Abstract
Background
Mitral stenosis (MS) is associated with prolonged inter- and intra-atrial electromechanical delays and increased P-wave dispersion, which are markers of atrial fibrillation (AF) risk. This study was conducted to assess the immediate effect of successful percutaneous transvenous mitral commissurotomy (PTMC) on these parameters.
Methods
This single center observational study included 25 patients with severe MS (aged 34.1 ± 7.1 years, with mean mitral valve area (MVA) of 0.74 ± 0.13 cm2), in sinus rhythm, who underwent successful PTMC at our hospital. P-wave dispersion (PWD) was calculated by subtracting minimum P-wave duration (Pmin) from maximum P-wave duration (Pmax), measured on a 12-lead surface ECG obtained from each patient in supine position at a paper speed of 50 mm/s and 20 mm/mV. Inter-atrial (AEMD), left intra-atrial (L-IAEMD), and right intra-atrial (R-IAEMD) electromechanical delays were measured on tissue Doppler imaging. PTMC was performed using the standard Inoue Balloon technique. All these parameters were evaluated and compared before and 24–48 h after PTMC.
Results
Successful PTMC led to significant reduction in AEMD (p < 0.001), L-IAEMD (p < 0.001), and R-IAEMD (p < 0.001). There were no changes in Pmax, Pmin, and PWD immediately after PTMC.
Conclusions
Successful PTMC has a favorable early impact on inter- and intra-atrial electromechanical delays, which are considered as novel parameters of atrial electromechanical remodeling in MS patients. Prospective large-scale studies are required to confirm whether improvement in these markers translates into reduced long-term AF risk.
Keywords: Atrial electromechanical delay, Mitral stenosis, P-wave dispersion, Percutaneous transvenous mitral commissurotomy
1. Introduction
Rheumatic mitral stenosis (MS) is an acquired progressive form of valvular heart disease, characterized by diffuse thickening of mitral valve leaflets, fusion of commissures and shortening and fusion of chordae tendinae. The combination of mitral valve disease and atrial inflammation, secondary to rheumatic carditis leads to left atrial (LA) dilation, fibrosis of atrial wall, and disorganization of atrial muscle bundles.1 These structural changes lead to electrical inhomogeneity, non-uniform conduction velocities, and inhomogenous refractory periods within the atrial myocardium.2, 3 The resultant electrical dyssynchrony and electromechanical dysfunction are associated with increased risk of atrial fibrillation (AF).4
Several electrocardiographic and echocardiographic markers reflecting the electrophysiological and electromechanical abnormalities of atria prone to develop AF have been studied with an idea of early identification of patients who are susceptible to develop AF. Increased maximum P-wave duration (Pmax) on surface ECG has been reported to be associated with left atrial size and risk of developing AF.5, 6 P-wave dispersion (PWD) is an ECG marker of non-uniform and heterogeneous atrial conduction with ECG leads of different orientation.5 It has been defined as the difference between maximum and minimum P-wave duration (Pmax and Pmin). Previous investigations have shown that Pmax and PWD are increased in patients with rheumatic MS.7, 8
Atrial electromechanical delay (AEMD) has been defined as the temporal delay between the detected onset of electrical activity (P-wave on ECG) and the realization of force in the atrial myocardium (onset of A′ wave on tissue Doppler imaging (TDI).9 Advances in TDI technology have facilitated the detection of atrial mechanical activity from different atrial regions with high temporal resolution and allowed calculation of inter- and intra-atrial electromechanical delays.10, 11, 12 Prolonged AEMD is considered as a novel marker of atrial electromechanical remodeling and has been associated with increased AF risk in various disease states including MS.9, 13, 14, 15
Percutaneous transvenous mitral commissurotomy (PTMC) is currently the treatment of choice for patients with symptomatic severe MS and favorable valve morphology. Successful PTMC leads to increase in mitral valve area (MVA), cardiac index, and decrease in transmitral diastolic pressure gradient, left atrial pressure, left atrial volume, and pulmonary artery pressures.16, 17 It also leads to sustained improvement in the functional class of the patients.18, 19 Recent studies have demonstrated that reduction in left atrial pressure and relief of chronic stretch following PTMC reverses left atrial direction-dependent conduction abnormalities, thus increasing the chances of MS patients retaining their normal sinus rhythm.20, 21, 22 This study was conducted with an aim of studying the immediate effect of successful PTMC on inter- and intra-atrial electromechanical delays and PWD in patients with severe rheumatic MS, in normal sinus rhythm. To the best of our knowledge, there is only one such study published in literature so far.23
2. Methods
2.1. Study design
This was a hospital-based prospective, non-randomized, observational study.
2.2. Study population
The study population included 25 consecutive patients admitted in our hospital with symptomatic severe MS [NYHA class II–IV, MVA of ≤1 cm2 by planimetry, pressure half time (PHT) ≥ 220 ms, and/or mean transmitral diastolic gradient (MDG) of ≥10 mmHg],24, 25, 26 in normal sinus rhythm, with favorable valve morphology (Wilkins's score ≤ 8, no or mild mitral regurgitation, and no commissural calcification or LA thrombus)27, 28 who underwent a successful PTMC (immediate post-procedural MVA of ≥1.5 cm2 or ≥50% increase from pre-procedural MVA, and no more than moderate mitral regurgitation after the procedure).29, 30 Exclusion criteria included: (i) Documented history or peri-procedural occurrence of AF or any other sustained arrhythmia. (ii) Significant involvement of valves other than mitral valve (aortic valve, pulmonary valve, tricuspid valve) or evidence of mitral annular calcification on echocardiography. (iii) Known history or clinical evidence of coronary artery disease, primary cardiomyopathy, left ventricular (LV) dysfunction, conduction abnormalities, pericarditis, thyroid dysfunction, anemia, renal failure (serum creatinine of >1.5 mg/dl), pulmonary disease, hypertensive cardiovascular disease, systemic inflammatory disease. (iv) Exposure to any known cardiotoxin (i.e. doxorubicin chemotherapy, ethanol, etc.), electrolyte imbalance, use of medications known to affect atrial conduction (i.e. digitalis, antiarrythmic drugs), and patients on pacemakers. (v) Clinical or laboratory evidence of rheumatic activity in preceding 6 months. (vi) Indiscernible P-waves in more than four leads on the baseline 12-lead ECG. (vii) Past history of any mitral valve intervention (PTMC, closed mitral valvotomy, open mitral valvotomy) or other cardiac surgeries.
