve survival.”10 However, the primary survival outcome (for example, mortality from all causes, cardiovascular disease, or coronary heart disease) was not explicitly defined. The original sample size calculations were not recovered. For our analyses, all cause mortality was selected as the primary outcome, with deaths from cardiovascular disease and coronary heart disease as secondary outcomes.
Statistical analyses were performed using Stata version 11 (Stata Corporation, 2009). We computed and compared descriptive statistics for baseline demographics, clinical characteristics, and dietary intakes by randomization assignment. We used survival analysis methodology to compare mortality from all causes, cardiovascular disease, and coronary heart disease between intervention and control groups on an intention to treat basis. Cumulative death rates were summarized using Kaplan Meier curves, and hazard ratios were estimated using Cox proportional hazards models.
To examine whether longitudinal changes in the prescribed dietary nutrients (PUFA, SFA, and PUFA:SFA ratio) were associated with mortality, we calculated hazard ratios for the whole sample and stratified by group for each mortality outcome as a function of time varying change from baseline. Nutrient values were expressed as a percentage of total energy intake. We used continuous variables after testing the linearity assumption. For these analyses, only participants with baseline dietary measurements were included (n=429, 63 deaths). To evaluate which of the prescribed nutrient changes could have mediated the increased mortality observed in the LA intervention group, we repeated the same analysis limiting the sample to the intervention group (n=207, 35 deaths). Since safflower oil is a concentrated source of n-6 LA containing no other reported PUFAs (table 1), this intervention group analysis of the change in PUFA (calculated by subtracting baseline values from values at each subsequent visit) estimates the effects of selectively increasing n-6 LA.
Oxidation products of n-6 LA have been implicated in the pathogenesis of cardiovascular disease,34 35 36 37 38 39 40 41 42 43 44 45 46 and alcohol use and cigarette smoking are major sources of free radical mediated oxidation.46 39 47 48 Therefore, we hypothesized that alcohol use or smoking at baseline modified the association between longitudinal change in PUFA intake and mortality using likelihood ratio tests (α=0.15). The original SDHS investigators posited that the LA intervention would reduce serum cholesterol as an intermediate for the prevention of cardiovascular death. Thus, to examine whether the magnitude of postrandomization changes in total blood cholesterol were associated with mortality, we calculated hazard ratios for each mortality outcome as a function of time varying change from baseline in total blood cholesterol.
Updated meta-analysis of PUFA intervention randomized controlled trials
To put the SDHS findings into context, we updated our prior systematic review and meta-analysis of the effects of PUFA interventions on risk of coronary heart disease28 49 to include the previously missing mortality data for coronary heart disease and cardiovascular disease from the SDHS. Web appendix 8 shows the detailed methods,28 along with descriptions of each PUFA intervention trial, publication bias assessment, heterogeneity analyses, pooled risk estimates for mortality outcomes, and sensitivity analyses.
Results
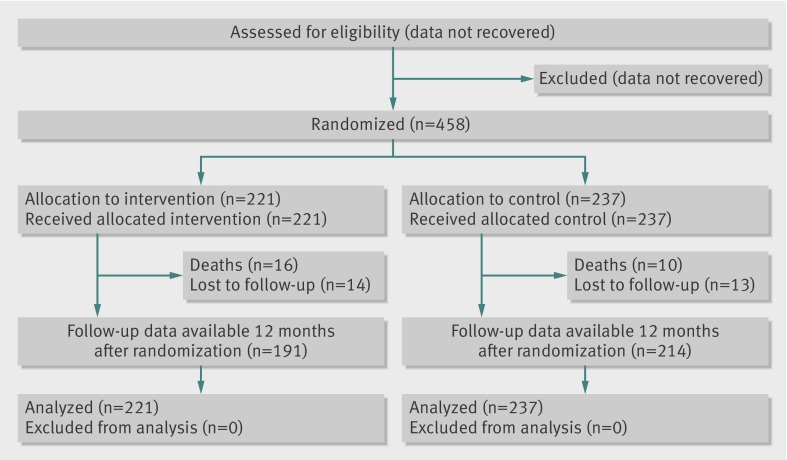
Figure 1 shows the trial profile. The two groups were well balanced at baseline, with no significant differences in any demographic, laboratory, or dietary variables (tables 2 and 3). Median follow-up was 39 months.
Fig 1 Trial profile. An intention to treat analysis included all randomized participants. Participant exclusion data from before randomization were not recovered. Numbers lost to follow-up were comparable in the two groups; reasons for dropout were not recovered
Table 2.
Baseline characteristics of 458 randomized participants
| Control group (n=237) | Intervention group (n=221) | |
|---|---|---|
| Age (years)* | 49.1 (6.55) | 48.7 (6.82) |
| Body mass index* | 25.4 (2.60) | 25.1 (2.39) |
| Systolic blood pressure (mm Hg)*† | 136.9 (21.1) | 136.6 (20.1) |
| Diastolic blood pressure (mm Hg)*† | 88.5 (12.0) | 88.5 (12.5) |
| Total cholesterol (mg/dL; 1 mg/dL=0.03 mmol/L)* | 282.0 (55.6) | 281.3 (63.4) |
| Triglycerides (mg/dL; 1 mg/dL=0.01 mmol/L)* | 185.9 (132.9) | 189.0 (199.1) |
| Fasting glucose (mg/dL; 1 mg/dL=0.06 mmol/L)* | 82.2 (11.6) | 84.0 (12.9) |
| Uric acid (mg/dL; 1 mg/dL=59.48 µmol/L)* | 6.7 (1.55) | 6.7 (1.55) |
| Marital status | ||
| Married | 206 (86.9) | 204 (92.3) |
| Single/divorced/widowed | 31 (13.1) | 17 (7.7) |
| Presenting event related to coronary heart disease | ||
| Myocardial infarction | 203 (85.7) | 192 (86.9) |
| Acute angina or coronary insufficiency | 34 (14.3) | 29 (13.1) |
| Smoking status | ||
| Smokers at admission | 163 (68.8) | 158 (71.5) |
| Alcohol use at admission (kcal; 1 kcal=4.18 kJ) | ||
| Non-drinker | 64 (27.0) | 60 (27.2) |
| Light (<200 kcal/day) | 86 (36.3) | 78 (35.3) |
| Moderate (200-500 kcal/day) | 44 (18.6) | 37 (16.7) |
| Heavy (>200 kcal/day) | 43 (18.1) | 46 (20.8) |
| Dyspnea | ||
| No dyspnea | 148 (62.4) | 133 (60.2) |
| On severe exertion | 69 (29.1) | 62 (28.0) |
| On mild exertion/at rest | 20 (8.4) | 26 (11.8) |
| Glucose metabolism§ | ||
| Normal | 171 (72.2) | 157 (71.0) |
| Prediabetes | 53 (22.4) | 46 (20.8) |
| Diabetes | 13 (5.5) | 18 (8.1) |
Data are no (%) of participants unless stated otherwise.
*Data are mean (standard deviation).
†Blood pressure measured in 5 unit intervals.
§Glucose response classification based on results of a challenge of 50 mg oral glucose, according to 1968 criteria of Joplin and Wright.83
Table 3.
