Abstract
Background
An association between fibromyalgia and hepatitis C virus (HCV) has been previously described. However, the relationship between nonalcoholic steatohepatitis (NASH) and fibromyalgia symptoms has not been assessed, though they share several risk factors.
Aim
We aimed to assess the factors associated with fibromyalgia symptoms across etiologies of liver disease.
Methods
Patients with cirrhosis due to HCV, NASH, or alcohol were recruited from an outpatient hepatology clinic and administered the Hospital Anxiety and Depression Score, Pittsburgh Sleep Quality Index, and the modified 2010 American College of Rheumatology Diagnostic Criteria for Fibromyalgia. Serum inflammatory markers were measured with standard luminex assays.
Results
Of 193 participants, 53 (27 %) met criteria for fibromyalgia. Fibromyalgia symptoms were significantly associated with etiology of liver disease (HCV: 35 %, NASH: 30 %, alcohol-related liver disease: 12 %, p < 0.01). Using logistic regression, mood symptoms (OR 1.14, 95 % CI 1.06, 1.22), sleep disturbance (OR 1.32, 95 % CI 1.16, 1.52), and etiology of liver disease (NASH vs. HCV not different, alcohol vs. HCV OR 0.19, 95 % CI 0.05, 0.63) were associated with fibromyalgia symptoms. If abdominal pain was included in the model, etiology became nonsignificant, indicating that it may be central sensitization due to abdominal pain in patients with chronic liver disease that explains fibromyalgia symptoms rather than the etiology of liver disease or inflammation.
Conclusions
Fibromyalgia symptoms were significantly associated with HCV and NASH cirrhosis and with psychiatric symptoms. Future work should focus on the underlying pathophysiology and management of widespread pain in patients with cirrhosis.
Keywords: Widespread pain, Opioids, Steatohepatitis, Hepatitis C virus
Introduction
Fibromyalgia is characterized by a cluster of symptoms including widespread pain and mood and sleep symptoms. Central sensitization, whereby the central nervous system becomes increasingly responsive to a relatively benign stimulus, is thought to underlie this chronic widespread pain [1, 2]. Fibromyalgia is present in approximately 4 % of the US population and significantly contributes to functional impairment and disability [3]. The pathogenesis of fibromyalgia remains poorly understood. Reports of a higher-than-expected prevalence of fibromyalgia in patients with hepatitis C virus (HCV) compared with healthy controls (19 vs. 5 %) have raised the possibility of a virally mediated pathway to illness [4]. However, the relationship between HCV and fibromyalgia has remained challenging to elucidate due largely to differences in methodology across studies [5]. The currently available data do not convincingly show that it is HCV, rather than the presence of an underlying liver disease with associated psychological symptoms and sleep disturbance, that functions as a risk factor for fibromyalgia.
There are several reasons to think that fibromyalgia symptoms would be associated with a variety of liver diseases beyond HCV. Sleep disorders, psychiatric disease, and elevated levels of inflammatory cytokines are associated with both fibromyalgia and cirrhosis. Disordered sleep is a hallmark of both hepatic encephalopathy and fibromyalgia, with sleep disturbances thought to predispose people to more severe pain [6, 7]. Mood disorders that have been linked to fibromyalgia [8] are common in cirrhosis [9]. The role of inflammation remains unclear in fibromyalgia, but pro-inflammatory cytokines have been elevated in some studies [10]. Similarly, nonalcoholic steatohepatitis (NASH) [11], alcohol-related liver disease [12–14], and HCV [15] have all been associated with elevations in pro-inflammatory cytokines compared with controls. Despite the important overlap in potential disease mechanisms, no study has examined the prevalence of fibromyalgia in a large and more heterogeneous cohort of patients with advanced liver disease, controlling for these important variables.
The current data support a critical role for central sensitization in the development of fibromyalgia. In this model, the processing of peripheral pain is amplified by the central nervous system via excitatory amino acids, substance P, and neurotrophins, and abnormalities of the autonomic nervous system and neuroendocrine axis along with negative affect participate in this amplification [16]. A recent study demonstrated that localized pain was among the strongest predictors of subsequent widespread pain, indicating a possible central mechanism [17]. We have previously found that pain, particularly abdominal pain, is common in patients with chronic liver disease and may increase as liver disease advances [18]. The pain that is common in cirrhosis may predispose to fibromyalgia symptoms.
Thus, the aims of this investigation were to: (1) determine the prevalence of fibromyalgia using a validated diagnostic instrument in patients with three different etiologies for liver cirrhosis; and (2) investigate clinical correlates of fibromyalgia in the total sample. We hypothesized that psychiatric and sleep symptoms along with chronic abdominal pain, rather than HCV viral factors, would be associated with fibromyalgia in patients with cirrhosis.