2.3. Consent and ethical issues
An informed consent was obtained from each patient after explaining the study in detail. The study was cleared by the Institutional Ethics Committee.
2.4. Pre-procedural evaluation
A detailed clinical history was obtained and complete physical examination of each patient was performed after admission. Baseline investigations including complete blood count, serum biochemistry, coagulation profile, serum electrolytes, and chest X-ray were performed one day prior to procedure.
2.5. Electrocardiogram (ECG)
On the morning of the day of procedure, a 12-lead surface ECG of each patient in supine position at a paper speed of 50 mm/s and 20 mm/mV was obtained to calculate the pre-procedural P-wave durations and PWD. The P-wave durations were measured manually, by an investigator blinded to the clinical details of the patient, using calipers and magnifying lens (tenfold magnification) to define the electrocardiographic deflections. The onset of the P-wave was defined as the junction between the isoelectric line and the beginning of P-wave deflection. The offset of P-wave was defined as the junction between the end of the P-wave deflection and the isoelectric line. The time internal between the onset and offset of P-wave was the P-wave duration. In each ECG lead, P-wave duration was measured for three consecutive P-waves and the mean of three measurements was considered to be the P-wave duration of that lead. P-wave duration was measured in all 12-ECG leads. Patients with indiscernible P-waves in more than four leads on the baseline 12-lead ECG were excluded from the study. The longest P-wave duration measured on any of the 12 ECG leads was defined as the P maximum (Pmax) and the shortest P-wave duration on any lead was defined as the P minimum (Pmin). The difference between Pmax and Pmin was calculated and defined as PWD = Pmax − Pmin. After 24–48 h of the successful PTMC procedure, a similar ECG was obtained to calculate the post-procedural Pmax, Pmin, and PWD.
2.6. Echocardiography
A comprehensive pre-procedural echocardiographic examination of each patient was carried out in the morning of the day of procedure, using a commercially available cardiac ultrasound scanner equipped with 2.5 and 3.5 MHz transducers (Aloka, Prosound, SSD α-110, South Korea), by an experienced cardiologist who was blinded to the clinical details and results of other investigations of the patient. Each patient was examined in the left lateral decubitus and supine position by precordial 2-dimensional, M-mode, Doppler, and tissue Doppler echocardiography according to the criteria of American Society of Echocardiography.31, 32 During the echocardiographic examination, a 1-lead ECG was recorded continuously. An average of three consecutive cycles was analyzed for each parameter. Left ventricular end-systolic and end-diastolic diameters (LVIDS and LVIDD) and left atrial (LA) end-systolic diameter were measured by M-mode imaging in the parasternal long axis view. LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), and ejection fraction (LVEF) were estimated by Simpson's rule in the apical 4-chamber view. MS was quantified by estimation of MVA by 2D planimetry in parasternal short axis view and by the pressure half time method in the apical 4-chamber view using continuous wave (CW) Doppler.25, 26 Peak and mean transmitral diastolic pressure gradients (PDG and MDG) were measured by continuous wave Doppler in the apical four chamber view. Mitral valve morphology and the sub-valvular apparatus were assessed by 2D imaging, and Wilkins's score was calculated.27, 28 Pulmonary artery systolic pressure (PASP) was calculated by adding the estimated right atrial pressure to {(tricuspid regurgitation velocity)2 × 4}, measured by continuous wave Doppler in the apical four chamber view.33 Color flow Doppler imaging was used to detect and quantify the severity of mitral regurgitation according to the guidelines of the American Society of Echocardiography.34
LA volume was calculated by the modified Simpson's method from the apical four chamber view at end systole, and was corrected for body surface area (BSA).35
For the assessment of inter- and intra-atrial electromechanical delays, tissue Doppler echocardiography was performed by activating the TDI mode of the same machine. TDI was done with transducer frequencies of 3.5–4 MHz, adjusting the spectral pulse Doppler signal filters until a Nyquist limit of 15–20 cm/s was reached and using the minimal optical gain. The monitor sweep speed was set at 50–100 mm/s to optimize the spectral display of myocardial velocities.9, 10, 11, 12, 13, 14, 15 In the apical four chamber view, the pulsed Doppler sample volume was placed at the level of LV lateral mitral annulus (Fig. 1), septal mitral annulus (Fig. 2), and right ventricular (RV) tricuspid annulus (Fig. 3). Special effort was made to align the pulsed wave cursor in order to keep the Doppler angle of incidence as close to 0° as possible to the direction of these walls. Time interval from the onset of P-wave on surface ECG to the beginning of late diastolic wave (A′ wave) on TDI, named as PA′, was obtained from lateral mitral annulus (), septal mitral annulus (), and RV tricuspid annulus (), respectively.9, 10, 11, 12, 13, 14, 15 The difference between lateral PA′ and tricuspid PA′ () was defined as inter-atrial electromechanical delay (AEMD); difference between septal PA′ and tricuspid PA′ () was defined as right intra-atrial electromechanical delay (R-IAEMD); and the difference between lateral PA′ and septal PA′ () was defined as left intra-atrial electromechanical delay (L-IAEMD).9, 10, 11, 12, 13, 14, 15
Fig. 1.