Baseline and follow-up dietary data in the SDHS, for 426 participants with baseline and at least one follow-up diet record
| Nutrient | Baseline* | Follow-up† | P | |||||
|---|---|---|---|---|---|---|---|---|
| Control (n=221) | Intervention (n=205) | Control (n=221) | Change from baseline | Intervention (n=205) | Change from baseline | |||
| PUFA‡ | 6.2 (3.2-9.2) | 6.1 (3.0-9.2) | 8.4 (6.7-10.9) | +2.2 | 15.4 (12.3-17.9) | +9.3§ | <0.001 | |
| SFA‡ | 15.6 (13.0-18.7) | 16.2 (13.4-19.3) | 13.5 (11.4-15.6) | −2.1 | 9.3 (8.2-10.9) | −6.9 | <0.001 | |
| PUFA:SFA ratio | 0.41 (0.18-0.68) | 0.38 (0.16-0.65) | 0.63 (0.45-0.92) | +0.22 | 1.72 (1.31-2.08) | +1.34 | <0.001 | |
| MUFA‡ | 14.7 (12.8-16.9) | 14.6 (13.2-16.5) | 14.0 (12.3-15.2) | −0.7 | 11.2 (10.1-12.7) | −3.4 | <0.001 | |
| Total fat‡ | 39.2 (35.0-43.5) | 40.2 (36.6-43.4) | 38.1 (34.3-41.2) | −1.1 | 38.3 (36.1-46.3) | −1.9 | 0.87 | |
| Carbohydrate‡ | 40.5 (37.0-45.2) | 39.9 (35.2-46.1) | 40.6 (35.6-44.8) | +0.1 | 41.3 (36.1-46.3) | +1.4 | 0.31 | |
| Protein‡ | 14.1 (12.4-16.3) | 14.4 (12.6-16.5) | 15.3 (13.4-17.3) | +1.2 | 14.8 (13.4-16.8) | +0.4 | 0.25 | |
| Alcohol‡ | 2.3 (0.0-8.1) | 2.4 (0.0-8.9) | 4.0 (0.9-8.7) | +1.7 | 3.1 (0.7-8.9) | +0.7 | 0.42 | |
| Energy (kcal/day; 1 kcal=4.18 kJ) | 2384 (2072-2770) | 2423 (1972-2860) | 2194 (1804-2524) | −190 | 2256 (1958-2574) | −167 | 0.07 | |
| Cholesterol (mg/day) | 439 (344-593) | 477 (355-621) | 331 (269-408) | −108 | 238 (203-283) | −239 | <0.001 | |
*Data are median (interquartile range) from a single seven day food record administered before randomization.
†Data are median summaries (interquartile range), with each participant assigned one value based on the average of their seven day food records after randomization. Comparisons between diets were calculated with the Mann Whitney U test.
‡Data are percentage of food energy.
§From safflower oil.
Table 3 summarizes nutrient intakes of the two groups at baseline and follow-up. During follow-up, the intervention group had a larger increase in PUFA than the control group, and larger reductions in SFAs, dietary cholesterol, and monounsaturated fatty acids (P<0.001). Part 5 of the web appendix shows intakes of target nutrients at each study time point.
Table 4 summarizes risk factors for cardiovascular disease at baseline and at 12 months of follow-up. Serum total cholesterol decreased more in the LA intervention group than in the control group (−13.3% v −5.5%; P<0.001). There were no significant differences between groups in triglycerides, body mass index, or systolic or diastolic blood pressure at baseline or during follow-up.
Table 4.
Risk factors for cardiovascular disease
| Baseline | 12 month follow-up | |||||
|---|---|---|---|---|---|---|
| Control (n=237) | Intervention (n=221) | Control (n=192) | Intervention (n=179) | P* | ||
| Total cholesterol (mg/dL; 1 mg/dL=0.026 mmol/L) | 282.0 (274.8 to 289.1) | 281.3 (272.9 to 289.7) | 266.5 (259.1 to 273.8) | 243.9 (237.4 to 250.4) | <0.001 | |
| Triglycerides (mg/dL; 1 mg/dL=0.011 mmol/L) | 185.9 (168.8 to 202.9) | 189.0 (162.6 to 215.4) | 151.8 (133.9 to 169.7) | 135.5 (126.0 to 145.1) | 0.06 | |
| Body mass index | 25.4 (25.1 to 25.8) | 25.1 (24.8 to 25.3) | 24.5 (24.1 to 24.9) | 24.3 (24.0 to 24.6) | 0.26 | |
| Systolic blood pressure (mm Hg) | 136.9 (134.2 to 139.6) | 136.6 (133.9 to 139.3) | 136.5 (133.4 to 139.5) | 136.4 (133.8 to 139.0) | 0.49 | |
| Diastolic blood pressure (mm Hg) | 88.5 (86.9 to 90.0) | 88.5 (86.9 to 90.2) | 87.9 (86.0 to 89.9) | 87.5 (85.7 to 89.3) | 0.38 | |
Data are mean (95% confidence interval) at baseline and 12 months after randomization.
*P values=between group differences, assessed by t test.
Cumulative death rates
Compared with the control group, the intervention group had an increased risk of all cause mortality (17.6% v 11.8%; hazard ratio 1.62 (95% confidence interval 1.00 to 2.64); P=0.051), cardiovascular mortality (17.2% v 11.0%; 1.70 (1.03 to 2.80); P=0.037), and mortality from coronary heart disease (16.3% v 10.1%; 1.74 (1.04 to 2.92); P=0.036) (fig 2 ).
Fig 2 Kaplan-Meier estimates of five year cumulative death rates after randomization to the intervention or control group. Results of Cox proportional hazards model include all follow-up data (≤83 months) on an intention to treat basis
Association of change in PUFA and saturated fat with mortality
Table 5 shows results of Cox models examining the relation between mortality risk and longitudinal changes in dietary n-6 LA, PUFA, and SFA. Among intervention patients (in whom the PUFA increase was n-6 LA from safflower oil), an increase of 5% of food energy from n-6 LA predicted 35% and 29% higher risk of cardiovascular death and all cause mortality, respectively (models adjusted for age, dietary cholesterol, body mass index at baseline, smoking, alcohol use, and marital status). Increases in the LA:SFA ratio in the intervention group were also significantly associated with higher cardiovascular death and all cause mortality; however, the reduction in SFA was not significantly related to any mortality outcome.
Table 5.