Materials and Methods
Study Design and Cohort Definitions
This cross-sectional survey was approved by the institutional review board of the University of Pittsburgh. As described previously [19] patients seen in the Center for Liver Diseases between January 2013 and March 2013 were recruited if they were at least 18 years of age. Outpatients of varying disease etiologies and stages are seen in the clinic. Given that they are the most prevalent etiologies of cirrhosis, we recruited patients with cirrhosis due to alcohol (ETOH), hepatitis C virus (HCV), or nonalcoholic steatohepatitis (NASH). Eligibility was assessed prior to their clinic visit and was based on a review of the electronic medical record. The determination of cirrhosis was made using the hepatologists’ notes and confirmed with blood work, imaging, and histology when available. Considering the effects on key endpoints, we excluded patients who were on interferon or had known inflammatory conditions (e.g., rheumatoid arthritis or multiple sclerosis). In addition, cancer (including hepatocellular carcinoma), overt hepatic encephalopathy, or prior organ transplant functioned as exclusion criteria. After recruitment and signing informed consent, participants filled out a battery of instruments (see self-report measures below) and provided blood for analyses.
Instruments and Measures
Medical Record Review
Relevant disease-specific parameters were then abstracted from the electronic medical record; these variables included disease severity, captured by the Model for End-Stage Liver Disease (MELD) score. Current medications were obtained from the charts and confirmed with participants. We assessed the use of prescription opioids, selective serotonin reuptake inhibitors (SSRIs), and selective serotonin and norepinephrine reuptake inhibitors (SNRIs). SSRI and SNRI medications were assessed separately because SNRIs are thought to have effects on pain and particularly on fibromyalgia, while SSRIs are not. Viral clearance of HCV was assessed.
Self-Report Items and Instruments
Patients were asked to self-report demographic information. The Hospital Anxiety and Depression Score (HADS) [20, 21] was designed to quantify symptoms of depression and anxiety. The scale for both depression and anxiety ranges from 0 to 21 and can be used continuous scale. When used with a cut-point of >7/21 for anxiety or depression the sensitivity ranges from 0.83 to 0.90, with a specificity of 0.78 to 0.79 [21]. This instrument was chosen because it does not include items related to physical, neurovegetative symptoms such as fatigue, energy, sleep, or pain. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) [22], which has been validated in a population with fibromyalgia [23]. Scores on the PSQI range from 0 to 21, and when a score of 5 is used as a cutoff, the PSQI has a 90 % sensitivity and 87 % specificity for identifying sleep-disordered patients [24]. Abdominal pain was deemed present if patients reported having abdominal location of pain on the McGill Pain Questionnaire (MPQ) [25]. We defined fibromyalgia using the modified 2010 American College of Rheumatology Diagnostic Criteria for Fibromyalgia (ACR 2010) [26], which has been designed and validated as a self-reported method for measuring fibromyalgia in a population with chronic pain. It has been recommended for use in a primary care setting using a cutoff of ≥13 with a sensitivity of 93.1 % and specificity of 91.7 % [27]. The burden of comorbid disease was quantified using the Charlson Comorbidity Index [28].
Pro-inflammatory Biomarkers
Systemic inflammatory response was assessed based on a commercially available luminex assay that quantified several important pro-inflammatory markers associated with pain, disturbances in sleep, and depression including C-reactive protein (CRP), IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α). Samples were obtained as part of the routine blood draws and processed, plated, and run in a standard fashion [29]. Standard control samples were run with each plate.
Statistical Analysis
The R statistical program (version 2.15.2) was used for data analysis [30]. Proportions of fibromyalgia were compared by etiology of liver disease using Chi-square testing. Baseline characteristics were compared for patients who did and did not meet fibromyalgia criteria using Student’s t and Fisher’s exact tests for continuous data and Chi-square tests for categorical data. Potential covariates included demographic variables, disease-related variables (MELD and complications and etiologies of cirrhosis), and psychiatric symptoms. Cytokine levels were compared using Wilcoxon rank-sum tests. Pairwise comparisons were made for fibromyalgia prevalence by etiology of liver disease using a Pearson’s Chi-squared test. Variables with a p value of < 0.2 in univariate testing (other than medications) were entered into an automated logistic regression model using AIC optimization to come to a final parsimonious model of factors significantly and independently associated with fibromyalgia. Multi-level variables in the final model were compared with the baseline in a pairwise fashion using p values corrected with a Holm-Bonferroni correction if the p for the overall variable was significant. The PSQI and HADS scores were entered into multivariable analyses as continuous variables. Logistic regression models were completed with and without inclusion of abdominal pain, as defined by circling this location on the body map portion of the MPQ. Subsequently, the subgroup of patients with HCV was evaluated to determine the role of viral clearance on the presence of fibromyalgia. In order to assess the predictors of widespread pain without the elements of fatigue and depressive symptoms in the fibromyalgia scale, negative binomial regression models were made to look at the number of locations of pain from the ACR 2010. Models were made first without then with abdominal pain included and then with an interaction term between HADS score and abdominal pain. All models were checked for multicollinearity using a pre-specified variance inflation factor >5.
