Abstract
AIM To investigate the protective effects of polydatin (PD) against injury to primarily cultured rat hepatocytes induced by CCl4.
METHODS Rat hepatocytes were separated by methods of liver infusion in vivo and cultured medium (7.5 × 105 cells/mL). Two mL or 0.2 mL was added into 24-well or 96-well plates respectively. Twenty-four hours after cell preculture, PD at concentrations of 10-7 mol/L-10-4 mol/L was added into each plate. At the same time injury to hepatocytes was induced by adding 10 mmol/L-CCl4. Then, 0.1 mL or 1 mL-culture solution was removed from the 96-well or 24-well plates at 6 h, 12 h, 24 h and 48 h after CCl4 intox-ication respectively for the determination of GPT, GSH and MDA. At 48 h, the survivability of rat hepatocytes was assayed by the MTT colormetric method.
RESULTS After CCl4 challenge, the release of GPT and the form ation of MDA in rat hepatocytes markedly increased and maintained at a high level in 48 h, whereas PD with different concentrations could markedly inhibit this elevation with 10-5 mol/L PD having the strongest effects and inhibiting rate was over 50%. PD could also im-prove the decreased content of GSH caused by CCl4 in accordance with the doses used. CCl4 ev-idently decreased the he patocyte survivability from 91.0% ± 7.9% to 35.4% ± 3.8%. On the other hand, PD at 10-7 mol/L-10-4 mol/L could reverse this change and improve t he cell survival rates to 56.1% ± 5.2%, 65.8% ± 5.0%, 88.7% ± 6.8% and 75.2% ± 7.3%, respectively.
CONCLUSION PD at 10-7 mol/L-10-4 mol/L could protect primarily cultured rat hepatocytes against CCl4 induced injury.
Keywords: polydatin; injury, hepatocyte; CC14
INTRODUCTION
Polygonum cuspidatum Sieb. et Zucc. (Polygo-naceae) is a traditional Chinese herbal drug, with bitter taste and cold nature. It mainly acts upon the liver, gallbladder and lung meridians. It is well known that P. cuspidatum has various activities such as promoting blood circulation, relieving swelling and pain, eliminating phlegm, alleviating cough, clearing away heat, and removing dampness and toxin. The drug has been widely used for car-diovascular and liver diseases. Its active compounds mainly consist of free anthraquinones which include emodin, physcion and chrysophanol. Another im-portant compound is resveratrol[1].
Polydatin (PD), 3, 4’, 5-trihydroxystibene-3-β-mono-D-gluc oside, also named piceid, is the glyco-side of resveratrol[1]. Some previous studies demon-strated that PD could lower the level of blood lipid, inhibit the platelet aggregation, dilate blood ves-sels, protect cardiocytes, reduce cerebral ischemic damage and inhibit lipid peroxidation[2-6]. However, the effects of PD on hepatocytes and its mecha-nisms have not been reported up to date. In this paper we report the details of protective effects of polydatin against injury to primarily cultured rat hepatocytes induced by CCl4.
MATERIALS AND METHODS
Materials
Collagenase (type IV), 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT), dex-amethasone, N-2-hydroxyethyl-piperazine-N’-2’-ethane sulfonic acid (HEPES), insulin, penicillin and streptomycin were purchased from Sigma Chemical Corp (St. Louis, USA). RPMI 1640 was a product of Gibco Life T echnologies INC (Grand Island, NY). Fetal calf serum was obtained from Institute of Hemopathy, Chinese Academy of Medical Sciences (Tianjin). PD (Purity > 90%), which was isolated from the root and rhizome of P. cuspi-datum[7], provided by the Department of Chem-istry, the First Military Medical University.
Animals
Wistar rats, male, 6 weeks old, weighing 160 g-180 g, were used for hepatocyte isolation. They were provided by Laboratory Animal Center, Guangzhou Un iversity of TCM.
Isolation and culture of rat hepatocytes
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Then the liver parenchymal cells of rat were isolated by the collagenase perfusion method following the procedure of Seglen and Ko-ji[8,9]. Simply, the portal vein of rat liver was exposed and cannulated with a teflon catheter. The liver was perfused with Ca2+ free solution containing NaCl 142, KCl 6.7, HEPES 10, NaOH 5.5 (mmol/L), pH 7.4, at 37, with a flow rate of 40 mL/min. Twelve minutes later, recirculation started with collagenase solution composed of NaCl 67, KCl 6-7, CaCl2·2H2O5, HEPES 100, NaOH 66, collagenase 0.2 g/L, pH 7.6. Isolated cells were cultured in RPMI 1640 containing 100 mL/L-fetal calf serum, 10 mmol/L-HEPES, 100 kU/L-penicillin and streptomycin, 10 mmol/L insulin and 10 mmol/L-dexamethasone. The content of hepato-cytes was adjusted to 7.5 × 108 cells/L with the above medium. Cultured medium 2 mL and 0.2 mL were added into 24-well and 96-well plates respectively. The cells were incubated for 4 h at 37 °C under 50 mL/L CO2 in air. Non-adherent hepatocytes were eliminated by replac ing the medium, and ad-herent hepatocytes continued to be incubated, and the medium was changed every 24 h.