Mortality outcomes according to longitudinal changes in dietary fatty acid intake
| Diet variable | Model§ | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All cause | Cardiovascular disease | Coronary heart disease | |||||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||||
| LA intervention group only (n=207)* | |||||||||
| PUFA (LA specific; per 5 en% increase) | 1 | 1.31 (1.00 to 1.71) | 0.05 | 1.37 (1.04 to 1.79) | 0.03 | 1.20 (0.90 to 1.59) | 0.22 | ||
| 2 | 1.29 (1.00 to 1.67) | 0.05 | 1.35 (1.03 to 1.75) | 0.03 | 1.21 (0.91 to 1.61) | 0.19 | |||
| SFA (per 5 en% increase) | 1 | 0.77 (0.52 to 1.13) | 0.18 | 0.77 (0.52 to 1.13) | 0.18 | 0.74 (0.50 to 1.11) | 0.15 | ||
| 2 | 0.81 (0.56 to 1.19) | 0.29 | 0.82 (0.56 to 1.21) | 0.31 | 0.78 (0.52 to 1.16) | 0.22 | |||
| LA:SFA ratio (per 1 unit increase) | 1 | 1.51 (1.00 to 2.30) | 0.05 | 1.59 (1.04 to 2.43) | 0.03 | 1.44 (0.93 to 2.23) | 0.11 | ||
| 2 | 1.55 (1.01 to 2.36) | 0.04 | 1.62 (1.05 to 2.48) | 0.03 | 1.47 (0.94 to 2.29) | 0.09 | |||
| Control group only (n=222)† | |||||||||
| PUFA (unspecified; per 5 en% increase) | 1 | 1.08 (0.71 to 1.66) | 0.71 | 1.07 (0.69 to 1.67) | 0.75 | 1.00 (0.62 to 1.61) | 0.99 | ||
| 2 | 1.10 (0.73 to 1.65) | 0.65 | 1.09 (0.72 to 1.66) | 0.68 | 1.02(0.65 to 1.60) | 0.93 | |||
| SFA (per 5 en% increase) | 1 | 0.77 (0.48 to 1.24) | 0.28 | 0.89 (0.54 to 1.47) | 0.65 | 0.98 (0.58 to 1.65) | 0.93 | ||
| 2 | 0.76 (0.48 to 1.21) | 0.25 | 0.86 (0.53 to 1.39) | 0.54 | 0.95 (0.57 to 1.58) | 0.84 | |||
| PUFA:SFA ratio (per 1 unit increase) | 1 | 0.84 (0.39 to 1.80) | 0.66 | 0.78 (0.36 to 1.70) | 0.53 | 0.56 (0.27 to 1.18) | 0.13 | ||
| 2 | 1.05 (0.56 to 1.97) | 0.88 | 1.00 (0.52 to 1.93) | 0.99 | 0.77 (0.39 to 1.52) | 0.46 | |||
| Whole sample (n=429)‡ | |||||||||
| PUFA (unspecified; per 5 en% increase) | 1 | 1.26 (1.04 to 1.52) | 0.02 | 1.29 (1.07 to 1.57) | <0.01 | 1.19 (0.97 to 1.47) | 0.09 | ||
| 2 | 1.31 (1.09 to 1.58) | <0.01 | 1.35 (1.12 to 1.63) | <0.01 | 1.26 (1.02 to 1.54) | 0.03 | |||
| SFA (per 5 en% increase) | 1 | 0.74 (0.57 to 0.97) | 0.03 | 0.77 (0.58 to 1.01) | 0.06 | 0.77 (0.58 to 1.03) | 0.08 | ||
| 2 | 0.70 (0.53 to 0.91) | <0.01 | 0.72 (0.54 to 0.95) | 0.02 | 0.72 (0.54 to 0.97) | 0.03 | |||
| PUFA:SFA ratio (per 1 unit increase) | 1 | 1.35 (1.01 to 1.80) | 0.04 | 1.39 (1.03 to 1.87) | 0.03 | 1.27 (0.93 to 1.73) | 0.14 | ||
| 2 | 1.53 (1.14 to 2.05) | <0.01 | 1.58 (1.17 to 2.13) | <0.01 | 1.44 (1.04 to 1.98) | 0.03 | |||
En%=percentage of food energy. Analysis includes 429 patients with diet data at baseline. Missing follow-up data were imputed for three patients with baseline data who died within two months after randomization; median values were used for their respective group assignment.
*No of deaths: 35 (all cause), 34 (cardiovascular), 32 (coronary heart disease).
†No of deaths: 28 (all cause), 26 (cardiovascular), 24 (coronary heart disease).
‡No of deaths: 63 (all cause), 60 (cardiovascular), 56 (coronary heart disease).
§Model 1: crude model. Model 2: adjusted for age, dietary cholesterol intake, baseline body mass index, smoking, alcohol use, and marital status.
Among controls (in whom PUFA changes were not specific to n-6 LA), changes in PUFA and SFA consumption were not significantly related to risk of death. Among the control and intervention groups combined, an increase of 5% of food energy from unspecified PUFA predicted about 30% higher risk of cardiovascular death and all cause mortality. A reduction in SFA and increase in the PUFA:SFA ratio were also associated with increased risks of all cause and cardiovascular mortality. Risk of death from coronary heart disease was significantly associated with longitudinal changes in PUFA and SFA in the adjusted (but not crude) models.
Table 6 shows the hazard ratios for the association between longitudinal changes in dietary PUFA and cardiovascular mortality, according to alcohol intake and smoking (sources of oxidative stress) at baseline. There was evidence of effect modification by alcohol and smoking (likelihood ratio test P=0.04 and P=0.13, respectively) among the whole sample, but not when restricted to the LA intervention group only. The positive association between longitudinal increases in n-6 LA and unspecified PUFAs and cardiovascular mortality was significant among moderate or heavy drinkers and smokers, but not among non-drinkers and non-smokers.
Table 6.
Risk of cardiovascular death according to longitudinal changes in dietary PUFA, stratified by sources of oxidative stress
| Subgroup | Risk of cardiovascular death | ||
|---|---|---|---|
| n | Hazard ratio (95% CI) | P | |
| LA intervention group only (n=207; per 5 en% increase in n-6 LA†) | |||
| Alcohol use* (kcal/day; 1 kcal=4.18 kJ) | |||
| Moderate/heavy (>200 kcal/day) | 79 | 1.70 (1.18 to 2.46) | <0.01 |
| Light (<200 kcal/day ) | 71 | 1.09 (0.69 to 1.72) | 0.71 |
| None | 57 | 1.40 (0.79 to 2.50) | 0.25 |
| Smoking status* | |||
| Yes | 147 | 1.55 (1.12 to 2.12) | 0.01 |
| No | 60 | 1.08 (0.67 to 1.68) | 0.75 |
| Whole sample (n=429; per 5 en% increase in unspecified PUFA) | |||
| Alcohol use* (kcal/day) | |||
| Moderate/heavy (>200 kcal/day) | 162 | 1.66 (1.23 to 2.25) | <0.01 |
| Light (<200 kcal/day) | 150 | 1.43 (1.00 to 2.04) | 0.05 |
| None | 117 | 0.91 (0.65 to 1.28) | 0.60 |
| Smoking status* | |||
| Yes | 301 | 1.46 (1.15 to 1.86) | <0.01 |
| No | 128 | 1.08 (0.79 to 1.49) | 0.62 |
En%=percentage of food energy.
*Assessed at acute hospital admission.
†From safflower oil.
Discussion
In this evaluation of data from the SDHS, selectively increasing the n-6 PUFA LA from safflower oil and safflower polyunsaturated margarine increased rates of death from cardiovascular disease, coronary heart disease, and all cause mortality compared with a control diet rich in SFA from animal fats and common margarines. This is the first published report to show an increase in mortality from cardiovascular disease and coronary heart disease, comparing this LA intervention to the control group, and demonstrating that the magnitude of increased n-6 LA intake was associated with higher risk of death. Although increased all cause mortality was reported in a 1978 publication,10 cardiovascular disease and coronary heart disease clinical outcomes, rather than all cause mortality, are the most relevant endpoints considered when evaluating the evidence base25 and formulating dietary guidelines for cardiovascular risk reduction.26 Therefore, recovery of these missing data has filled a critical gap in the published literature archive, allowing for a more comprehensive risk-benefit assessment for n-6 LA, including all known datasets from randomized controlled trials.
