Abstract
AIM: To investigate the pathogenic effect of SEB and D-GalN on liver and the protection of cyclosporin A, the relationship between hepatic apoptosis and necrosis and the possible mechanism of acute hepatic necrosis.
METHODS: After staphylococcal enterotoxin B (SEB) mixed with D-galactosamine (D-GalN) were injected intraperitoneally into Balb/c mice and those previously treated with cyclosporin A, blood samples were collected and livers were isolated at 2, 6, 12, 24h. Patterns of hepatocellular death were studied morphologically and biochemically, circulating cytokines (TNF-α, IFN-γ) and mice mortality within 24h was assessed.
RESULTS: The SEB could induce the typical apoptotic changes of hepatocytes, the D-GalN could induce hepatocytes apoptosis and degeneration at the same time, and the mice having received the SEB + D-GalN injections developed apoptosis at 2 and 6h, but after 12h hepatocytes were characterized by severe injury, whereas all the examinations in the cyclosporin A treated mice were normal.
CONCLUSION: Hepatic cell apoptosis might be related to necrosis, and massive hepatocyte apoptosis is likely the initiating step of acute hepatic necrosis in mice. The effects induced by SEB and D-GalN on hepatocytes might be mediated by T cells, and could be prevented by cyclosporin A.
Keywords: cyclosporin A, liver necrosis, apoptosis, staphylococcal enterotoxin B, D-galactosamine
INTRODUCTION
Patterns of cell death are defined as apoptosis and necrosis[1]. After staphylococcal enterotoxin B (SEB) together with D-Galactosamine (D-GalN) were injected ip. into BALB/c mice as well as those previously treated with cyclosporin A, we studied the patterns of hepatocellar death to investigate the pathogenic effect of SEB and D-GalN on liver and the protection of cyclosporin A, the relationship between hepatic apoptosis and necrosis, and the possible mechanism of acute hepatic necrosis.
MATERIALS AND METHODS
Main reagents
Cyclosporin A was given by Prof. Fan Li-An, Shanghai Institute of Immunology. D-GalN was purchased from Sigma Chemical Co. SEB was purchased from Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences.
Mice
Six-week-old male Balb/c mice weighing 20 g were purchased from the Department of Experimental Animal, Shanghai Institute of Biological Products, Ministry of H ealth of China.
Groups
Mice were randomly divided into 6 groups: NS, CSA, SEB, D-GalN, SEB+D-GalN and CSA+SEB+D-GalN. Each group was divided into 4 subgroups each containing 6 mice according to 2, 6, 12 and 24 h. After pretest, SEB amout was set at 50 μg/mice; D-GalN, 16 mg/mice; and CSA, 0.5 mg/mice. CSA was injected 0.5 h earlier. After mixed in vitro, SEB and D-GalN were injected. Control group was treated with normal saline in the same way. All drugs were injected intraperitoneally.
Light microscopic examination
Livers were isolated at 2, 6, 12 and 24 h, and immediately fixed in 100 mL/L formalin, 5 μm thick sections were stained with HE for light microscopic examination. Incidence of apoptosis bodies (ABs) was counted[2].
Transmission electron microscopic examination
Immediately after sacrifice, 1 mm3 section from the liver were fixed in 20 g/L glutaradehyde. After stained, ultrathin sections were examined under a 200CX electron microscope.
Agarose gel electrophoresis of hepatocellular DNA
DNA was purified from hepatocytes and subsequently analyzed on 10 g/L agarose gels[3].
Serum alanine aminotransferase (ALT) assay
Serum ALT activities were measured by a CX4 automatic biochemical analyzer (Beck man).
Serum tumor necrosis factor (TNF-α ) assay
It was performed according to L929 cell-killing method[4].
Serum interferon γ (IFN-γ ) assay
IFN-γ assay was made by cytopathic effect reduction method[5].
Statistical analysis
Statistical significance was evaluated according to paired student’s t test .
RESULTS
Tissue histology
Hepatocytes of SEB 2 h and 6 h groups showed nuclear pycnosis (Figure 1A) and no necrosis was found in 12 h and 24 h, suggesting that SEB only induce hepatocyte apoptosis in Balb/c-mice. Hepatocytes of D-GalN 2 h group developed chromatin condensation and organella edema (Figure 1B), 6 h group showed hepatocyte coexistence of degeneration and apoptosis bodies, no necrosis occurred after 12 h. Hepatocytes of SEB+D-GalN 2 h group showed nuclear pycnosis, nucleus was fragmented in 6 h group, after 12 h, besides some hepatocytes apoptosis, necrosis characters such as widespread destruction of the liver stracture, massive erythrocyte agglutination, etc (Figure 1C) were found. Hepatocytes of the CSA+SEB+D-GalN group were normal (Figure 1D).