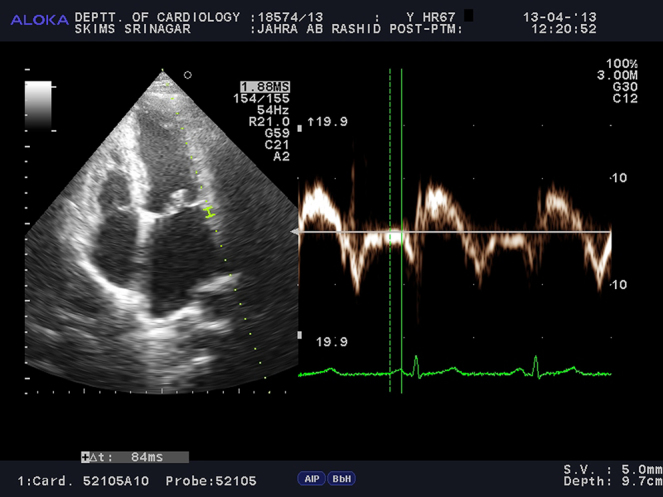
Measurement of by Tissue Doppler Imaging (TDI).
Fig. 2.
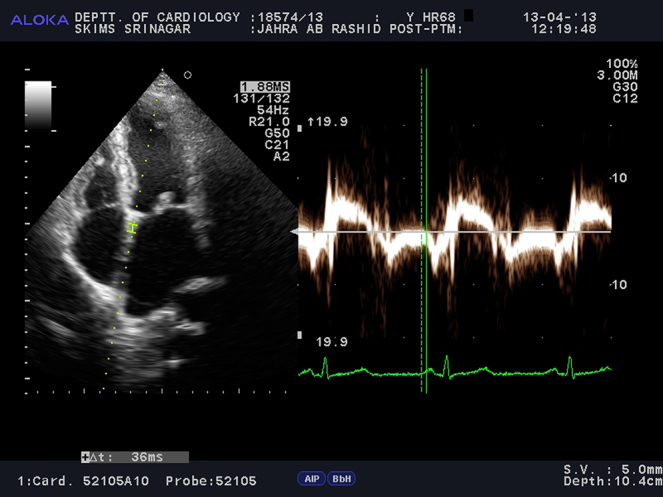
Measurement of by Tissue Doppler Imaging (TDI).
Fig. 3.
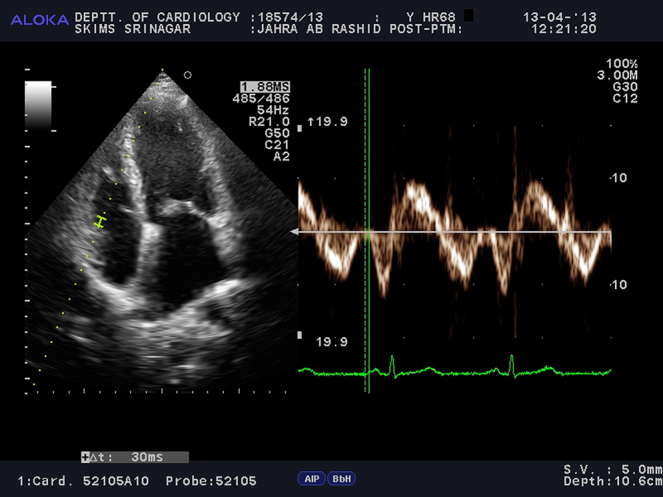
Measurement of by Tissue Doppler Imaging (TDI).
Immediately after the PTMC procedure, a repeat echocardiogram was performed to assess the post-procedural MVA, presence and severity of mitral regurgitation, peak and mean transmitral diastolic pressure gradients, PASP, and to define the success of procedure as described previously.29, 30 All patients, in whom PTMC was successful, underwent a repeat echocardiographic evaluation 24–48 h after the procedure for the measurement of post-procedural LA diameter; LV dimensions, volumes and EF; LA volume; and inter- and intra-atrial electromechanical delays.
2.7. PTMC procedure
PTMC was performed using a standard trans-septal approach with an Inoue balloon.36 Pre- and post-procedural catheterization data were not included in this study.
2.8. Collection and recording of data
All the patient data including demographic, clinical, diagnostic data as well as pre- and post-procedural electromechanical parameters, procedural outcome, complications, and discharge diagnosis were recorded on a specially pre-designed patient record form.
2.9. Statistical analysis
Statistical analysis was performed by SPSS software package (version 20.0, SPSS Inc, Chicago, Illinois, USA). All continuous variables were expressed as mean ± SD, and categorical variables were reported as frequency and percentages. Pearson's correlation coefficients were used to assess the strength of relationship between continuous variables. Paired t-test was used to study the difference of means of various continuous variables. Statistical significance was defined as a p value of <0.05.
3. Results
The initial study population included 30 patients of symptomatic severe MS, in normal sinus rhythm, who fulfilled the other inclusion criteria before the procedure. Out of these, 5 patients were excluded from the study after PTMC (2 patients did not give consent for the study, 1 patient had a failed trans-septal puncture, 1 patient developed severe post-procedural mitral regurgitation, and 1 patient developed peri-procedural AF which reverted to sinus rhythm after DC cardioversion). Final study sample consisted of the remaining 25 patients. Immediate procedural success rate was 93.3% (peri-procedural AF was not considered to be a procedural failure).