Comparison with other randomized controlled trials and updated meta-analysis
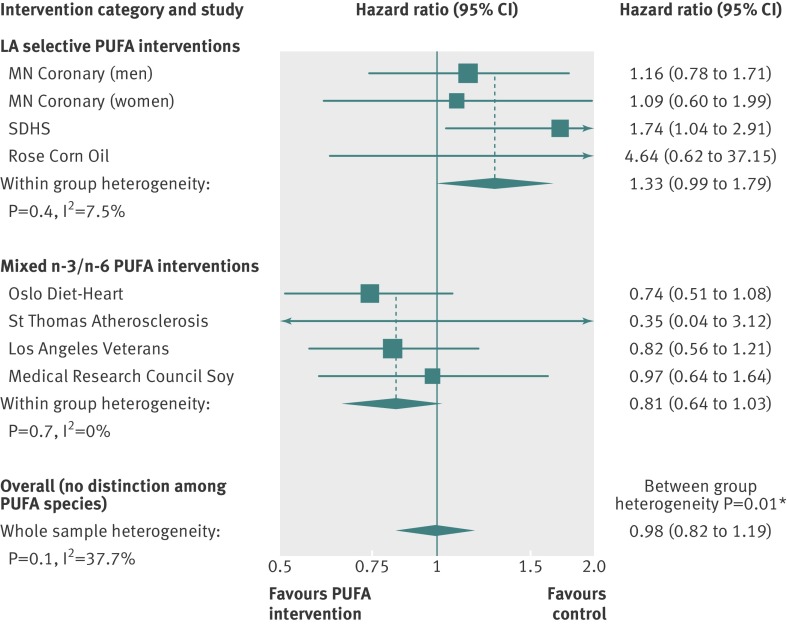
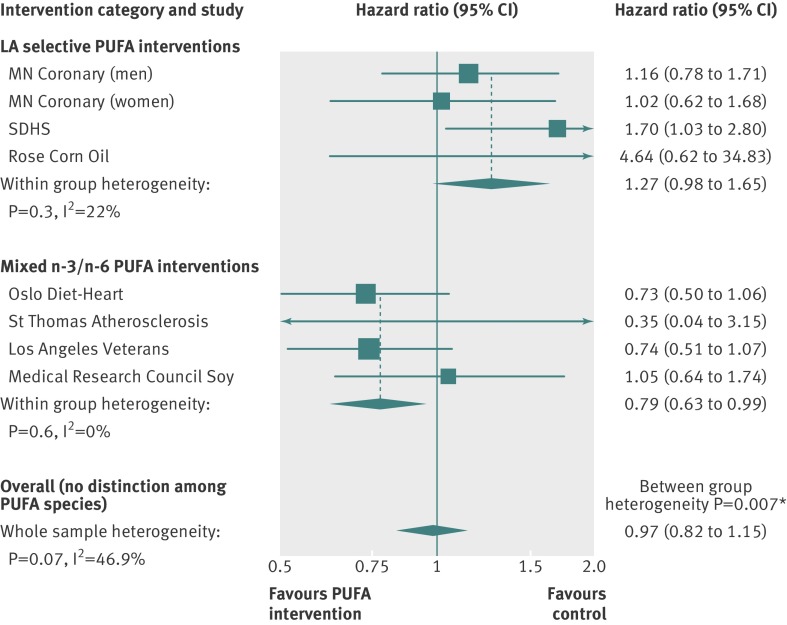
These unfavorable effects of n-6 LA shown in the SDHS are consistent with two other randomized controlled trials, in which experimental dietary conditions selectively increased n-6 LA in the place of SFAs by replacing animal fats and common margarines with corn oil.50 51 Together, these three trials provide a rare opportunity to evaluate the specific effects of increasing n-6 LA without confounding from concurrent increases in n-3 PUFAs. In a pooled analysis, the increased risks of death from coronary heart disease (hazard ratio 1.33 (95% confidence interval 0.99 to 1.79); P=0.06; fig 3 ) and cardiovascular disease (1.27 (0.98 to 1.65); P=0.07; fig 4 ) approached significance. Secondary prevention trials showed significant adverse effects of n-6 LA on coronary heart disease mortality (1.84 (1.11 to 3.04); P=0.02; web appendix, part 8). By contrast, pooled analysis of the four randomized controlled trials52 53 54 55 that increased n-3 PUFAs alongside n-6 LA showed reduced cardiovascular mortality (0.79 (0.63 to 0.99); P=0.04; fig 4).
Fig 3 Updated meta-analysis of effects of LA selective interventions and mixed n-3/n-6 PUFA interventions on risk of death from coronary heart disease. LA selective interventions selectively increased n-6 LA without a concurrent increase in n-3 PUFAs. Mixed PUFA interventions increased n-3 PUFAs and n-6 LA. PUFA interventions replaced high SFA control diets in each trial. *Significant heterogeneity between groups. Full methods and results in part 8 of the web appendix
Fig 4 Updated meta-analysis of effects of LA selective interventions and mixed n-3/n-6 PUFA interventions on risk of cardiovascular death. LA selective interventions selectively increased n-6 LA without a concurrent increase in n-3 PUFAs. Mixed PUFA interventions increased n-3 PUFAs and n-6 LA. PUFA interventions replaced high SFA control diets in each trial. *Significant heterogeneity between groups. Full methods and results in part 8 of the web appendix
The specific PUFA content of intervention oils was identified as a major source of heterogeneity for all mortality outcomes (web appendix, part 8). Therefore, benefits previously attributed to greater intake of total PUFA might be specifically attributable to n-3 PUFAs, and are not necessarily generalisable to n-6 LA, or PUFAs in general. Alongside the SDHS findings, these meta-analytic results provide supporting evidence that the specific PUFA content of intervention oils is a critical determinant of clinical cardiovascular outcomes, and that selective substitution of n-6 LA for SFA is unlikely to be beneficial, particularly in patients with established coronary heart disease. These findings should be interpreted with some caution, owing to the relatively small number of trials investigating PUFA interventions and differences in design and population characteristics of each trial. Part 8 of the web appendix presents the complete methods, results, and limitations of the analysis.
Among these PUFA intervention trials, the SDHS was unique because it collected longitudinal diet data using serial seven day food records, providing an opportunity to examine which nutrients might have mediated the increased risk of death observed in the study’s intervention group. Restriction of the analysis to the intervention group—which was provided safflower oil (a concentrated source of n-6 LA lacking n-3 PUFAs)—allowed us to estimate the specific effects of increasing n-6 LA only. Among patients in this intervention group, the increase in n-6 LA was associated with higher all cause and cardiovascular mortality, providing supporting evidence that LA itself was a key component mediating the unfavorable effects.