Figure 1.
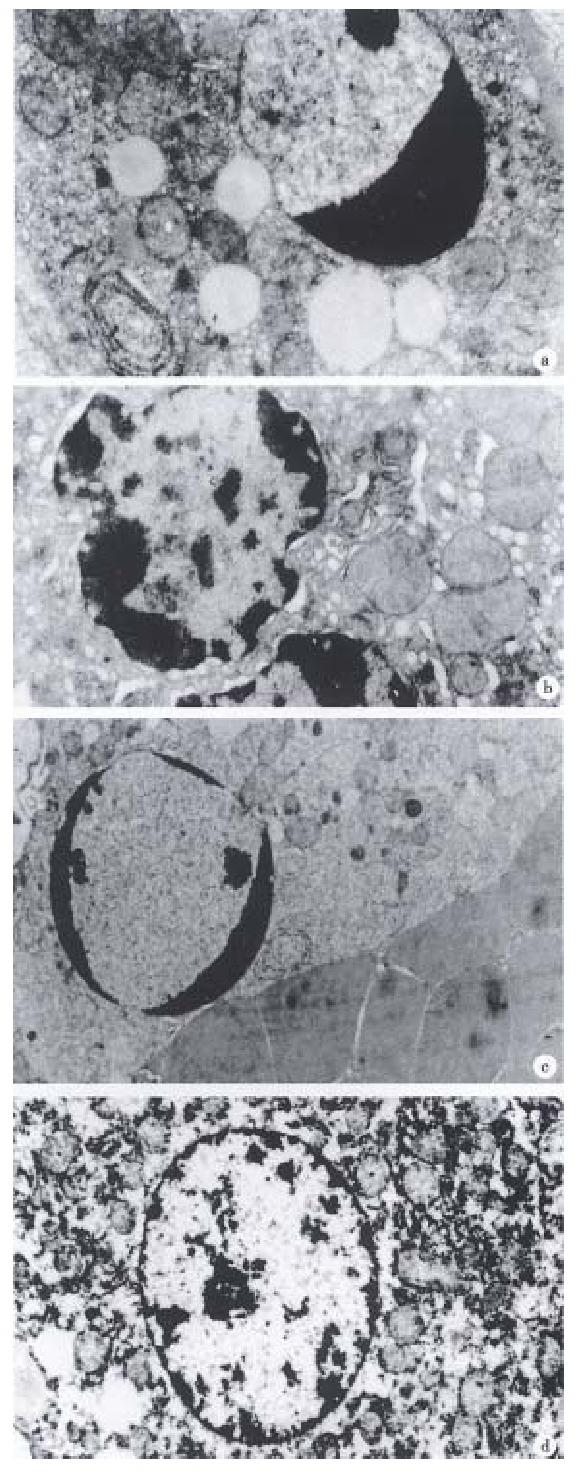
Hepatocytes of Balb/c mice under TEM. A. SEB 6 h, chromatin condensation, × 10000; B. D-GalN 2 h, nuclear pycnosis and organelle edema, × 10000; C. SEB + D-GalN 12 h, nuclear pycnosis and erythrocyte agglutination, × 4000; D. CSA + SEB + D-GalN 6 h, nuclear of hepatocyte, × 6000
In SEB and D-GalN group, the incidence of ABs was strikingly raised at 6 h , rapidly decreased after 6 h, and decreased to normal in 24 h. But in SEB + D-GalN group it continuously increased till 24 h, and in CSA + SEB + D-G alN group it had no obvious changes.
Agarose gel electrophoresis of hepatocellular DNA
The groups of SEB 6 h, D-GalN 6 h, SEB + D-GalN 6 h and, 12 h showed typical “ladder” pattern, but SEB + D-GalN 24 h present “smear” patt ern, and no abnormalities were found in CSA + SEB + D-GalN group (Figure 2).
Figure 2.
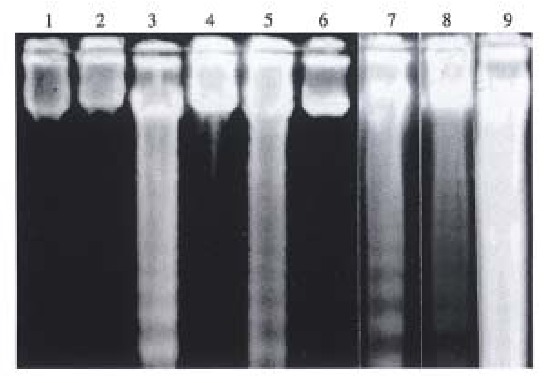
DNA agarose gel electrophoresis in livers of Balb/c mice. 1. CSA 6 h; 2. CSA + SEB + D-GalN 6 h; 3. SEB + D-GalN 6 h; 4. CSA + SE B + D-GalN 12 h; 5. SEB + D-GalN 12 h; 6. NS; 7. SEB 6 h; 8. D-Gal N 6 h; 9. SEB + D-GalN 24 h.