3.1. Patient characteristics
Mean age of the patients was 34.1 years, ranging from 21 to 45 years. Among the 25 patients recruited in the study sample, 7 (28%) were male while 18 (72%) patients were female. Mean body surface area of the patients was 1.45 m2, ranging from 1.29 m2 to 1.75 m2.
3.2. Pre-PTMC parameters
3.2.1. Routine echocardiographic parameters
Baseline echocardiographic parameters of the patients were as follows: LA diameter – 4.82 ± 0.51 cm; LVIDD – 4.39 ± 0.34 cm; LVIDS – 2.73 ± 0.36 cm; LVEDV – 60.99 ± 10.79 ml/m2; LVESV – 19.37 ± 5.77 ml/m2; LVEF – 68.52 ± 5.48%; Wilkins score – 6.04 ± 1.27; MVA-2D – 0.74 ± 0.13 cm2; MVA-PHT – 0.79 ± 0.14 cm2; PDG – 27.76 ± 6.25 mmHg; MDG – 15.60 ± 4.23 mmHg; PASP – 58.68 ± 13.14 mmHg; and LA volume – 79.0 ± 12.30 ml/m2.
3.2.2. P-wave durations and PWD
Baseline P-wave durations were: Pmax – 136.40 ± 13.19 ms; and Pmin – 91.60 ± 12.48 ms. PWD was 44.80 ± 5.86 ms.
3.2.3. PA′ intervals, Inter- and Intra-atrial electromechanical delays
Baseline PA′ intervals were: – 44.96 ± 5.57 ms; – 106.24 ± 18.48 ms; and – 32.48 ± 4.81 ms. Inter- and intra-atrial electromechanical delays were: AEMD – 73.76 ± 15.67 ms; R-IAEMD – 12.48 ± 2.66 ms; and L-IAEMD – 61.28 ± 14.27 ms.
3.3. Pearson correlation analysis of inter- and intra-atrial electromechanical delays (Table 1)
Table 1.
Pearson correlation analysis of inter- and intra-atrial electromechanical delays.
| Parameter | AEMD |
R-IAEMD |
L-IAEMD |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | 0.701 | <0.001 | 0.419 | 0.037 | 0.691 | <0.001 |
| BSA | −0.139 | 0.508 | −0.211 | 0.312 | −0.113 | 0.590 |
| LA diameter | 0.757 | <0.001 | 0.445 | 0.026 | 0.749 | <0.001 |
| LVIDD | −0.166 | 0.428 | 0.061 | 0.772 | −0.193 | 0.354 |
| LVIDS | −0.153 | 0.466 | 0.106 | 0.613 | −0.188 | 0.369 |
| LVEDV | −0.112 | 0.593 | 0.152 | 0.469 | −0.151 | 0.470 |
| LVESV | −0.133 | 0.525 | 0.166 | 0.428 | −0.177 | 0.396 |
| LVEF | 0.135 | 0.521 | −0.155 | 0.460 | 0.177 | 0.398 |
| Wilkins score | 0.564 | 0.003 | 0.338 | 0.099 | 0.556 | 0.004 |
| MVA-2D | −0.421 | 0.036 | −0.151 | 0.470 | −0.434 | 0.030 |
| MVA-PHT | −0.424 | 0.035 | −0.261 | 0.208 | −0.417 | 0.038 |
| PDG | 0.513 | 0.009 | 0.217 | 0.297 | 0.523 | 0.007 |
| MDG | 0.504 | 0.010 | 0.284 | 0.169 | 0.500 | 0.011 |
| PASP | 0.662 | <0.001 | 0.309 | 0.132 | 0.669 | <0.001 |
| LA volume | 0.733 | <0.001 | 0.535 | 0.006 | 0.706 | <0.001 |
| Pmax | 0.742 | <0.001 | 0.288 | 0.162 | 0.760 | <0.001 |
| Pmin | 0.616 | 0.001 | 0.277 | 0.180 | 0.625 | 0.001 |
| PWD | 0.358 | 0.079 | 0.060 | 0.776 | 0.382 | 0.060 |
Note: r, correlation coefficient; p, p value; BSA, body surface area; LA, left atrium; LVIDD, left ventricular diastolic diameter; LVIDS, left ventricular systolic diameter; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; MVA, mitral valve area-planimetry; MVA-PHT, mitral valve area – pressure half time; PDG, peak diastolic gradient; MDG, mean diastolic gradient; PASP, pulmonary artery systolic pressure; Pmax, maximum P-wave duration; Pmin, minimum P-wave duration; PWD, P-wave dispersion. Bold entries represent significant p values.
AEMD and L-IAEMD showed a positive correlation with age, LA diameter, Wilkins score, PDG, MDG, PASP, LA volume, Pmax, and Pmin. AEMD and L-IAEMD were negatively correlated with MVA. AEMD and L-IAEMD did not show any significant correlation with PWD. R-IAEMD showed a positive correlation with age, LA diameter, and LA volume. There was no significant correlation between R-IAEMD and MVA MDG, RVSP, Pmax, Pmin, or PWD (Table 1).
3.4. Impact of PTMC on various parameters
3.4.1. Routine echocardiographic parameters (Table 2)
Table 2.