This advantage of PUFA specificity from safflower oil in the SDHS could account for the discrepancy between our findings of increased mortality risk with selective increase in n-6 LA, and the reported benefits of the general category of PUFAs in pooled analyses of randomized controlled trials25 and observational cohorts,56, which did not clearly distinguish between n-6 LA and other PUFA species. Importantly, these prior analyses of unspecified PUFAs have been considered decisive evidence for the benefits of n-6 LA26 57 58 59 60 and a key evidence base for advice to maintain or increase n-6 LA consumption.26 57 Therefore, our finding that selectively increasing n-6 LA from safflower oil increased the risk of death from all causes, coronary heart disease, and cardiovascular disease in the SDHS might have important implications for worldwide dietary advice.
Other dietary considerations
Although our analysis focused on evaluating the effects of the targeted reductions in SFAs and increases in n-6 LA from safflower oil, participants in the LA intervention group also significantly reduced their intake of monounsaturated fatty acids (MUFA) and dietary cholesterol. This reduction in MUFA was probably a byproduct of the reduction in foods high in SFAs that were also rich sources of MUFA (for example, animal fats, common margarine, shortening). Dietary cholesterol levels decreased, owing to reductions in animal fat and egg yolk consumption. Individual PUFA species were not recorded as separate entities in the seven day food records. Therefore, we cannot exclude the possibility that other PUFA species were altered in either group. However, the safflower oil given to intervention patients had large quantities of n-6 LA without other PUFA species.
Another factor that could have been altered by the intervention is dietary trans fatty acids, which are known to raise total and low density lipoprotein cholesterol 61 and have been associated with increased cardiovascular risk in observational studies.62 This association was not widely appreciated during the SDHS, and the trans fatty acid content of participants’ diets was not recorded. Restriction of common margarines and shortenings (major sources of trans fatty acids) in the intervention group would be expected to substantially reduce consumption of trans fatty acids compared with the control group.
Conversely, some of this reduction in trans fatty acids in the intervention group may have been offset by small amounts of trans fatty acids in the safflower oil polyunsaturated margarine. Although the precise composition of this margarine was not specified, it was selected for the study because of its ability to lower blood cholesterol and its high PUFA to SFA ratio,22 two characteristics of margarines that contain comparatively low amounts of trans fatty acids.63 Because dietary trans fatty acids are predominantly 18-carbon MUFA isomers,64 the recorded changes in MUFAs probably included small amounts of trans fatty acids in both groups.
Statistical adjustment for changes in MUFAs (an imperfect surrogate for trans fatty acids) in sensitivity analyses did not noticeably alter the observed relation between LA and increased risk of cardiovascular death in the intervention group (data not shown). Collectively, these observations indicate that changes in trans fatty acid were unlikely to play a substantial role in the findings reported here. Nevertheless, the SDHS dataset does not contain sufficient information to rule out the possibility that changes in nutrients other than n-6 LA and SFAs could have contributed to, or reduced, the observed unfavorable effects of the LA intervention.
Reconciling results from the SDHS with the traditional diet-heart hypothesis
Increasing dietary n-6 LA in place of SFA lowers serum total cholesterol, primarily by reducing low density lipoprotein with little or no effect on high density lipoprotein.61 The traditional diet-heart hypothesis predicts that these favorable, diet induced changes in blood lipids will diminish deposition of cholesterol in the arterial wall, slow progression of atherosclerosis, reduce clinical cardiovascular risk, and eventually improve survival.18 65 As expected, increasing n-6 LA from safflower oil in the SDHS significantly reduced total cholesterol; however, these reductions were not associated with mortality outcomes (results not shown). Moreover, the increased risk of death in the intervention group presented fairly rapidly and persisted throughout the trial. These observations, combined with recent progress in the field of fatty acid metabolism, point to a mechanism of cardiovascular disease pathogenesis independent of our traditional understanding of cholesterol lowering.
Proposed mechanistic model linking dietary LA to cardiovascular pathogenesis
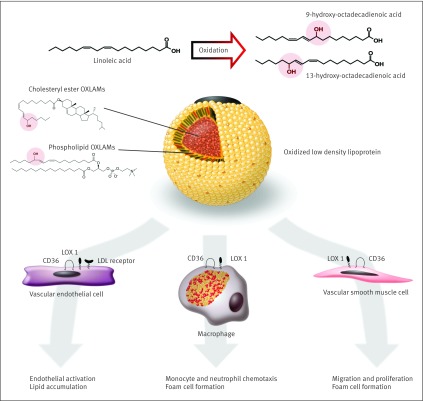
Omega-6 LA is the most abundant fatty acid in native low density lipoprotein particles.66 Oxidized LA metabolites (OXLAMs) are the most abundant oxidized fatty acids in oxidized low density lipoprotein,34 67 which is potentially more atherogenic than unmodified low density lipoprotein.46 A potential mechanism contributing to higher cardiovascular mortality in the LA intervention group is a diet induced increase in the production of bioactive OXLAMs, including 9- and 13-hydroperoxy-octadecadienoic acid, and 9- and 13-hydroxy-octadecadienoic acid. These OXLAMs are enriched in the lipid laden, macrophage foam cells; vascular endothelial cells; and migrating vascular smooth muscle cells of atherosclerotic lesions.35 36 37 38 OXLAMs, particularly the isomers and enantiomers produced by free radical mediated oxidation,35 39 68 have been mechanistically linked to cardiovascular disease pathogenesis. Mechanisms include inducing the formation of macrophage foam cells41 42; endothelial cell activation43; migration, proliferation, and foam cell formation of vascular smooth muscle cells44 69; and inhibition of lysosomal hydrolysis of low density lipoprotein cholesteryl esters70 (fig 5 ).
Fig 5 Proposed mechanistic model linking dietary LA to cardiovascular disease pathogenesis.34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 Conversion of LA to OXLAMs can proceed enzymatically, or via free radical mediated oxidative stress. Major sources of oxidative stress such as cigarette smoking and chronic alcohol exposure facilitate LA oxidation and production of oxidized low density lipoprotein (LDL). OXLAMs are the most abundant oxidized fatty acids in oxidized LDL and in atherosclerotic lesions. OXLAMs have been mechanistically linked to cardiovascular pathogenesis via multiple mechanisms. LDL=low density lipoprotein; CD36=cluster of differentiation 36 scavenger receptor; LOX 1=lectin like oxidized LDL receptor 1
Major sources of free radical mediated oxidative stress, such as cigarette smoking and chronic alcohol exposure, increase the oxidation of low density lipoprotein fatty acids46 47 48; smokers and drinkers are reported to have increased concentrations of LA oxidation products in atherosclerotic lesions.39 OXLAMs have both proatherogenic and antiatherogenic actions, although the isomers and enantiomers that predominate in such oxidation are suspected to have deleterious properties, particularly in established atherosclerotic lesions.35 39 68
Our model proposed here predicts that oxidative stress combined with diets high in n-6 LA facilitate this oxidation, leading to OXLAM mediated atherosclerotic progression and increased cardiovascular mortality. Consistent with this model, the link between the magnitude of increase in LA and mortality was robust in smokers and drinkers in the SDHS, suggesting that diets high in n-6 LA may be particularly detrimental in the context of oxidative stress induced by smoking or alcohol.
