Abstract
AIM: To improve the cultivation efficiency and yield of human liver cell line Cl-1.
METHODS: High-density cultivation of Cl-1 on microcarriers was carried out with periodic observation of their growth and proliferation. The specific functions of human liver cell were also determined.
RESULTS: Cells of Cl-1 cell line grew well on microcarrier Cytodex-3 and on the 7th day the peak was reached. The amount of Cl-1 cells was 2.13 × 108 and the total amount of albumin synthesis reached 71.23 μg, urea synthesis 23.32 mg and diazepam transformation 619.7 μg respectively. The yield of Cl-1 on microcarriers was 49.3 times that of conventional cultivation. The amounts of albumin synthesis, urea synthesis and diazepam transformation were 39.8 times, 41.6 times and 33.3 times those of conventional cultivation, respectively.
CONCLUSION: The human liver cell line Cl-1 can be cultivated to a high density with Cytodex-3 and has better biological functions. High-density cultivation of Cl-1 on microcarriers can act as the biological material of bioartificial liver.
Keywords: bioartificial liver, liver cell lines, microcarrier
INTRODUCTION
The animal experiments of extracorporeal bioartificial liver suggested that the device could provide special assistance to hepatic functions, and the effects of its primary clinical application was encouraging[1-3]. Although few successful studies were reported on human cell line acting as the biological material of bioartificial liver, it is rather conspicuous[4,5], and has opened up a new path for the study of bioartificial liver.
To meet the principal needs of bioartificial liver functions, microcarrier technique was used to cultivate high density human liver cell line to improve the cultivation efficiency and yield in this study. The growth of liver cells on microcarriers was observed and the specific functions of liver cells were determined periodically. The feasibility and value of human liver cell line cultivated on microcarriers as the biological material of bioartificial liver were inquired.
MATERIALS AND METHODS
Materials
The tissue of human liver cell line Cl-1 was taken from normal adult liver. Microcarrier Cytodex-3 was produced by Pharmacia in Sweden. Magnetic stirrer (0 r/min-200 r/min) and stirring culture vessel was made by Bellco Biotechnology in USA. The culture matrix consisted of DMEM was soluted in 10% NCS and L-Glutamine at a concentration of 3 g/L, products of Gibco.
Methods
Common culture of Cl-1 Cl-1 cells 100 mL, at a concentration of 2 × 105 mL, were inoculated into a cubic culture flask. On the 1st, 3rd, 5th and 7th day the growth of cells was observed on an upside-down microscope and the cells were counted respectively. The amount of the cells in the culture system was also calculated. superficial clear liquid was obtained periodically to determine the functions of the liver cells.
Microcarrier culture of Cl-1 Cell suspension 100 mL, at a concentration of 2 × 105 mL, was inoculated into a stirring culture vessel containing 500 mg Cytodex-3 and stirred intermittently for 8 h. Then the culture system was placed into a fixed temperature culture case at a temperature of 37 °C and stirred continuously at a speed of 300 r/min. From the 2nd day on, whether to change the culture substrate or not and how much volume to change were determined by the color of the matrix and the value of its pH and the interval of changing liquid was about 24 h-36 h.
Morphological observation and counting of the cells cultivated on microcarriers On the 1st, 3rd, 5th, 7th and 9th day 0.1 mL samples were taken at a well-distributed state of stirring, and growth of the cells was observed on the upside-down microscope. One mL samples were collected every other day to calculate the amount of cells in the culture system by means of crystal ester-calculating.
Observation of cells cultivated on microcarriers under electron scanning microscope On the 7th day cell sample was taken at a well-distributed state and culture matrix was discarded. After it was ri nsed with phosphate buffer solution and fixed for 0.5 h with 2 mL 2% pentanal, it was rinsed with phosphate buffer solution again, fixed for 0.5 h with 1% osmic acid, dehydrated gradiently for 10 min each stage, exchanged with acetic isopental ester for 4 h, and dried by CO2 drier (HITACHI HCP-2, JAPAN). Finally it was splashed with ion platinum vacantly. Growth of the cells was observed under S-450 electron scanning microscope (JAPAN).
Determination of albumin synthesis When the culture substrate was changed, superficial clear liquid was obtained to determine the concentration of human albumin by radio-immunity competition.
Determination of diazepam transformation Standard diazepam was added to the culture vessel at a concentration of 20 μg/mL. The superficial clear liquid was taken periodically and the concentration of diazepam was determined according to Xue Guo-Zhu et al[6]. The amounts of diazepam transformation were calculated.