Serum ALT assay
Compared with control group, there was very obvious difference in the activities of serum ALT of SEB + D-GalN 6 h and 12 h groups (P < 0.01), and there was obvious difference in D-GalN 2, 6 h and SEB + D-GalN 2 h groups (P < 0.05), but there was no difference in each of SEB groups, CSA, CSA + SEB + D-GalN, D-GalN 12 h and 24 h groups (P < 0.05). Among these groups, activities of serum ALT of SEB + D-GalN 12 h even up to several thousand units (Table 1).
Table 1.
Serum ALT activities of Balb/c mice in different time (IU/L, 1IU/L = 16.67 nmol·s-1/L)
| t/h | NS | CSA | SEB | D-GalN | SEB + D-GalN | CSA + SEB + D-GalN |
| 2 | 51.8 ± 16.4 | 51.5 ± 17.8 | 59.5 ± 18.8 | 71.3 ± 25.7a | 69.0 ± 24.6a | 54.2 ± 24.5 |
| 6 | 56.0 ± 21.3 | 63.0 ± 18.1 | 60.5 ± 20.3 | 81.3 ± 24.7a | 95.7 ± 38.7b | 60.7 ± 29.4 |
| 12 | 54.8 ± 14.9 | 55.5 ± 27.5 | 56.5 ± 16.9 | 63.8 ± 22.8 | 4109.2 ± 1910.1b | 60.5 ± 26.7 |
| 24 | 59.7 ± 24.5 | 59.0 ± 24.7 | 59.5 ± 24.7 | 60.7 ± 26.3 | 65.7 ± 24.2 |
P < 0.05,
P < 0.01 vs normal saline.
Circulating cytokine levels
Serum TNF-α levels of SEB 2 h (210 ng/L ± 80 ng/ L ) and SEB+D-GalN 2 h (300 ng/L ± 110 ng/L) reached a peak, thereafter sharply decreased in SEB 6 h (50 ng/L ± 30 ng/L) and SEB + D-GalN 6 h (50 ng/L ± 20 ng/L). Serum IFN-γ levels of SEB and SEB+D-GalN groups began to increase after 2 h of administration (SEB 2 h 400 ng/L ± 120 ng/L, SEB+D-GalN 2 h 400 ng/L ± 150 ng/L), and peaked at 6 h-12 h (SEB 6 h 1480 ng/L ± 480 ng/L, 12 h 1620 ng/L ± 590 ng/L, 24 h 780 ng/L ± 350 ng/L, SEB + D-GalN 6 h 1360 ng/L ± 520 ng/L, 12 h 1860 ng/L ± 680 ng/L 24h 790 ng/L ± 320 ng/L). Our data showed that there was no difference in the kinetics of appearance of serum cytokines between mice of SEB and SEB+D -GalN groups ( P < 0.05) . In CSA + SEB + D-GalN groups both serum of TNF-α or IFN-γ levels were negative.
Twenty-four lethality
None of the Balb/c mice died at a dose of SEB up to 150 μg/ mouse alone, and D-GalN of 16 mg/mouse within 24 h, whereas the lethality was elevated within 24 h with increased dose of D-GalN when the two were used in combination, for example, 50 μg SEB caused death in about half of the mice, 150 μg S E B up to 100%. But pretremented with CSA 0.5 h earlier, the mice were all survived (Table 2).
Table 2.
Lethality within 24 h
| D-GalN (mg/mice) | SEB (μg/mice) | CSA (mg/mice) | 24 h lethality (death/total) |
| 10 | 0/3 | ||
| 50 | 0/6 | ||
| 100 | 0/3 | ||
| 150 | 0/3 | ||
| 16 | 0/6 | ||
| 16 | 10 | 0/3 | |
| 16 | 50 | 3/6 | |
| 16 | 100 | 2/3 | |
| 16 | 150 | 3/3 | |
| 16 | 50 | 0.5 | 0/6 |
| 0.5 | 0/6 | ||
| 0/6a |
Mice treated with normal saline.