CCl4-induced hepatocytes injury
After pre-culture for 24 h, the hepatocytes were exposed to fresh medium containing 10 mmol/L-CCl4 and various concentrations of PD. At 6, 12, 24 and 48 h after CCl4 intoxication, 0.1 mL and 1 mL cul-ture solution were removed from 96-well and 24-well plates respectively for determination.
Measurement of glutamic pyruvic transami-nase (GPT)
The kits of GPT analysis, provided by the Shanghai Institute of Biological Products of Ministry of Health, were used to measure the activity of GPT in 0.1 mL- culture medium.
Determination of reduced glutathione (GSH) and malondialdehyde (MDA)
Utilizing the kits of GSH analysis and the kits of MDA analysis, all purchased from Nanjing Jiancheng Bio-engineering Institute, the content of GSH in 1mL culture medium and the level of MDA in 0.1mL culture medium we re measured.
Cell survivability assay
The survivability of rat hepatocytes was assayed by the MTT colormetric method[10]. At 48h after CCl4 challenge, 20 μL/well- MTT stock solution (5 g/L) was added into each well of 96-well plates. The cells were continuously incubated for another 4 h before 0.1 mL/well dimethyl sulfoxide was added to all wells and mixed thoroughly to dissolve the brown-black crystals. The plates were read on microplate reader, using a test wavelength of 570 nm with a reference wavelength of 655 nm.
Statistical analysis
The results were expressed as -x ± s and significant difference was a ssessed by Student’s t test.
RESULTS
Effects of PD on GPT activity in culture medium
The concentration of GPT in culture medium signif-icantly increased after CCl4 challenge, and main-tained at a high level in 8 h (Table 1). Further-more, a progressively elevated trend existed with time-dependence. PD could significantly inhibit the level of GPT in accordance with the doses used. Es-pecially, PD 10 μmol/L had the strongest effects and the inhibiting rate was over 50%.
Table 1.
Effect of PD on GPT activity in culture medium (-x ± s, n = 8)
| Group c/(mol/L) |
GPT(U) |
||||
| 6 h | 12 h | 24 h | 48 h | ||
| Normal | 13.5 ± 2.5b | 13.8 ± 3.1b | 13.7 ± 5.6b | 14.1 ± 3.3b | |
| Control | 72.3 ± 14.1 | 79.7 ± 10.3 | 85.4 ± 9.2 | 88.3 ± 19.6 | |
| P D | 10-7 | 60.3 ± 17.1a | 62.0 ± 15.6a | 68.8 ± 17.5a | 71.4 ± 20.5a |
| P D | 10-6 | 55.0 ± 10.3a | 58.3 ± 16.7a | 64.1 ± 13.6a | 69.1 ± 19.2a |
| P D | 10-5 | 30.6 ± 10.6b | 38.3 ± 5.5b | 42.5 ± 7.0b | 45.0 ± 7.6b |
| P D | 10-4 | 42.1 ± 7.8a | 47.5 ± 9.8a | 56.8 ± 11.3a | 59.2 ± 10.7a |
P < 0.05,
P < 0.01, vs CCl4-treated control group.
Effects of PD on GSH content in culture medium (Table 2).
Table 2.
Effects of PD on GSH content in culture supernatant (-x ± s, n = 8)
| Group c/(mol/L) |
GSH (ng/L) after CCl4 challenge |
||||
| 6 h | 12 h | 24 h | 48 h | ||
| Normal | 9.8 ± 0.8b | 10.1 ± 0.8b | 10.4 ± 0.7b | 10.6 ± 1.2b | |
| Control | 4.2 ± 0.6 | 4.1 ± 0.7 | 4.1 ± 0.3 | 3.8 ± 0.6 | |
| P D | 10-7 | 5.0 ± 0.3 | 5.4 ± 0.5 | 5.6 ± 0.9 | 6.1 ± 1.0a |
| P D | 10-6 | 5.3 ± 0.8 | 5.6 ± 0.9 | 6.4 ± 0.6a | 6.8 ± 1.1a |
| P D | 10-5 | 8.4 ± 1.2b | 5.9 ± 1.3a | 7.7 ± 0.8a | 9.0 ± 1.2b |
| P D | 10-4 | 6.7 ± 0.4a | 6.1 ± 1.0a | 6.8 ± 0.7a | 7.6 ± 0.9a |
P < 0.05,
P < 0.01, vs CCl4 treated control group.
The content of GSH in culture medium decreased obviously as compared with that in normal hepato-cytes after 6 h incubation with CCl4 (Table 2). On the other hand, PD of various concentrations could improve GSH in a dose-dependence manner, and 10 μmol/L PD showed a most signifi cant activity.
Effects of PD on MDA formation in rat hepato-cytes
CCl4 challenge obviously elevated the MDA forma-tion in rat hepatocytes, with a marked rise in time-dependence manner, whereas MDA formation of rat hepatocytes decreased significantly at various concentrations of PD as compared with that in CCl4 control group, and it reached minimum value at 10-5 mol/L and slightly elevated when PD concen-tration was up to 10-4-mol/L (Table 3).