Determination of urea in superficial clear liquid Superficial clear liquid (0.2 mL) was taken and determined by BECKMAN bioche mical auto-detector. The amount of urea was calculated on the basis of the culture volume.
RESULTS
Comparison of cell amounts after expanded by cultivation
After 1-3 days of cultivation, the Cl-1 cells in common culture adhered and grew slowly, and part of them in suspension was devitalized. From then on the adhering Cl-1 cells grew rapidly, and cell amount reached 4.32 × 106/100 mL on the 5th day. On the 7th day the amount decreased to 3.83 × 106/100 mL while in the microcarrier culture the growth of Cl-1 accelerated on the 3rd day and reached the peak of 2.13 × 108 on the 7th day, and decreased to 1.83 × 108 on the 9th day (Figure 1). The ratio of the peak amounts of Cl-1 between microcarrier culture and common culture was 49.3:1.
Figure 1.
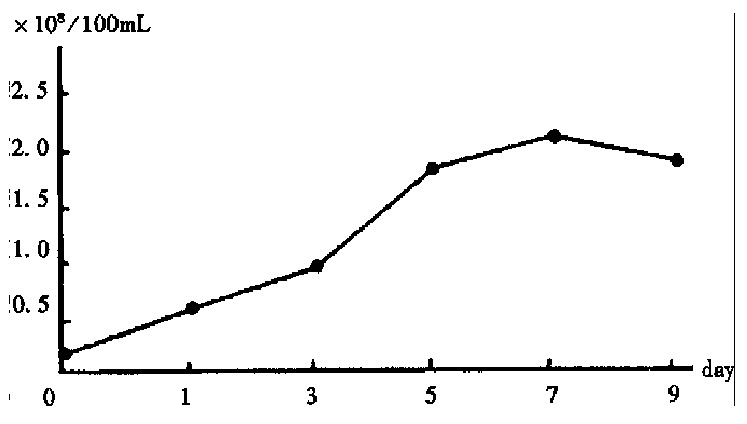
Change of the amount of Cl-1 cultivated on microcarriers.
Morphological observation of Cl-1 cultivated on microcarriers
Observation under upside-down microscope On the 1st day over 50% microcarriers were attached with cells and the cells were beginning to expand. On the 5th day more than 80% microcarriers were covered with cells (Figure 2). The multimorphological growth of liver cells could be observed under high-amplification microscope. On the 7th day the phenomenon of bridge-link could be observed between microcarriers, i.e., the microcarriers were linked to each other through cells (Figure 3).
Figure 2.
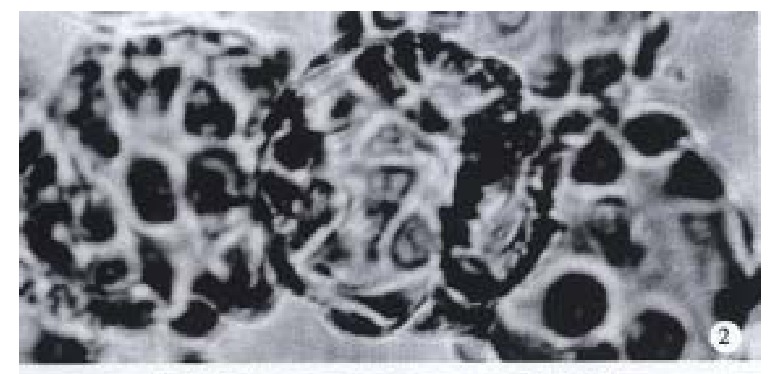
On the 5th day more than 80% microcarriers were covered with Cl-1 cells under upside-down microscope. × 120
Figure 3.
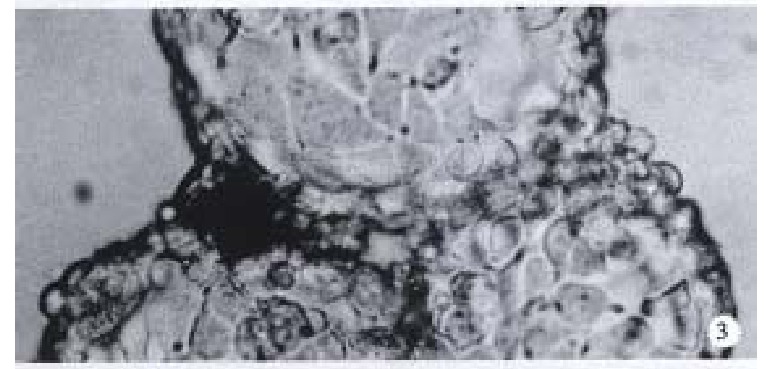
On the 7th day the phenomenon of bridge-link could be observed between microcarriers under upside-down microscope. × 200
Observation under electron microscope On the 7th day of cultivation, electron microscopy showed that the cells adhered fast to the microcarriers semi-spherically (Figure 4). The microvilli on the surfaces of the cells could be observed clearly.