Impact of PTMC on routine echo parameters.
| Parameter | Pre-PTMC (mean ± SD) | Post-PTMC (mean ± SD) | Difference (mean) | p-Value |
|---|---|---|---|---|
| LA diameter (cm) | 4.82 ± 0.51 | 4.46 ± 0.58 | 0.36 | <0.001 |
| LA volume (ml/mm2) | 79.00 ± 12.30 | 67.95 ± 14.93 | 11.05 | <0.001 |
| LVIDD (cm) | 4.39 ± 0.34 | 4.54 ± 0.29 | −0.15 | <0.001 |
| LVIDS (cm) | 2.73 ± 0.36 | 2.78 ± 0.27 | −0.05 | 0.136 |
| LVEDV (ml/m2) | 60.99 ± 10.79 | 65.82 ± 8.97 | −4.83 | <0.001 |
| LVESV (ml/m2) | 19.37 ± 5.77 | 20.15 ± 4.44 | −0.78 | 0.193 |
| LVEF (%) | 68.52 ± 5.48 | 69.56 ± 4.44 | −1.04 | 0.143 |
| MVA-2D (cm2) | 0.74 ± 0.13 | 1.77 ± 0.25 | −1.03 | <0.001 |
| MVA-PHT (cm2) | 0.79 ± 0.14 | 1.80 ± 0.22 | −1.01 | <0.001 |
| PDG (mmHg) | 27.76 ± 6.25 | 13.12 ± 4.43 | 14.64 | <0.001 |
| MDG (mmHg) | 15.60 ± 4.23 | 6.40 ± 1.91 | 9.20 | <0.001 |
| PASP (mmHg) | 58.68 ± 13.14 | 45.92 ± 11.56 | 12.76 | <0.001 |
Note: LA, left atrium; LVIDD, left ventricular diastolic diameter; LVIDS, left ventricular systolic diameter; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; MVA, mitral valve area-planimetry; MVA-PHT, mitral valve area – pressure half time; PDG, peak diastolic gradient; MDG, mean diastolic gradient; PASP, pulmonary artery systolic pressure. Bold entries represent significant p values.
Immediately after PTMC, there was a statistically significant decrement in LA diameter, LA volume, PDG, MDG, and PASP. There was a statistically significant increase in MVA-2D, MVA-PHT, LVIDD, and LVEDV. There was no significant change in LVIDS, LVESV, and LVEF after PTMC (Table 2).
3.4.2. P-wave durations and PWD (Table 3)
Table 3.
Impact of PTMC on P-wave durations and PWD.
| Parameter | Pre-PTMC (mean ± SD) | Post-PTMC (mean ± SD) | Difference (mean) | p-Value |
|---|---|---|---|---|
| Pmax (ms) | 136.40 ± 13.19 | 136.40 ± 13.19 | 0.00 | 1.000 |
| Pmin (ms) | 91.60 ± 12.48 | 91.60 ± 12.48 | 0.00 | 1.000 |
| PWD (ms) | 44.80 ± 5.86 | 44.80 ± 5.86 | 0.00 | 1.000 |
Note: Pmax, maximum P-wave duration; Pmin, minimum P-wave duration; PWD, P-wave dispersion.
There was no change in Pmax, Pmin, and PWD in the immediate post-PTMC phase (Table 3).
3.4.3. PA′ intervals, inter- and intra-atrial electromechanical delays (Table 4)
Table 4.
Impact of PTMC on PA′ intervals, inter- and intra-atrial electromechanical delays.
| Parameter | Pre-PTMC (mean ± SD) | Post-PTMC (mean ± SD) | Difference (mean) | p-Value |
|---|---|---|---|---|
| 44.96 ± 5.57 | 42.08 ± 5.67 | 2.88 | <0.001 | |
| 106.24 ± 18.48 | 89.28 ± 15.00 | 16.96 | <0.001 | |
| 32.48 ± 4.81 | 31.36 ± 4.72 | 1.12 | 0.016 | |
| AEMD (ms) | 73.76 ± 15.67 | 57.92 ± 11.73 | 15.84 | <0.001 |
| R-IAEMD (ms) | 12.48 ± 2.66 | 10.72 ± 2.51 | 1.76 | <0.001 |
| L-IAEMD (ms) | 61.28 ± 14.27 | 47.20 ± 10.89 | 14.08 | <0.001 |
Note: AEMD, atrial electromechanical delay; R-IAEMD, right intra-atrial electromechanical delay; L-IAEMD, left intra-atrial electromechanical delay. Bold entries represent significant p values.
There were statistically significant reductions in , , intervals, AEMD, R-IAEMD, and L-IAEMD in the immediate post-PTMC phase (Table 4).
4. Discussion
The main findings of our study were:
-
1.
Successful PTMC led to a statistically significant decrease in inter- and intra-atrial electromechanical delays.
-
2.
There was no change in P-wave durations or P-wave dispersion immediately after PTMC.
-
3.
Inter- and intra-atrial electromechanical delays showed a strong positive correlation with P-wave durations but not with PWD.
In rheumatic MS, the left atrial anatomy, physiology, and electrophysiology are adversely affected secondary to increased left atrial afterload and direct involvement of left atrium by rheumatic carditis.1, 2, 3, 37 These changes are associated with an increased risk of development of AF which significantly increases the long-term mortality and morbidity of these patients.4, 37, 38
Introduced first in 1984 by Inoue et al.,36 PTMC has currently become the treatment of choice in patients with moderate to severe MS, with favorable valve morphology, who are symptomatic or have new onset AF or significant pulmonary hypertension.39 Over the past three decades, extensive clinical experience has established the safety and efficacy of this procedure in both short and long term.16, 17, 18, 19, 27, 28, 29, 30 Some studies have shown that PTMC has a favorable impact on long-term incidence of AF in MS patients.20, 40 In the present study, we not only described the proven acute hemodynamic benefits of PTMC but also demonstrated its favorable effect on novel parameters of left atrial electromechanical remodeling. We also described the correlation of these parameters with each other and with various clinical and hemodynamic variables.