Results
A total of 1,551 patients were scheduled to be seen at the Center for Liver Diseases over the 3-month period of recruitment. Out of this pool, 326 were eligible and came to their appointments, 192 were new patients, 658 were non-cirrhotic, 174 had other etiologies of liver disease, 40 had cancer, 23 were on interferon, 11 had other inflammatory diseases (e.g., acute alcoholic hepatitis, psoriatic arthritis, pancreatitis), 11 had undergone prior hepatic transplantation, and 7 were ineligible for other reasons (e.g., non-English speaking). Subsequently, 109 did not come to their scheduled appointments. Of the 326 possible subjects, 210 participants were recruited, 193 people making up the final cohort after 17 were excluded after consent because they were found later to meet exclusion criteria (9 with pancreatobiliary disease on imaging, 5 with cancer, and 1 each with multiple sclerosis, encephalitis, and failure to complete research surveys). The average age was of the sample was 58 ± 9 years, 77 (40 %) were women, and the mean MELD score was 12.3 ± 5.0. Among participants, HCV was the most common etiology (N = 78), followed by nonalcoholic steatohepatitis (NASH; N = 66), and alcohol-related liver disease (ETOH; N = 49).
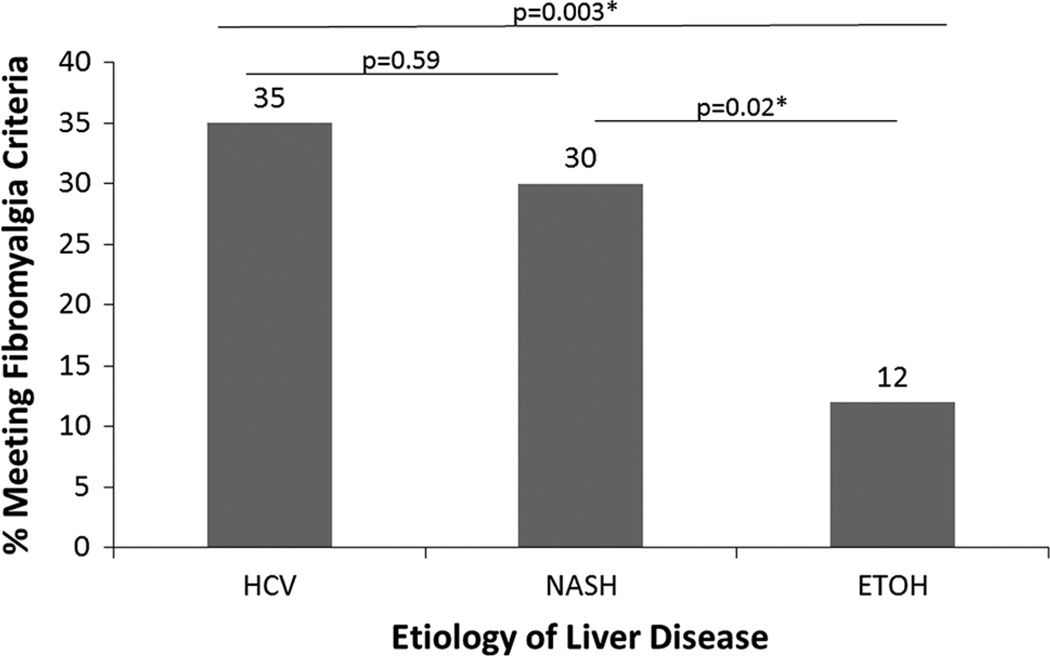
The overall point prevalence of fibromyalgia as operationally defined for the study was 27 % (Table 1). Patients with fibromyalgia were significantly younger and had increased abdominal pain and psychiatric and sleep symptoms. There were no significant differences in MELD or overall comorbidity levels by fibromyalgia status. The prevalence of meeting fibromyalgia criteria differed between etiologies of liver disease with significantly lower rates in ETOH patients (Fig. 1). Those participants who met fibromyalgia criteria were significantly more likely to be taking prescription opioids or SSRI, but not SNRI antidepressant medications (Table 1).