Figure 4.
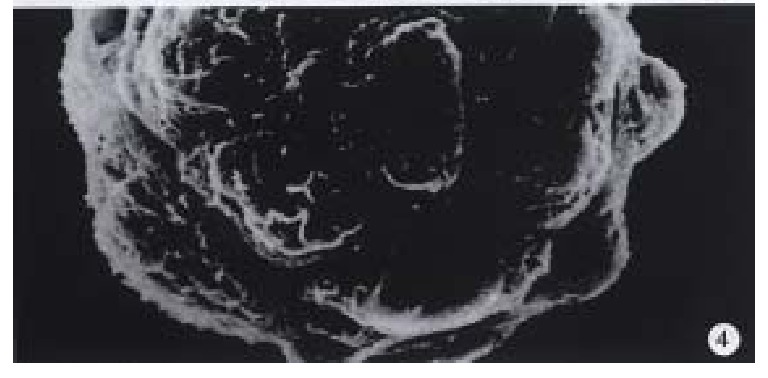
On the 7th day of cultivation, the electron microscope showed that the cells adhered fast to the microcarriers semi-spherically under scanning electronic microscope. × 950
Changes of specific functions of cells When Cl-1 cells were cultivated on microcarriers, the specific functional indexes such as albumin synthesis, urea synthesis and diazepam transformation rose gradually along with the prolonging of the cultivation. On the 7th day these indexes peaked, and then decreased gradually, while Cl-1 cells were commonly cultivated, the functional indexes as described above reached their peaks on the 5th day and decreased noticeably on the 7th day. All the functional indexes determined at various time points in microcarrier culture were obviously higher than those in common culture (Tables 1 and 2). When functional indexes of Cl-1 cells reached their peaks on the 7th day, the albumin synthesis, urea synthesis and diazepam transformation of Cl-1 in microcarrier culture were 39.8, 41.6 and 33.3 times over those in common culture on the 5th day.
Table 1.
Changes of specific functions of Cl-1 under two culture con ditions
| Indexes of functions | 1st day | 3rd day | 5th day | 7th day | 9th day |
| Albumin synthesis (μg) | |||||
| Microcarrier culture | 21.34 | 35.87 | 58.35 | 71.32 | 67.98 |
| Common culture | 0.43 | 1.21 | 1.79 | 1.65 | |
| Urea synthesis (mg) | |||||
| Microcarrier culture | 7.45 | 11.49 | 19.62 | 23.32 | 20.18 |
| Common culture | 0.08 | 0.34 | 0.56 | 0.42 | |
| Diazepam transformation (μg) | |||||
| Microcarrier culture | 112.2 | 271.3 | 544.1 | 619.7 | 573.3 |
| Common culture | 3.4 | 8.9 | 18.6 | 13.5 |
Table 2.
Comparison of peak function values of Cl-1 under two culture conditions
| Indexes of functions | Albumin synthesis(μg) | Urea synthesis(mg) | Diazepam transformation(μg) |
| Microcarrier culture | 71.23 | 23.32 | 619.7 |
| Common culture | 1.79 | 0.56 | 18.6 |
| Ratio | 39.8:1 | 41.6:1 | 33.3:1 |
DISCUSSION
Microcarrier culture can improve the culture efficiency and yield. As the biolo gical material of bioartificial liver, the liver cells must meet two needs: (1) possessing the specific functions of liver; and (2) providing biological function enough to satisfy the patients. The former demands high differentiation of the cells, and the latter sufficient amount of liver cells. Our primary study on the human liver cell line at early stage showed the characteristics of high differentiation and good specific liver functions of Cl-1. It is extremely valuable as the biological material of bioartificial liver[6]. In common culture, however, the yield of human cell line can only reach the level of 106-107, not enough to meet the need of necessary amount to survive an individual. So the urgent question on the biological material of bioartificial liver is how to increase the amount of the cells. The technique of microcarrier culture in cell engineering makes cultivating the human liver cell line possible in high-density in that it possesses a high ratio of surface area to volume, which provides a comparatively large area for cells to adhere in small culture volume. Few successful cultivations of animal primary liver cells on microcarriers have been reported both at home and abroad[7,8], while there was no report on cultivation of human liver cells on microcarriers yet . Microcarrier culture of Cl-1 was studied by using Cytodex-3 and slow stirring in our work. After 100 mL suspension of Cl-1 was inoculated at a concentration of 2 × 105/mL, the cells grew and adhered to the microcarriers in great amount and reached the peak on the 7th day. The maximal yield of cells cultivated on microcarrier was 49.3 times over that in 100 mL common cubic flask. The reasons for the increase of cell yield in a big margin when Cl-1 was cultivated on microcarrier were as follows: (1) The surface area in the culture system for cells to grow was greatly improved by using microcarriers. 0.5 g Cytodex-3 was added into 100 mL culture matrix each and the cultivating surface area could reach 2300 cm2; while the efficient surface area of the common culture flask was only 28 cm2. The quantity of the cells increased correspondingly with the improvement of the cultivating surface; (2) Cytodex-3 is primarily characterized by a thin layer of collagen chemically coupled to a matrix of cross-linked dextran, so that it can attach the liver cells strongly, stimulate the expanding and growth of them and promote their growth[5,9]; and (3) Low speed stirring employed to cultivate Cl-1 cells made them easy to adhere to the surfaces of the suspending microcarriers and expand to mono-layers gradually.