Our study, in consistency with the previous well-established studies, demonstrated significant increase in MVA, LV end-diastolic dimensions and end-diastolic volumes, and significant decrease in LA diameter, trans-mitral diastolic pressure gradients and PASP immediately after PTMC.16, 17, 18, 19, 27, 28, 29, 30
Increased Pmax and PWD are well-known ECG markers of non-uniform and heterogeneous atrial conduction.13 Various studies have demonstrated their association with left atrial size and risk of developing AF.5, 6 Previous investigations have also shown that PWD is increased in patients with rheumatic MS.7, 8 In a study conducted on patients with mild to moderate MS, Guntekin et al. demonstrated that Pmax and PWD increase progressively in accordance with the progression of MS.41 They also showed that Pmax, Pmin, and PWD were significantly correlated with MVA, mean mitral gradient, LA size, and PASP. Likewise, we also found that baseline Pmax and Pmin had a significant correlation with these parameters. Additionally, we also demonstrated that Pmax and Pmin had positive correlation with age, Wilkins score, and left atrial volume. These findings suggest that increasing age and greater distortion of mitral valve apparatus (suggestive of greater rheumatic activity process) and larger LA volume are associated with more severe structural changes in the left atrium, leading to greater electrical inhomogeneity, non-uniform conduction velocities, and inhomogeneous refractory periods within the atrial myocardium, which manifests on the ECG as increased P-wave duration. These observations are supported by the findings of Kabukcu et al., who suggested that AF is a marker of widespread rheumatic damage in patients with MS.42 Although PWD was prolonged in our patients, we did not find any significant correlation between PWD and other parameters. Rezaian et al. have also made a similar observation in the past.43 They attributed this finding to the fact that majority of the patients in their study had mild MS and a substantial proportion of them were on beta blockers (known to reduce PWD).8 Since we excluded the patients with non-severe MS and those taking beta blockers from our study, these reasons could not explain our finding, which needs further clarification. Furthermore, we did not observe any change in P-wave durations and PWD in the immediate post-PTMC period. While some authors have demonstrated an acute decrease in these parameters after successful PTMC,7 others have found that such regression occurs somewhat late (≥6 months) after the procedure.44 We believe that surface ECG manifestations of delayed and heterogeneous atrial conduction may take time to resolve after successful relief of MS. This needs confirmation on follow-up.
AEMD is a novel, simple, and inexpensive alternative to invasive electrophysiological studies for assessing atrial electromechanical remodeling.9, 10, 11, 12, 13, 14, 15 Recent studies have revealed that AEMD is prolonged in patients with paroxysmal AF and other disease states associated with increased risk of AF.9, 10, 11, 12, 13, 14, 15 These studies indicated that prolonged AEMD seems to reflect atrial remodeling for an arrhythmogenic substrate. Ozer et al. demonstrated that AEMD is prolonged in patients with MS and is correlated with PWD.13 They also showed that AEMD is related with left atrial size but not with the severity of MS. Our study revealed that AEMD was positively correlated with age, LA diameter, Wilkins score, trans-mitral pressure gradients, pulmonary artery pressure, LA volume, Pmax, and Pmin. Although AEMD had a positive correlation with PWD, this was not statistically significant in our study. Additionally, AEMD showed a negative correlation with MVA. This was in contrast to the observation made by Ozer et al.13 This discrepancy could partly be explained by the fact our patients had more severe MS compared to those included in that study (MVA – 0.74 ± 0.13 cm2 vs 1.5 ± 0.36 cm2). Univariate correlations of L-IAEMD were similar to those of AEMD, while R-IAEMD was positively correlated with age, LA diameter, and LA volume. These findings suggest that like P-wave duration and PWD, inter- and intra-atrial electromechanical delays are markers of left atrial remodeling in patients with MS and prolong progressively with increasing age, severity of MS, and left atrial volumes. Furthermore, a strong positive correlation between these electrocardiographic and echocardiographic parameters suggests their complementary role in identifying patients at increased risk of developing AF.
We also demonstrated a significant decrease in AEMD, L-IAEMD, and R-IAEMD immediately after successful PTMC. Till date, there is only one published study that has demonstrated such a finding.23 Since we did not observe any corresponding reductions in P-wave durations and PWD, it may indicate that TDI is superior to surface ECG in reflecting the changes in atrial electrical milieu after relief of MS. Such a speculation needs validation by invasive electrophysiological studies. Another interesting finding of our study was that R-IAEMD contributed to 17% of the total AEMD before PTMC and this contribution increased to 19% after PTMC. Thus, despite the fact that both R-IAEMD and L-IAEMD decreased significantly after PTMC, the reduction in total AEMD was attributable mainly to the decrease in L-IAEMD. This can be logically explained to be a consequence of reduction in left atrial size and pressure following relief of MS. The increased contribution of R-IAEMD to the total AEMD after PTMC could be hypothesized to be secondary to right atrial volume overload due to left to right interatrial shunt across the acquired atrial septal defect. Whether this contribution changes after the defect gets sealed needs to be addressed in the long-term follow-up.
5. Limitations
The main limitations of our study were as follows:
-
(i)
This study was carried out at a single center and the study sample was relatively small.