Table 1.
Baseline characteristics by fibromyalgia score >12
| Characteristic | No fibromyalgia (N = 140) |
Fibromyalgia (N = 53) |
p value |
|---|---|---|---|
| Age | 58.9 ± 8.8 | 55.2 ± 9.0 | 0.01 |
| Female | 50 (36) | 27 (51) | 0.08 |
| Non-white race | 12 (9) | 5 (9) | 1.00 |
| Latino | 4 (3) | 0 | 0.58 |
| BMI | 30.7 ± 5.3 | 29.2 ± 6.0 | 0.59 |
| Tobacco use | 26 (19) | 16 (30) | 0.12 |
| Charlson Comorbidity Index | 3.9 ± 1.1 | 4.0 ± 1.2 | 0.46 |
| Abdominal pain | 43 (31) | 37 (70) | <0.01 |
| Liver-related variables | |||
| Etiology of liver disease | 0.02 | ||
| HCV | 51 (36) | 27 (51) | |
| ETOH | 43 (31) | 6 (11) | |
| NASH | 46 (33) | 20 (38) | |
| MELD | 12.3 ± 5.0 | 11.5 ± 4.2 | 0.25 |
| Ascites | 72 (51) | 21 (40) | 0.19 |
| Varices | 44 (31) | 15 (28) | 0.77 |
| Encephalopathy | 106 (76) | 38 (72) | 0.91 |
| Psychiatric symptoms | |||
| Anxiety (>7) | 28 (20) | 41 (77) | <0.01 |
| Depression (>7) | 34 (24) | 35 (66) | <0.01 |
| HADS anxiety score | 5.1 ± 3.7 | 10.2 ± 4.2 | <0.01 |
| HADS depression score | 4.8 ± 3.4 | 9.2 ± 3.9 | <0.01 |
| Global HADS | 9.9 ± 6.2 | 19.4 ± 7.5 | <0.01 |
| Global PSQI | 9.0 ± 3.4 | 14.1 ± 3.5 | <0.01 |
| Medications | |||
| Prescription opioids | 19 (14) | 26 (49) | <0.01 |
| SSRI | 27 (29) | 24 (53) | <0.01 |
| SNRI | 13 (9) | 3 (6) | 0.56 |
Categorical variables are shown as N(column %) with p derived from Chi-square or Fisher’s exact tests
Continuous variables are shown as mean ± SD with p derived from Student’s t test
Bold values are statistically significant (p < 0.05)
Fig. 1.
Prevalence of fibromyalgia by etiology of liver disease
We examined whether inflammation was associated with fibromyalgia but found no statistically significant differences (Table 2). Table 3 shows the final multivariable logistic regression for predictors of fibromyalgia symptoms in total sample of patients with cirrhosis. The most important factors were the mood and sleep symptoms. Etiology was also significant in the final model. NASH and HCV had the same association with fibromyalgia, while with alcohol-related disease had less. When abdominal pain was included in the model, it was a strong predictor of fibromyalgia. Interestingly, the overall etiology variable became nonsignificant in this model, though still with a trend toward significance. When we assessed a potential interaction between HADS score and abdominal pain, this was nonsignificant.
Table 2.
Inflammatory markers in study patients meeting versus not meeting fibromyalgia criteria
| Standard controls | No fibromyalgia (N = 141) | Fibromyalgia (N = 52) | p value | |
|---|---|---|---|---|
| CRP (mg/dl) | 1.0 (0.3, 1.1) | 1.4 (0.6, 3.2) | 1.1 (0.6, 2.0) | 0.22 |
| IL-1β (pg/ml) | 18.0 (17.2, 20.9) | 23.2 (16.1, 43.7) | 21.9 (14.4, 35.9) | 0.44 |
| IL-6 (pg/ml) | 50.4 (43.9, 65.1) | 234.0 (98.5, 592.2) | 194.0 (95.6, 478.0) | 0.33 |
| IL-8 (pg/ml) | 707 (503, 807) | 1,528.0 (830.5, 2,558.0) | 1,403.0 (971.0, 2,736.0) | 0.63 |
| TNFα (pg/ml) | 228 (221, 465) | 364.0 (241.0, 524.8) | 353.0 (238.0, 563.0) | 0.93 |
Standard control values are for reference and were not included in statistical comparisons
Medians (first quartile, third quartile), p based on Wilcoxon rank-sum test
Table 3.