Lowering dietary LA as a strategy for cardiovascular risk reduction
If OXLAM mediated atherosclerotic progression did contribute to the increased cardiovascular mortality seen in the SDHS, then diet induced reductions in OXLAMs could potentially reduce cardiovascular risk. We recently showed that lowering n-6 LA in human diets for 12 weeks reduced OXLAMs and their precursor LA in circulation,71 and increased erythrocyte n-3 eicosapentaenoic acid and docosahexaenoic acid.72 Higher levels of these n-3 fatty acids are associated with reduced risk of coronary heart disease in some73 74 75 but not all76 observational studies. These findings provide biochemical mechanisms that are relevant to our proposed model, linking dietary LA to the increased cardiovascular mortality seen in the SDHS, and also suggest a dietary strategy (that is, LA lowering) that may warrant evaluation for risk reduction of cardiovascular disease.
Limitations and strengths of the SDHS
The SDHS had several important strengths. The combination of randomization, provision of a specific study oil (safflower) delivering n-6 LA but not n-3 PUFAs, and detailed longitudinal diet assessments allowed for estimation of the specific effects of LA. Although several observational studies have reported associations between LA and risk of coronary heart disease,77 78 79 the food frequency questionnaires used may have limited ability to distinguish the respective intakes of n-6 LA and n-3 α linolenic acid.80 81 Discrimination among PUFA species is limited in observational studies, owing to the wide variability in n-6 LA and n-3 α linolenic acid contents of apparently similar food items (table 1); the lack of vegetable oil and PUFA labeling requirements on packaged foods; and the lack of consumer appreciation for the specific vegetable oils used when dining away from home, which accounts for a large share of total PUFA consumption.82 Therefore, provision of a study oil containing a large quantity of n-6 LA without other PUFA species was a distinct advantage of the design of the SDHS. Other SDHS strengths included the relatively long term follow-up (median 39 months) and the use of ICD coded mortality outcomes as objective endpoints.
The SDHS also had several important limitations. Some control participants began substituting polyunsaturated margarine for butter—leading to substantial, but comparatively modest, dietary changes in the same direction as the intervention group. These changes may have attenuated the observed differences in mortality between the groups, leading to an underestimation of the adverse effects of the intervention. Consistent with other trials investigating the secondary prevention of coronary heart disease, both groups made healthy lifestyle modifications (for example, smoking reduction), probably from personal reassessment that accompanies a coronary event.
Importantly, these non-dietary components of the intervention were designed to be equivalent in both groups. Among 29 participants with missing diet data at baseline, there were a disproportionate number of deaths in the intervention group (four deaths from coronary heart disease) compared with the control group (no deaths). This disproportionate loss could have attenuated the association reported here, between increase in dietary n-6 LA with higher mortality. Consistent with this interpretation, a sensitivity analysis that imputed missing data by randomization group found more precise and slightly stronger associations between increases in LA and mortality (web appendix, part 7).
Because the fatty acid content of blood or adipose tissue was not measured, changes in these fatty acid pools could not be used to validate the data obtained by serial administration of seven day food records. However, the significant reduction in blood cholesterol seen in the LA intervention group provides biological data that is broadly consistent with the longitudinal diet data.
Without access to the original study protocol, we cannot fully appraise the accuracy of outcome ascertainment and other quality aspects of the SDHS. Another potential limitation was incomplete data recovery, owing to our inability to identify some of the study variables recorded on punch cards. However, we were able to identify, confirm, and verify each of the key variables for the dietary, laboratory, and coded mortality outcomes that were required to interpret the main study findings (web appendix, part 1).
The adverse effects of increasing n-6 LA from 6% of food energy to 15% in this cohort are not necessarily generalizable to lower LA intakes; therefore, some caution should be used when extrapolating results to other populations. Finally, because the SDHS investigated secondary prevention of coronary heart disease in men aged 30-59 years, results are not necessarily generalizable to women, men aged younger than 30 years or older than 59 years, or populations without established coronary heart disease.
Conclusions
In this cohort, substituting dietary n-6 LA in place of SFA increased the risks of death from all causes, coronary heart disease, and cardiovascular disease. An updated meta-analysis of LA intervention trials showed no evidence of cardiovascular benefit. These findings could have important implications for worldwide dietary advice to substitute n-6 LA, or PUFAs in general, for SFA.
What is already known on this topic?
Increasing dietary omega-6 linoleic acid in the place of saturated fat lowers total cholesterol and low density lipoprotein cholesterol
Advice to substitute linoleic acid for saturated fat is one component of dietary guidelines to reduce the risk of coronary heart disease; however, clinical benefits specific to linoleic acid have not been established
A comprehensive analysis of the effects of linoleic acid on death from coronary heart disease and cardiovascular disease was previously not possible, owing to missing outcome data from the Sydney Diet Heart Study, a randomized controlled clinical trial
What this study adds
In this cohort, substituting omega 6 linoleic acid for saturated fat did not provide the intended benefits, but increased all cause morality, cardiovascular death, and death from coronary heart disease
An updated meta-analysis incorporating these missing data showed no evidence of benefit, and suggested a possible increased risk of cardiovascular disease from replacing saturated fat with omega-6 linoleic acid
These findings could have important implications for worldwide dietary advice to substitute omega-6 linoleic acid (or polyunsaturated fatty acids in general) for saturated fatty acids
We thank the original SDHS team of researchers for their contributions: Ralph Blacket (principal investigator), Joan Woodhill (coprincipal investigator, lead dietitian), Jean Palmer (coinvestigator), Charles McGilchrist (statistician), Lear Bernstein (senior dietitian), and Janet Aitken (lipid laboratory); the physicians affiliated with the University of New South Wales teaching hospitals for their contributions and referral of participants; all the patients who participated in the study; John Svee (Data Conversion Resource, Westminster, CO, USA), and Steve Morgan and John Glauvitz (Data Recovery Systems, Morgan Hill, CA, USA) for providing technical expertise in data recovery and conversion; Toni Calzone for advice regarding recovery of magnetic tape data; Paul Blacket for efforts searching for study data; and Natalie Kress, Michael Donovan, Jie Qu, and Katherine Ness for proofreading the manuscript.
Contributors: CER designed and directed the project; located, managed, and validated the recovered data; developed the proposed mechanistic model; and was the main writer of the manuscript. BL was an investigator in the original trial, provided the nine track magnetic tape, verified the study methodology and ethical considerations, confirmed validity of recovered data, and revised the manuscript. SFMH performed the literature review, managed and validated recovered data, and assisted in writing and revising the manuscript. John Svee of the Data Conversion Resource completed the data recovery and conversion to modern spreadsheet format, in collaboration with CER and SFMH. DZ conducted the statistical analyses of the main trial, and KRF conducted the meta-analyses, in collaboration with SFMH, CER, JMD, and CMS. JMD, DZ, KRF, and CMS revised the manuscript. AR prepared the illustration of the proposed model and revised the manuscript. JRH directed the project and critically revised the manuscript. All authors contributed to analyses or interpretation of results and to the intellectual content of the manuscript. CER is the guarantor.
Funding: The Life Insurance Medical Research Fund of Australia and New Zealand provided a grant in support of this the SDHS. The Intramural Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, supported data recovery and evaluation. The funding source had no role in study design, data collection, analysis, interpretation, or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: support from the Life Insurance Medical Research Fund of Australia and New Zealand and the Intramural Program of the National Institute on Alcohol Abuse and Alcoholism for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The SDHS study protocol and patient consent forms were approved by the Dean of the Faculty of Medicine, University of New South Wales, Sydney, Australia. Medical research and clinical practice procedures were carried out according to the June 1964 World Medical Association Declaration of Helsinki and the Australian National Health and Medical Research Council guidelines, which provided the most current ethical principles for medical research involving humans. Patients provided consent to participate without coercion, and were free to refuse participation or withdraw at any time. The Office of Human Research Protection for the National Institutes of Health reviewed these conditions and determined that these de-identified data were suitable for the current analyses (exemption no 5744).