-
(ii)
We only studied the immediate impact of successful PTMC on the electrocardiographic and echocardiographic markers of AF risk. We presently do not have any long-term follow-up data of these patients in terms of the changes in these parameters and their actual predictive value with regard to the risk of development of AF. Long-term prospective studies in larger groups of patients are required to arrive at such definitive conclusions.
-
(iii)
We measured the conduction times only with TDI and did not use the gold standard, i.e. electrophysiological study to validate our results.
-
(iv)
Finally, it is well recognized that the development of clinical AF is complex and depends not only on substrate but also on other factors such as triggers and initiators. The effect of reversal of stretch on these other factors was not addressed by this study.
6. Conclusion
Successful PTMC has a favorable early impact on inter- and intra-atrial electromechanical delays, which are considered as novel parameters of atrial electromechanical remodeling in MS patients. Prospective large-scale studies are required to confirm whether improvement in these markers translates into reduced long-term AF risk.
Conflicts of interest
The authors have none to declare.
References
- 1.Otto C.M., Bonow R.O. Valvular heart disease. In: Libby P., Bonow R.O., Mann D.L., Zipes D.P., Braunwald E., editors. Braunwald's Heart Disease: A Text Book of Cardiovascular Medicine. 8th ed. Saunders Elsevier; Philadelphia: 2008. pp. 1646–1657. [Google Scholar]
- 2.Alpert J.S., Sabik J., Casgrove D.M. Mitral valve disease. In: Topol E.J., editor. Textbook of Cardiovascular Medicine. Lippincott-Raven; New York: 1998. pp. 505–506. [Google Scholar]
- 3.Braunwald E. Valvular heart disease. In: Braunwald E., Zipes D.P., Libby P., editors. Heart Disease: A Textbook of Cardiovascular Disease. 6th ed. WB Saunders; Philadelphia: 2001. pp. 1643–1653. [Google Scholar]
- 4.Josephson M.E., Kastor J.A., Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol. 1977;39:967–971. doi: 10.1016/s0002-9149(77)80209-9. [DOI] [PubMed] [Google Scholar]
- 5.Dilaveris P.E., Gialafos E.J., Sideris S.K. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–738. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 6.Dilaveris P.E., Gialafos E.J., Andrikopoulos G.K. Clinical and electrocardiographic predictors of recurrent atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:352–358. doi: 10.1111/j.1540-8159.2000.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 7.Turhan H., Yetkin E., Senen K. Effects of percutaneous mitral balloon valvuloplasty on P-wave dispersion in patients with mitral stenosis. Am J Cardiol. 2002;89:607–609. doi: 10.1016/s0002-9149(01)02307-4. [DOI] [PubMed] [Google Scholar]
- 8.Erbay A.R., Turhan H., Yasar A. Effects of long-term beta blocker therapy on P-wave duration and dispersion in patients with rheumatic mitral stenosis. Int J Cardiol. 2005;102:33–37. doi: 10.1016/j.ijcard.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 9.Cui Q.Q., Zhang W., Wang H. Assessment of atrial electromechanical coupling and influential factors in nonrheumatic paroxysmal atrial fibrillation. Clin Cardiol. 2008;31:74–78. doi: 10.1002/clc.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merckx K.L., De Vos C.B., Palmans A. Atrial activation time determined by transthoracic Doppler tissue imaging can be used as an estimate of the total duration of atrial electrical activation. J Am Soc Echocardiogr. 2005;18:940e4. doi: 10.1016/j.echo.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Rein A., O’Donnel C.P., Colan S.D., Marx G.R. Tissue velocity Doppler assessment of atrial and ventricular electromechanical coupling and atrioventricular time intervals in normal subjects. Am J Cardiol. 2003;92:1347–1350. doi: 10.1016/j.amjcard.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Deniz A., Sahiner L., Aytemir K. Tissue Doppler echocardiography can be a useful technique to evaluate atrial conduction time. Cardiol J. 2012;19:487–493. doi: 10.5603/cj.2012.0089. [DOI] [PubMed] [Google Scholar]
- 13.Ozer N., Yavuz B., Can I. Doppler tissue evaluation of intra-atrial and interatrial electromechanical delay and comparison with P-wave dispersion in patients with mitral stenosis. J Am Soc Echocardiogr. 2005;18:945–948. doi: 10.1016/j.echo.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Omi W., Nagai H., Takamura M. Doppler tissue analysis of atrial electromechanical coupling in paroxysmal atrial fibrillation. J Am Soc Echocardiogr. 2005;18:39–44. doi: 10.1016/j.echo.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Pekdemir H., Cansel M., Yagmur J. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in patients with mitral annulus calcification. J Electrocardiol. 2010;43:339–343. doi: 10.1016/j.jelectrocard.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Hung J.S., Chern M.S., Wu J.J. Short- and long-term results of catheter balloon percutaneous transvenous mitral commissurotomy. Am J Cardiol. 1991;67:854–862. doi: 10.1016/0002-9149(91)90619-v. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura R.A., Holmes D.R., Jr., Reeder G.S. Efficacy of percutaneous mitral valvuloplasty with the Inoue balloon. Mayo Clin Proc. 1991;66:276–282. doi: 10.1016/s0025-6196(12)61009-x. [DOI] [PubMed] [Google Scholar]