Factors associated with fibromyalgia symptoms
| Covariate | No abdominal pain | Abdominal pain included |
||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| Age | 0.95 | 0.90, 1.00 | 0.05 | 0.96 | 0.90, 1.01 | 0.14 |
| Etiology (vs. HCV) | 0.90, 1.00 | <0.01a | 0.07 | |||
| NASH | 0.79 | 0.29, 2.10 | 0.63 | 0.61 | 0.21, 1.75 | |
| ETOH | 0.19 | 0.05, 0.63 | 0.02 | 0.24 | 0.06, 0.78 | |
| HADS Score | 1.14 | 1.06, 1.22 | <0.01 | 1.11 | 1.03, 1.20 | <0.01 |
| PSQI Score | 1.32 | 1.16, 1.52 | <0.01 | 1.36 | 1.19, 1.59 | <0.01 |
| Abdominal pain | 3.01 | 1.16, 8.19 | 0.03 | |||
Bold values are statistically significant (p < 0.05)
For the overall variable from Wald test. When this was statistically significant, pairwise calculations were assessed for each etiology versus HCV and corrected with the Holm-Bonferroni method
In order to assess the predictors of fibromyalgia symptoms without the associated fatigue and depression in the fibromyalgia score, models were made using negative binomial regression looking at the predictors of the number of locations of pain in the body (Table 4). Other than age becoming nonsignificant, the other findings were stable. However, the etiology remained significant in all models, with patients with alcohol-related liver disease having significantly less locations of pain than those with HCV or NASH. An interaction term between the HADS score and abdominal pain was significant in this model. We also assessed how taking psychological symptoms out of the pool of potential predictors would affect the results and the predictors were otherwise the same. As a secondary analysis, we assessed whether active viremia in patients with HCV (vs. those who had successfully cleared the HCV) affected pain, and there was no association in uni- or multivariable models. There was no evidence of multicollinearity in any of the models (VIF < 2).
Table 4.
Final model of number of pain locations
| Covariate | No abdominal pain | Abdominal pain | Interaction term | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IRR | 95 % CI | p | IRR | 95 % CI | p | IRR | 95 % CI | p | |
| Etiology (vs. HCV) | 0.02* | 0.01* | <0.01 | ||||||
| NASH | 0.86 | 0.68, 1.14 | 0.30 | 0.82 | 0.63, 1.06 | 0.14 | 0.85 | 0.66, 1.09 | 0.20 |
| ETOH | 0.62 | 0.45, 0.83 | <0.01 | 0.64 | 0.48, 0.86 | 0.01 | 0.63 | 0.47, 0.85 | <0.01 |
| HADS Score | 1.03 | 1.02, 1.05 | <0.01 | 1.03 | 1.01, 1.05 | <0.01 | 1.05 | 1.03, 1.08 | <0.01 |
| PSQI Score | 1.06 | 1.02, 1.09 | <0.01 | 1.06 | 1.03, 1.09 | <0.01 | 1.06 | 1.03, 1.10 | <0.01 |
| Abdominal Pain | 1.43 | 1.12, 1.82 | <0.01 | 2.60 | 1.61, 4.20 | <0.01 | |||
| Abdo pain × HADS | 0.96 | 0.93, 0.99 | <0.01 | ||||||
Three models were made using negative binomial regression to explore the predictors of the number of locations of pain among patients with cirrhosis. One model did not include abdominal pain, one model included abdominal pain, and the third included an interaction term between psychiatric symptoms and abdominal pain
Bold values are statistically significant (p < 0.05)
Discussion
Consistent with prior investigations of patients with HCV [4], we identified a high prevalence of fibromyalgia in patients with cirrhosis, affecting nearly one-third of our large patient cohort. However, our data also provide new and important insights. First, widespread chronic pain meeting criteria for fibromyalgia was not limited to HCV, but also affected patients with other underlying causes of liver disease. Second, in HCV-infected individuals, viremia was not associated with widespread pain. Third, NASH and HCV had a comparable prevalence of fibromyalgia. Taking together with the fact that mood disorders and sleep disturbances were the best independent predictors of fibromyalgia in our cohort, this suggests that central sensitization rather than inflammatory or viral processes plays a key role in this common comorbitity.