Data sharing: The dataset is available from the corresponding author at chris.ramsden@nih.gov. Data sharing consent was not obtained, but the presented data are anonymised and risk of identification is low.
Cite this as: BMJ 2013;346:e8707
Web Extra. Extra material supplied by the author
Web appendix: Supplementary material
References
- 1.Dietary fat and its relation to heart attacks and strokes. Report by the Central Committee for Medical and Community Program of the American Heart Association. JAMA 1961;175:389-91. [PubMed] [Google Scholar]
- 2.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in diet: iodine value of dietary fat versus 2S-P. Metab Clin Exptl 1965;14:747-58. [DOI] [PubMed] [Google Scholar]
- 3.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 2011;364:2439-50. [DOI] [PubMed] [Google Scholar]
- 4.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999;354:447-55. [PubMed] [Google Scholar]
- 5.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090-8. [DOI] [PubMed] [Google Scholar]
- 6.Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309-18. [DOI] [PubMed] [Google Scholar]
- 7.Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med 2012;172:686-94. [DOI] [PubMed] [Google Scholar]
- 8.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024-33. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Agriculture, Agricultural Research Service. 2012. USDA National Nutrient Database for Standard Reference, Release 25. www.ars.usda.gov/nutrientdata.
- 10.Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist C, Blacket RB. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Adv Exp Med Biol 1978;109:317-30. [DOI] [PubMed] [Google Scholar]
- 11.Palmer J, Leelarthaepin B, McGilchrist C, Blacket R. Coronary heart disease profile in Australian men and some factors influencing survival. Adv Exp Med Biol 1977;82:115-7. [DOI] [PubMed] [Google Scholar]
- 12.McGilchrist CA, Leelarthaepin B, Blacket RB. Measures on qualitative variables in coronary heart disease studies. J Chronic Dis 1976;29:285-8. [DOI] [PubMed] [Google Scholar]
- 13.Blacket RB, Leelarthaepin B, Palmer AJ, Woodhill JM. Coronary heart disease in young men: a study of seventy patients with a critical review of etiological factors. Aust N Z J Med 1973;3:39-62. [DOI] [PubMed] [Google Scholar]
- 14.Blacket RB, Leelarthaepin B. Coronary disease in young men. Singapore Med J 1973;14:344-6. [PubMed] [Google Scholar]
- 15.Blacket RB. Diet in prevention of heart disease. Med J Australia 1973;1:969-73. [PubMed] [Google Scholar]
- 16.Palmer J, Woodhill J, Blacket R. Strict modified fat diet in coronary heart disease. The problem of nonresponders. Isr J Med Sci 1969;5:754-9. [PubMed] [Google Scholar]
- 17.Palmer AJ, Blacket RB, Leelarthaepin.B. Hyperlipidemia in a group of coronary subjects in Sydney. Med J Aust 1973;2:19-23. [PubMed] [Google Scholar]
- 18.Blacket RB, Woodhill J, Mishkel MA. Diet, hypercholesterolaemia and coronary heart disease. Med J Aust 1965;1:59-63. [PubMed] [Google Scholar]
- 19.Woodhill JM, Palmer AJ, Blacket RB. Dietary habits and their modification in a coronary prevention programme for Australians. Food Technol Aust 1969;21:264-71. [Google Scholar]
- 20.Woodhill JM, Bernstein L. Lowering serum cholesterol levels by dietary modification. A change in food habits, not a special diet. Med J Aust 1973;1:973-9. [PubMed] [Google Scholar]
- 21.Fisher M. How the ‘Miracle’ was cowed: margarine quotas and politics. Aust Q 1970;42:20-33. [Google Scholar]
- 22.Significant points from the annual report of Marrickville Holdings. Sydney Morning Herald 1965. Nov 19.
- 23.Nestel PJ, Hipsley EH, Blacket RB. Diet and heart disease. Med J Aust 1971;1:1144-8. [PubMed] [Google Scholar]
- 24.Woodhill J. Australian dietary surveys with special reference to vitamins. Int Z Vitaminforsch 1970;40:520-40. [PubMed] [Google Scholar]
- 25.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902-7. [DOI] [PubMed] [Google Scholar]
- 27.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2012;5:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr 2010;104:1586-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. International classification of diseases: seventh revision. WHO, 1957.
- 30.Abell LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem 1952;195:357-66. [PubMed] [Google Scholar]
- 31.Van Handel E, Zilversmit DB. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med 1957;50:152-7. [PubMed] [Google Scholar]
- 32.Woodhill JM, Bernstein L. Lowering serum-cholesterol levels by dietary modification—change in food habits, not a special diet. Med J Aust 1973;1:973-9. [PubMed] [Google Scholar]
- 33.Blacket RB, Leelarthaepin B, McGilchrist CA, Palmer AJ, Woodhill JM. The synergistic effect of weight loss and changes in dietary lipids on the serum cholesterol of obese men with hypercholesterolaemia: implications for prevention of coronary heart disease. Aust N Z J Med 1979;9:521-9. [DOI] [PubMed] [Google Scholar]
- 34.Folcik VA, Cathcart MK. Predominance of esterified hydroperoxy-linoleic acid in human monocyte-oxidized LDL. J Lipid Res 1994;35:1570-82. [PubMed] [Google Scholar]
- 35.Shibata N, Toi S, Shibata T, Uchida K, Itabe H, Sawada T, et al. Immunohistochemical detection of 13(R)-hydroxyoctadecadienoic acid in atherosclerotic plaques of human carotid arteries using a novel specific antibody. Acta Histochem Cytochem 2009;42:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harland WA, Gilbert JD, Steel G, Brooks CJW. Lipids of human atheroma. 5. Occurrence of a new group of polar sterol esters in various stages of human atherosclerosis. Atherosclerosis 1971;13:239. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter KL, Taylor SE, van der Veen C, Williamson BK, Ballantine JA, Mitchinson MJ. Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim Biophys Acta 1995;1256:141-50. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn H, Belkner J, Wiesner R, Schewe T, Lankin VZ, Tikhaze AK. Structure elucidation of oxygenated lipids in human atherosclerotic lesions. Eicosanoids 1992;5:17-22. [PubMed] [Google Scholar]
- 39.Waddington EI, Croft KD, Sienuarine K, Latham B, Puddey IB. Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis 2003;167:111-20. [DOI] [PubMed] [Google Scholar]
- 40.Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 2010;1:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998;93:229-40. [DOI] [PubMed] [Google Scholar]
- 42.Xie S, Lee YF, Kim E, Chen LM, Ni J, Fang LY, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci U S A 2009;106:13353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 2009;50:204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barlic J, Zhang Y, Murphy PM. Atherogenic lipids induce adhesion of human coronary artery smooth muscle cells to macrophages by up-regulating chemokine CX3CL1 on smooth muscle cells in a TNFalpha-NFkappaB-dependent manner. J Biol Chem 2007;282:19167-76. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan R, Reddy MA, Malik KU, Fatima S, Khan BV. Signaling mechanisms of nuclear factor-kappab-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2001;21:1408-13. [DOI] [PubMed] [Google Scholar]
- 46.Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, et al. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci 2001;947:199-205. [DOI] [PubMed] [Google Scholar]
- 47.Yokode M, Ueyama K, Arai NH, Ueda Y, Kita T. Modification of high- and low-density lipoproteins by cigarette smoke oxidants. Ann N Y Acad Sci 1996;786:245-51. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, et al. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem 2010;285:22211-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsden CE, Hibbeln JR, Majchrzak-Hong SF, Davis JM. Don’t disregard the essential distinction between PUFA species. Br J Nutr 2011;106:953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose GA, Thomson WB, Williams RT. Corn oil in treatment of ischaemic heart disease. BMJ 1965;1:1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 1989;9:129-35. [DOI] [PubMed] [Google Scholar]
- 52.Leren P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Med Scand Suppl 1966;466:1-92. [PubMed] [Google Scholar]
- 53.Medical Research Council. Controlled trial of soya-bean oil in myocardial infarction. Lancet 1968;2:693-9. [PubMed] [Google Scholar]
- 54.Watts GF, Lewis B, Brunt JN, Lewis ES, Coltart DJ, Smith LD, et al. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas’ Atherosclerosis Regression Study (STARS). Lancet 1992;339:563-9. [DOI] [PubMed] [Google Scholar]
- 55.Dayton S, Pearce ML, Goldman H, Harnish A, Plotkin D, Shickman M, et al. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet 1968;2:1060-2. [DOI] [PubMed] [Google Scholar]
- 56.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Heart Foundation of Australia. Position statement: Dietary fats and dietary sterols for cardiovascular health. 2009. www.heartfoundation.org.au/SiteCollectionDocuments/Dietary-fats-position-statement-LR.pdf.