DISCUSSION
Hepatocytes of SEB groups only showed apoptosis morphologically, and the course seems to finish within 12 h, DNA agarose gel electrophoresis of hepatocytes exhibited the “ladder” pattern at 6 h-12 h, but activities of serum ALT were always normal. This result indicates that SEB caused hepatocytes nuclear pycnosis while membrane was intact. Mice lethality within 24 h is 0. Bec ause SEB did not exhibit any direct toxic effect on hepatocytes[6], we analysed the mechanism of SEB-induced hepatocyte apoptosis may be related to biological features of superantigen nonspecific stimulating massive T cell proliferation and releasing cytokines (TNF-α, IFN-γ, etc.). D-GalN could induce both hepatocyte apoptosis and degeneration morphologically. The result of DNA agarose gel electrophoresis was similar to that of SEB groups, but activities of ALT rose between 2 and 6 h, probably due to (P < 0.05), hepatocyte degeneration which elavated membrane permeability. Mice lethality within 24 h is 0. D-GalN is a kind of indirect toxin to liver, which can causes a selective depletion of urine nucleotides in mouse liver, thus leading to secondary hepatic injury. High doses can cause hepatocyte injury in mice resembling human viral hepatitis[1].
Balb/c mice treated with SEB + D-GalN showed typical features of hepatocyte apoptosis within 6 h, but after 12 h, extensive cell necrosis was promi nent besides apoptosis in a few hepatocyte. After 12 h, the incidence of ABs became too high to count because a majority of hepatocytes were destructed. In biochemistry, DNA agarose gel electrophoresis demoustrated typical “ladder” be tween 6 and 12 h, but a “smear” pattern at 24 h. Serum ALT increased from the 2nd h (0.01 < P < 0.05 at 2 h, as compared P < 0.01, at 6 h 68 μmol·s-1·L-1 to at 12 h. This indicated a great amount of hepatocytes were destructed, with a 24 h lethality of 50% whereas the mice pretreated with CSA were all normal. Superantigen had no direct toxic effect on hepatocytes, only nonspecifically stimulated massive T cell proliferation and released cytokines while CSA can inhibit T lymphocyte releasing cytokines. Therefore, we thich that the acute injury to hepatocytes is mediated by T cells thus it can be inhibited by CSA.
Leist[7] reported that D-GalN + LPS/TNF induced hepatocyte apoptosis in Balb/c mice in the early stage and necrosis in the late stage. Our results are in agreement with Leist’s. Based on the studies of transgenic mice in lit erature[8] and our experiment, we assume that the process of hepatic injury induced by SEB + D-GalN is: massive hepatocyte apoptosis occurred, then the neutrophils and macrophages were attracted by endogenous mediators and activated by apoptotic hepatocytes releasing massive cytokines, and finally, the secondary acute hepatic necrosis, occurred leading to the death of mice. D-GalN in addition to its sensitization of hepatocyte, D-GalN may prevent the rapid uptake of apoptotic cells by neighboring hepatocytes and Kupffer’s cells[7]. Gantner[9] reported apoptotic hepatocytes can induce procoagulant activities in endothelial cells and platelets that eventually result in thrombin deposition and sinusoidal congestion. Therefore, there might be some relationship between apoptosis and necrosis. If the number of apoptotic hepatocytes is small and can be rapidly phagocytized by the nearby hepatocytes and Kupffer’s cells, secondary necrosis will not occur while if it is massive and can not be phagocytized rapidly, secondary necrosis will develop.
Footnotes
Supported by Shanghai Institute of Immunology Foundation, No.9508.
Edited by Jing-Yun Ma
References
- 1.Alison MR, Sarraf CE. Liver cell death: patterns and mechanisms. Gut. 1994;35:577–581. doi: 10.1136/gut.35.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faa G, Ledda-Columbano GM, Ambu R, Congiu T, Coni P, Riva A, Columbano A. An electron microscopic study of apoptosis induced by cycloheximide in rat liver. Liver. 1994;14:270–278. doi: 10.1111/j.1600-0676.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 3.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, 2nd ed. Beijing: Science and Technology Press. 1993:309. [Google Scholar]
- 4.Hu BY, Zhang BZ, Zhu YM. Assay of TNF induced by whole blood. Shanghai J Immunol. 1991;11:160. [Google Scholar]
- 5.Yong TP, Yi XN. Practical immunology, 1st ed. Changchun: Changchun Publishing House. 1994:596. [Google Scholar]
- 6.Uchiyama T, Yan XJ, Imanishi K, Yagi J. Bacterial superantigens--mechanism of T cell activation by the superantigens and their role in the pathogenesis of infectious diseases. Microbiol Immunol. 1994;38:245–256. doi: 10.1111/j.1348-0421.1994.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 7.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari FV. Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology. 1995;22:1316–1325. doi: 10.1016/0270-9139(95)90645-2. [DOI] [PubMed] [Google Scholar]
- 9.Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995;109:166–176. doi: 10.1016/0016-5085(95)90282-1. [DOI] [PubMed] [Google Scholar]