The role of pro-inflammatory cytokines in fibromyalgia remains unclear, likely related to differences in measurement techniques. For example, monocyte cytokine evaluation from supernatants has been proposed as a more reliable marker than serum cytokine measurement [31–35]. Other investigators advocate the use of chemokines, which were not measured in this study, as the best markers for inflammation related to fibromyalgia [33]. The current study design also cannot differentiate subtle inflammation related to fibromyalgia from that associated with liver disease itself. Despite these caveats, we did not find fibromyalgia to be related to inflammatory markers. This study leaves open the possibility that widespread pain in cirrhosis is related to changes in the central processing of pain, which could be potentially related to opioid-related hyperalgesia or possibly modulated by the changes in the hypothalamic–pituitary–adrenal axis [31]. Lower cortisol levels have been associated with decreased pain thresholds and increased functional pain [36–38]. In addition, adrenal insufficiency is not uncommon in cirrhosis [39, 40]. We did not measure cortisol levels in our study and can thus not address the potential correlation between liver disease, adrenal function, and fibromyalgia symptoms in this population.
While we noted some difference in the prevalence of widespread pain by etiology of liver disease, these differences became less pronounced when we controlled for abdominal pain. We had previously demonstrated high rates of abdominal pain in patients with chronic liver disease, which was at least in part dependent on disease severity [18]. The relative homogeneity of MELD scores in this study likely explains the disparity. Our study design does not enable us to identify causes of abdominal pain and place them into a potential conceptual framework for the development of fibromyalgia. However, it is unlikely that this symptom is simply a manifestation of fibromyalgia, as about one-third of the patients without more widespread pain had at least some abdominal pain. Considering current thinking about mechanisms of central sensitization, it is possible that abdominal pain plays an important triggering role, leading to hypervigilance and progressive spread of pain-related complaints in susceptible individuals. Abdominal pain was found in 36 % of those without a history of ascites, 56 % of those with active ascites on exam, and 44 % of participants with a history of ascites that had been controlled with diet or medications and was not present at the time of the survey. Ascites and abdominal pain were not significantly associated (p = 0.17), but this may have been partially related to the small number of participants with large ascites at the time of enrollment. Ascites was not significantly associated with fibromyalgia or widespread pain in the models. Larger studies with longitudinal design will be needed to more conclusively address the roles of ascites and abdominal pain in triggering widespread pain.
While all subgroups in our cohort had a higher prevalence of fibromyalgia compared with healthy controls in other studies, we certainly saw higher numbers in NASH and HCV. Inflammatory activity with elevation of chemokines and cytokines characterizes NASH and contributes to the progression to cirrhosis [41]. While conceptually appealing, the missing correlation between biomarkers of inflammation and pain argues against this explanation. Alternatively, our findings may reflect the known association between obesity and fibromyalgia [42, 43]. Obesity as defined by the body mass index (BMI) was abstracted from the chart, but is notoriously unreliable in patients with cirrhosis given the third spacing of fluids and ascites. However, BMI was significantly associated with NASH cirrhosis and may serve as a link between NASH and fibromyalgia. This study challenges the idea that HCV mediates pain through viral factors given that participants with active viremia were not more likely to meet fibromyalgia criteria than participants with HCV and viral clearance. Additionally, our data also raise questions about the presumed close relationship between fibromyalgia and HCV, which has been seen or even labeled as entity by some [4]. The participants with alcohol-related liver disease were the least likely to meet criteria for fibromyalgia. This finding requires further evaluation, as the relationship between alcohol use and pain has yet to be fully elucidated. However, some have posited that alcohol’s modulation of gamma aminobutyric acid (GABA) transmission makes it a candidate therapy for fibromyalgia [44].
Consistent with the known link between affect, pain, and opioid use [18, 45], participants who met criteria for fibromyalgia and had ongoing pain were more likely to be on opioids. Despite analgesic therapy, chronic opioid users still described ongoing symptoms. While we may see this as an indication of the limited effectiveness of opioids in benign disorders characterized by pain [46], we can certainly not exclude skewing of data with more severely affected individuals receiving opioids but only experiencing a limited benefit. A better understanding of this complex relationship is especially important in patients with cirrhosis, given an association of these agents with worsening encephalopathy [47]. Of note, SNRIs use was uncommon in this population, though this is an accepted treatment for fibromyalgia-associated pain [48]. SNRIs, along with exercise and cognitive behavioral therapy, may be a possible intervention for patients with cirrhosis who meet fibromyalgia criteria.