The overall functions of Cl-1 cultivated in microcarrier system were improved. Due to the marked increase of the amount of cells, the function indexes of Cl-1 cultivated on microcarrier were greatly improved and predominated over those in common culture. Such improvement was the prerequisite for Cl-1 cultivated on microcarrier to act as the biological material of bioartificial liver. Furthermore, the time for the highest cell amount and function indexes in microcarrier culture was later than that in common culture. It may be due to the improvement of efficient surface area for cultivating which delayed the peak of growth and reproduction of Cl-1 afterwards.
Further improvement is needed in microcarrier culture of liver cells. Matsmura held that about 25 g-75 g liver cell (2.5 × 109-7.5 × 109 liver cells) was enough to provide the necessary liver functions to survive an individual when his liver weight was 1200 g[10]. Although the amount of Cl-1 had been greatly improved to the level of 2 × 108 in a culture volume of 100 mL in this study, it can not meet the need of the bioar tificial liver yet. Expansion of the volume of microcarrier culture to several or even several tens of liters is to be further studied. Perfusion culture with m icrocarrier is promising.
Footnotes
Supported by the National Natural Science Foundation of China, No.39570212.
Edited by Xian-Lin Wang
References
- 1.Sussman NL, Finegold MJ, Kelly JH. Recovery from syncytial giant-cell hepatitis (SGCH) following treatment with an extracor-poreal liver assist device (ELAD) Hepatology. 1992;15:51A. [Google Scholar]
- 2.Rozga J, Holzman MD, Ro MS, Griffin DW, Neuzil DF, Giorgio T, Moscioni AD, Demetriou AA. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502–509; discussion 502-509;. doi: 10.1097/00000658-199305010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetriou AA, Rozga J, Podesta L, Lepage E, Morsiani E, Moscioni AD, Hoffman A, McGrath M, Kong L, Rosen H. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995;208:111–117. doi: 10.3109/00365529509107771. [DOI] [PubMed] [Google Scholar]
- 4.Sussman NL, Kelly JH. Artificial liver: a forthcoming attraction. Hepatology. 1993;17:1163–1164. doi: 10.1002/hep.1840170632. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220:59–67. [PMC free article] [PubMed] [Google Scholar]
- 6.Xue GZ, Gao Y, Yang JZ. Primary study on human cell line acting as the biological material of artificial liver. J Chin Infilt Artif Organ. 1996;7:1–4. [Google Scholar]
- 7.Rozga J, Williams F, Ro MS, Neuzil DF, Giorgio TD, Backfisch G, Moscioni AD, Hakim R, Demetriou AA. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258–265. [PubMed] [Google Scholar]
- 8.Wang YJ, Li MD, Wang YM. Cultivation of rat liver cells adhered on microcarriers. J Chin Exp Surg. 1997;14:61. [Google Scholar]
- 9.Kasai S, Sawa M, Mito M. Is the biological artificial liver clinically applicable A historic review of biological artificial liver support systems. Artif Organs. 1994;18:348–354. doi: 10.1111/j.1525-1594.1994.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura KN, Guevara GR, Huston H, Hamilton WL, Rikimaru M, Yamasaki G, Matsumura MS. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery. 1987;101:99–103. [PubMed] [Google Scholar]