- 58.Dietary Guidelines Advisory Committee. What is the effect of dietary intake of n-6 polyunsaturated fatty acids (PUFA) on increased risk of cardiovascular disease and type 2 diabetes, including intermediate markers such as lipid and lipoprotein markers and inflammation? USDA Nutrition Evidence Library, 2010. www.nutritionevidencelibrary.com/topic.cfm?cat=3141.
- 59.Czernichow S, Thomas D, Bruckert E. n-6 Fatty acids and cardiovascular health: a review of the evidence for dietary intake recommendations. Br J Nutr 2010;104:788-96. [DOI] [PubMed] [Google Scholar]
- 60.Katan MB. Omega-6 polyunsaturated fatty acids and coronary heart disease. Am J Clin Nutr 2009;89:1283-4. [DOI] [PubMed] [Google Scholar]
- 61.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146-55. [DOI] [PubMed] [Google Scholar]
- 62.Bendsen NT, Christensen R, Bartels EM, Astrup A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: a systematic review and meta-analysis of cohort studies. Eur J Clin Nutr 2011;65:773-83. [DOI] [PubMed] [Google Scholar]
- 63.Carpenter DL, Slover HT. Lipid composition of selected margarines. J Am Oil Chemists Soc 1973;50:372-6. [DOI] [PubMed] [Google Scholar]
- 64.Chardigny JM, Malpuech-Brugere C, Dionisi F, Bauman DE, German B, Mensink RP, et al. Rationale and design of the TRANSFACT project phase I: a study to assess the effect of the two different dietary sources of trans fatty acids on cardiovascular risk factors in humans. Contemp Clin Trials 2006;27:364-73. [DOI] [PubMed] [Google Scholar]
- 65.Sherwin R. Controlled trials of the diet-heart hypothesis: some comments on the experimental unit. Am J Epidemiol 1978;108:92-9. [DOI] [PubMed] [Google Scholar]
- 66.Esterbauer H, Jurgens G, Quehenberger O, Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res 1987;28:495-509. [PubMed] [Google Scholar]
- 67.Lenz ML, Hughes H, Mitchell JR, Via DP, Guyton JR, Taylor AA, et al. Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein. J Lipid Res 1990;31:1043-50. [PubMed] [Google Scholar]
- 68.Vangaveti V, Baune B, Kennedy R. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 2010;1:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brewer ER, Ashman PL, Kuba K. The Minnesota Coronary Survey: composition of their diets, adherence, and serum lipid response. Circulation 1975;51-52(suppl II):II-269.
- 70.Heltianu C, Robciuc A, Botez G, Musina C, Stancu C, Sima AV, et al. Modified low density lipoproteins decrease the activity and expression of lysosomal acid lipase in human endothelial and smooth muscle cells. Cell Biochem Biophys 2011;61:209-16. [DOI] [PubMed] [Google Scholar]
- 71.Ramsden CE, Ringel A, Feldstein AE, Taha AY, Macintosh BA, Hibbeln JR, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids 2012;87:135-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacIntosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, et al. Low n-6 and low n-6 plus high n-3 diets for use in clinical research. Br J Nutr 2013; published online Jan 18:1-10. [DOI] [PMC free article] [PubMed]
- 73.Harris WS, Kennedy KF, O’Keefe JH Jr, Spertus JA. Red blood cell fatty acid levels improve GRACE score prediction of 2-yr mortality in patients with myocardial infarction. Int J Cardiol 2012, 10.1016/j.ijcard.2012.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aarsetoey H, Ponitz V, Nilsen OB, Grundt H, Harris WS, Nilsen DW. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation 2008;78:258-64. [DOI] [PubMed] [Google Scholar]
- 75.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 2008;197:821-8. [DOI] [PubMed] [Google Scholar]
- 76.Aarsetoey H, Ponitz V, Grundt H, Staines H, Harris WS, Nilsen DW. (n-3) Fatty acid content of red blood cells does not predict risk of future cardiovascular events following an acute coronary syndrome. J Nutr 2009;139:507-13. [DOI] [PubMed] [Google Scholar]
- 77.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses’ Health Study. Am J Epidemiol 2005;161:672-9. [DOI] [PubMed] [Google Scholar]
- 78.Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, et al. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr 2012;96:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Goede J, Geleijnse JM, Boer JMA, Kromhout D, Verschuren WMM. Linoleic acid intake, plasma cholesterol and 10-year incidence of CHD in 20 000 middle-aged men and women in the Netherlands. Br J Nutr 2012;107:1070-6. [DOI] [PubMed] [Google Scholar]
- 80.Ramsden CE, Hibbeln JR, Majchrzak-Hong SF. All PUFAs are not created equal: absence of CHD benefit specific to linoleic acid in randomized controlled trials and prospective observational cohorts. World Rev Nutr Diet 2011;102:30-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr 2011;93:1337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.US Department of Agriculture, Agricultural Research Service, Beltsville Human Research Center, Food Surveys Research group (Beltsville, MD), and the US Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD). What we eat in America, NHANES 2007-2008 data: away from home—percent of nutrients by gender and age. www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0708/Table_9_AWY_GEN_07.pdf.
- 83.Joplin GF, Wright AD. Detection of diabetes in man. In: Dickens F, Randle PJ, Whelan WJ, eds. Carbohydrate metabolism and its disorders. Academic Press, 1968.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material