Despite recruiting a large patient cohort and using a complex assessment, the study has several limitations. We only focused on patients with liver disease and did not include a disease control group. However, the prevalence of fibromyalgia symptoms in the general population is well established [3], and the alcohol group served as a type of disease control group. Moreover, the absolute prevalence is not as important as the fact that we established that NASH is as strongly associated with fibromyalgia symptoms as is HCV. While we did not assess for minimal hepatic encephalopathy, which could have confounded our findings, the majority of subjects were on treatment for a history of encephalopathy and we found no association between history of encephalopathy and fibromyalgia symptoms, HADS, or PSQI score. Also, we relied on a validated survey tool to define the presence or absence of fibromyalgia and did not confirm our findings independently with rheumatologic assessments. While prior publications demonstrated a high sensitivity and specificity for the ACR 2010, it has not been independently validated in patients with chronic and advanced liver disease. Circumstantial evidence supports that we were measuring fibromyalgia in that fibromyalgia in this study was associated with psychiatric and sleep symptoms, younger age, and female gender, which are established as associated with fibromyalgia in the literature. Additionally, the ACR 2010 yielded similar rates of fibromyalgia in the HCV group as did prior studies with the ACR 1990 [4], which further validates our findings. It is, however, notable that we used this in all-comers and not just those in whom we suspected fibromyalgia.
In conclusion, this study demonstrates the high burden of chronic widespread pain among patients with cirrhosis. In part, this is related to sleep and psychiatric symptoms as well as chronic abdominal pain, possibly suggesting central sensitization as a mechanism. While more studies are needed to better understand underlying mechanisms and evaluate therapeutic approaches, concerns about detrimental effects of opioids together with our findings and evidence-based management strategies in fibromyalgia give at least some guidelines for alternative treatments in patients with liver disease.
Acknowledgments
We would like to thank Amy Schmotzer and Sharon Boggiano for their help with this project. This project was supported by the Starzl Transplantation Institute Young Investigator Award, NIH grant T32-DK063922.
Footnotes
Conflict of interest We have no disclosures relevant to the manuscript, but Dr. Szigethy has received travel support from Merck.
Contributor Information
Shari S. Rogal, Email: rogalss@upmc.edu, Center for Health Equity Research and Promotion, VA, Pittsburgh Healthcare System, University Drive (151C), Pittsburgh, PA 15240, USA; Department of Surgery, University of Pittsburgh, Pittsburgh, PA, USA.
Klaus Bielefeldt, Email: bielefeldtk@upmc.edu, Division of Gastroenterology, Hepatology, and Nutrition, University of Pittsburgh, Pittsburgh, PA, USA.
Ajay D. Wasan, Email: wasanad@upmc.edu, Department of Anesthesia, University of Pittsburgh, Pittsburgh, PA, USA.
Eva Szigethy, Email: szigetheye@upmc.edu, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Francis Lotrich, Email: lotrichfe@upmc.edu, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Andrea F. DiMartini, Email: dimartiniaf@upmc.edu, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Wolfe F. Fibromyalgia wars. J Rheumatol. 2009;36:671–678. doi: 10.3899/jrheum.081180. [DOI] [PubMed] [Google Scholar]
- 2.Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014 doi: 10.1016/j.semarthrit.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozanoglu E, Canataroglu A, Abayli B, Colakoglu S, Goncu K. Fibromyalgia syndrome in patients with hepatitis C infection. Rheumatol Int. 2003;23:248–251. doi: 10.1007/s00296-003-0290-7. [DOI] [PubMed] [Google Scholar]
- 5.Narvaez J, Nolla JM, Valverde-Garcia J. Lack of association of fibromyalgia with hepatitis C virus infection. J Rheumatol. 2005;32:1118–1121. [PubMed] [Google Scholar]
- 6.Moldofsky H. The significance of dysfunctions of the sleeping/waking brain to the pathogenesis and treatment of fibromyalgia syndrome. Rheum Dis Clin North Am. 2009;35:275–283. doi: 10.1016/j.rdc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Piedra C, Catena A, Miro E, Pilar Martinez M, Sanchez AI, Buela-Casal G. The impact of pain on anxiety and depression is mediated by objective and subjective sleep characteristics in fibromyalgia patients. Clin. J Pain. 2013 doi: 10.1097/AJP.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CA, Auger R, Enders FT, Felmlee-Devine D, Smith GE. The effects of poor sleep quality on cognitive function of patients with cirrhosis. J Clin Sleep Med. 2014;10:21–26. doi: 10.5664/jcsm.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12:245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 12.Torer N, Ozenirler S, Yucel A, Bukan N, Erdem O. Importance of cytokines, oxidative stress and expression of BCL-2 in the pathogenesis of non-alcoholic steatohepatitis. Scand J Gastroenterol. 2007;42:1095–1101. doi: 10.1080/00365520701286680. [DOI] [PubMed] [Google Scholar]