- 18.Lau K.W., Gao W., Ding Z.P., Hung J.S. Immediate and long-term results of percutaneous Inoue balloon mitral commissurotomy with use of a simple height-derived balloon sizing method for the stepwise dilatation technique. Mayo Clin Proc. 1996;71:556–563. doi: 10.4065/71.6.556. [DOI] [PubMed] [Google Scholar]
- 19.Arora R., Kalra G.S., Singh S. Percutaneous transvenous mitral commissurotomy: immediate and long-term follow up results. Catheter Cardiovasc Interv. 2002;55:450–456. doi: 10.1002/ccd.10109. [DOI] [PubMed] [Google Scholar]
- 20.Abe S., Matsubara T., Hori T. Effect of percutaneous transvenous mitral commissurotomy for the preservation of sinus rhythm in patients with mitral stenosis. J Cardiol. 2001;38:29–34. [PubMed] [Google Scholar]
- 21.Coronel R., Langerveld J., Boersma L.V. Left atrial pressure reduction for mitral stenosis reverses left atrial direction-dependent conduction abnormalities. Cardiovasc Res. 2010;85:711–718. doi: 10.1093/cvr/cvp374. [DOI] [PubMed] [Google Scholar]
- 22.John B., Stiles M.K., Kuklik P. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol. 2010;55:1217–1226. doi: 10.1016/j.jacc.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 23.Demirkan B., Guray Y., Guray U. The acute effect of percutaneous mitral balloon valvuloplasty on atrial electromechanical delay and P-wave dispersion in patients with mitral stenosis. Herz. 2013;38:210–215. doi: 10.1007/s00059-012-3672-3. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R.A., Rihal C.S., Tajik A.J., Holmes D.R., Jr. Accurate measurement of the transmitral gradient in patients with mitral stenosis: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1994;24:152–158. doi: 10.1016/0735-1097(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 25.Faletra F., Pezzano A., Jr., Fusco R. Measurement of mitral valve area in mitral stenosis: four echocardiographic methods compared with direct measurement of anatomic orifices. J Am Coll Cardiol. 1996;28:1190–1197. doi: 10.1016/S0735-1097(96)00326-9. [DOI] [PubMed] [Google Scholar]
- 26.Thomas J.D., Weyman A.E. Doppler mitral pressure half-time: a clinical tool in search of theoretical justification. J Am Coll Cardiol. 1987;10:923–929. doi: 10.1016/s0735-1097(87)80290-5. [DOI] [PubMed] [Google Scholar]
- 27.Abascal V.M., Wilkins G.T., O'Shea J.P. Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy. Circulation. 1990;82:448–456. doi: 10.1161/01.cir.82.2.448. [DOI] [PubMed] [Google Scholar]
- 28.Wilkins G.T., Weyman A.E., Abascal V.M., Block P.C., Palacios I.F. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palacios I.F., Sanchez P.L., Harrell L.C., Weyman A.E., Block P.C. Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long-term outcome. Circulation. 2002;105:1465–1471. doi: 10.1161/01.cir.0000012143.27196.f4. [DOI] [PubMed] [Google Scholar]
- 30.Fawzy M.E., Hegazy H., Shoukri M., El Shaer F., ElDali A., Al-Amri M. Long-term clinical and echocardiographic results after successful mitral balloon valvotomy and predictors of long-term outcome. Eur Heart J. 2005;26:1647–1652. doi: 10.1093/eurheartj/ehi226. [DOI] [PubMed] [Google Scholar]
- 31.Quiñones M.A., Otto C.M., Stoddard M. Recommendations for quantification of Doppler Echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 32.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Currie P.J., Seward J.B., Chan K.L. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 34.Zoghbi W.A., Enriquez-Sarano M., Foster E. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler Echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 35.Kircher B., Abbott J.A., Pau S. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography. Am Heart J. 1991;121:864–871. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K., Owaki T., Nakamura T., Kitamura F., Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 37.John B., Stiles M.K., Kuklik P. Electrical remodelling of the left and right atria due to rheumatic mitral stenosis. Eur Heart J. 2008;29:2234–2243. doi: 10.1093/eurheartj/ehn329. [DOI] [PubMed] [Google Scholar]
- 38.Selzer A., Cohn K.E. Natural history of mitral stenosis: a review. Circulation. 1972;45:878–890. doi: 10.1161/01.cir.45.4.878. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 40.Fawzy M.E., Shoukri M., Sergani H.A. Favorable effect of balloon mitral valvuloplasty on the incidence of atrial fibrillation in patients with severe mitral stenosis. Cathet Cardiovasc Interv. 2006;68:536–541. doi: 10.1002/ccd.20770. [DOI] [PubMed] [Google Scholar]
- 41.Guntekin U., Gunes Y., Tuncer M., Gunes A., Sahin M., Simsek H. Long term follow-up of P-wave duration and dispersion in patients with mitral stenosis. Pacing Clin Electrophysiol. 2008;31:1620–1624. doi: 10.1111/j.1540-8159.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 42.Kabukcu M., Arslantas E., Ates I., Demircioglu F., Ersel F. Clinical, echocardiographic, and hemodynamic characteristics of rheumatic mitral valve stenosis and atrial fibrillation. Angiology. 2005;56:159–163. doi: 10.1177/000331970505600206. [DOI] [PubMed] [Google Scholar]
- 43.Rezaian G.R., Rezaian S., Liaghat L., Zare N. P-wave dispersion in patients with rheumatic mitral stenosis. Int J Angiol. 2007;16:20–23. doi: 10.1055/s-0031-1278239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarastchuk J.C., Guérios E.E., Perreto S. Changes in P-wave after percutaneous mitral valvuloplasty in patients with mitral stenosis and left atrial enlargement. Arg Bras Cardiol. 2006;87:359–363. doi: 10.1590/s0066-782x2006001600020. [DOI] [PubMed] [Google Scholar]