- 13.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27:89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zampino R, Marrone A, Restivo L, et al. Chronic HCV infection and inflammation: clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–540. doi: 10.4254/wjh.v5.i10.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staud R, Rodriguez ME. Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheumatol. 2006;2:90–98. doi: 10.1038/ncprheum0091. [DOI] [PubMed] [Google Scholar]
- 17.McBeth J, Lacey RJ, Wilkie R. Predictors of new-onset widespread pain in older adults: results from a population-based prospective cohort study in the UK. Arthritis Rheumatol. 2014;66:757–767. doi: 10.1002/art.38284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogal SS, Winger D, Bielefeldt K, Szigethy E. Pain and opioid use in chronic liver disease. Dig Dis Sci. 2013;58:2976–2985. doi: 10.1007/s10620-013-2638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with Cirrhosis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.10.029. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Osorio CD, Gallinaro AL, Lorenzi-Filho G, Lage LV. Sleep quality in patients with fibromyalgia using the Pittsburgh Sleep Quality Index. J Rheumatol. 2006;33:1863–1865. [PubMed] [Google Scholar]
- 24.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- 25.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari R, Russell AS. A questionnaire using the modified 2010 American College of Rheumatology criteria for fibromyalgia: specificity and sensitivity in clinical practice. J Rheumatol. 2013;40:1590–1595. doi: 10.3899/jrheum.130367. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 30.R_Core_Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/ [Google Scholar]
- 31.Thompson ME, Barkhuizen A. Fibromyalgia, hepatitis C infection, and the cytokine connection. Curr Pain Headache Rep. 2003;7:342–347. doi: 10.1007/s11916-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Buchner M, Moser MT, Daniel V, Schiltenwolf M. The role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 months. Clin J Pain. 2009;25:1–4. doi: 10.1097/AJP.0b013e31817e13a3. [DOI] [PubMed] [Google Scholar]
- 33.Garcia JJ, Cidoncha A, Bote ME, Hinchado MD, Ortega E. Altered profile of chemokines in fibromyalgia patients. Ann Clin Biochem. 2013 doi: 10.1177/0004563213506413. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Moser M, Schiltenwolf M, Buchner M. Circulating cytokine levels compared to pain in patients with fibromyalgia—a prospective longitudinal study over 6 months. J Rheumatol. 2008;35:1366–1370. [PubMed] [Google Scholar]
- 35.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology. 2001;40:743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 36.Choi JC, Lee JH, Choi E, Chung MI, Seo SM, Lim HK. Effects of seasonal differences in testosterone and cortisol levels on pain responses under resting and anxiety conditions. Yonsei Med J. 2014;55:216–223. doi: 10.3349/ymj.2014.55.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfven G, de la Torre B, Uvnas-Moberg K. Depressed concentrations of oxytocin and cortisol in children with recurrent abdominal pain of non-organic origin. Acta Paediatr. 1994;83:1076–1080. doi: 10.1111/j.1651-2227.1994.tb12989.x. [DOI] [PubMed] [Google Scholar]
- 38.Choi JC, Chung MI, Lee YD. Modulation of pain sensation by stress-related testosterone and cortisol. Anaesthesia. 2012;67:1146–1151. doi: 10.1111/j.1365-2044.2012.07267.x. [DOI] [PubMed] [Google Scholar]
- 39.Fede G, Spadaro L, Tomaselli T, et al. Comparison of total cortisol, free cortisol, and surrogate markers of free cortisol in diagnosis of adrenal insufficiency in patients with stable cirrhosis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Aravinthan A, Al-Naeeb Y, Richardson P. Relative adrenal insufficiency in a patient with liver disease. Eur J Gastroenterol Hepatol. 2009;21:381–383. doi: 10.1097/MEG.0b013e328309c77e. [DOI] [PubMed] [Google Scholar]
- 41.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727–735. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senna MK, Sallam RA, Ashour HS, Elarman M. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: a randomized controlled trial. Clin Rheumatol. 2012;31:1591–1597. doi: 10.1007/s10067-012-2053-x. [DOI] [PubMed] [Google Scholar]
- 43.Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord-Trondelag Health Study. Arthritis Care Res. 2010;62:611–617. doi: 10.1002/acr.20118. [DOI] [PubMed] [Google Scholar]
- 44.Chung M, Wang C. Can alcohol consumption be an alternative treatment for fibromyalgia? Arthritis Res Therapy. 2013;15:126. doi: 10.1186/ar4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42:1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 47.Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the Cirrhotic Patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014 doi: 10.1111/jgh.12560. [DOI] [PubMed] [Google Scholar]
- 48.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]