Table 3.
Effects of PD on MDA formation in rat hepatocytes (-x ± s, n = 8)
| Group c/(mol/L) |
GSH (ng/L) after CCl4 challenge |
||||
| 6 h | 12 h | 24 h | 48 h | ||
| Normal | 4.0 ± 0.4b | 4.5 ± 0.6b | 4.8 ± 0.4b | 4.6 ± 0.7b | |
| Control | 15.5 ± 1.8 | 16.0 ± 2.7 | 17.5 ± 2.1 | 19.0 ± 2.4 | |
| P D | 10-7 | 13.1 ± 2.0 | 13.8 ± 3.3 | 13.0 ± 4.3a | 14.5 ± 1.8a |
| P D | 10-6 | 11.4 ± 1.7a | 12.0 ± 1.8a | 12.1 ± 3.1a | 12.5 ± 2.0a |
| P D | 10-5 | 6.5 ± 1.2b | 6.7 ± 1.2b | 7.5 ± 2.3b | 8.2 ± 2.7b |
| P D | 10-4 | 8.7 ± 3.5b | 8.9 ± 2.8b | 9.8 ± 2.6b | 10.3 ± 3.0b |
P < 0.05,
P < 0.01, vs CCl4 treated control group.
Effects of PD on cell survivability in primary culture rat hepatocytes
The results of MTT assay showed that normal hepa-tocytes had high level of cell viability (91.0% ± 7.9%) and CCl4 induced marked decrease of hepa-toc ytes survivability (35.4% ± 3.8%, P < 0.01 vs normal group), whereas the level of cell survivabili-ty could be significantly enhanced by PD at the con-centrations of 10-7 mol/L-10-4 mol/L to 56.1% ± 5.2% (P < 0.05, vs CCl4-treated control group), 65.8% ± 5.0% ( P < 0.05), 88.7% ± 6 .8% ( P < 0.001) and 75.2% ± 7.3% ( P < 0.01) respectively. It reached a maximum value at 10-5 mol/L and slightly declined when the concentration of PD was up to 10-4 mol/L (Figure 1).
Figure 1.
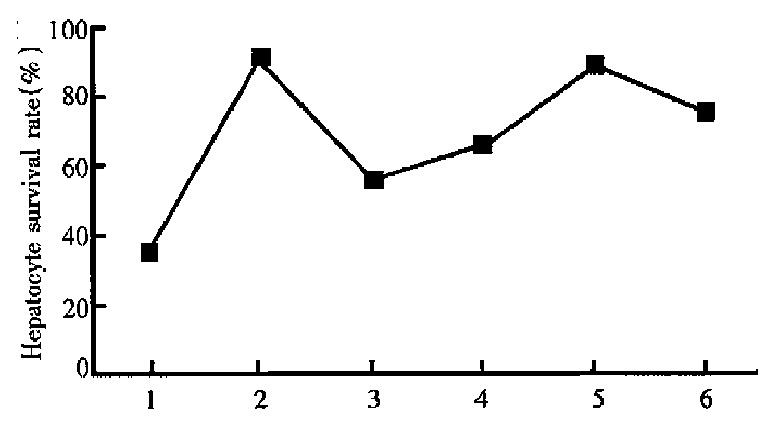
Effects of PD on cell survivability in primary culture rat hepatocytes. 1. CCl4-treated control group; 2. normal hepatocytes; 3. PD 10-7 mol/ L; 4. PD 10-6 mol/ L; 5. PD 10-5 mol/ L; 6. PD 10-4 mol/ L.
DISCUSSION
P. cuspidatum has been used to treat some chronic liver diseases such as hepatitis and hepatocirrhosis. We have been trying to search for hepatoprotective compounds of P. cuspidatum. Our previous in vitro studies showed that emodin, another active com-pound, had a hepatoprotective effect[11]. The pre-sent in vitro study also indicated that PD had a pro-tective effect against CCl4 induced injury to primarily cultured rat hepatocytes. Since the extraction and isolation of PD are relatively simple and have a high content of 1.23% in the root of P. cuspidatum[7], we may take these advantages to further study its mechanisms of hepatoprotective effect and develop a new drug from it.
CCl4 is a well-known example of a chemical that produces free radical-mediated liver injury. It generates CCl4 by the activation of liver cytochrome P-450, initiating lipid peroxidation of bio-mem-branes[12]. In the presente xperiment, it was found that CCl4 induced both the increase of GPT in super-nat ant and the elevation of MDA in rat hepato-cytes. However, administration of 10-7 mol/ L - 10-4 mol/L PD could partly reduce GPT and MDA. Therefore, there may be two possible mechanisms contributing to the hepatoprotective actions of PD. One is that PD inhibits further production of lipid peroxidation in rat hepatocytes, and the other is that it inhibits the destructive action of lipid peroxi-dation on liver cells.
GSH is an important endogenous anti-oxidant substance. The decrease of GSH content may be due to increased GSH consumption as it participates in the detoxifica tion system for the metabolism of CCl4, and results in an enhanced susceptibility of hepatocytes to CCl4 toxicity[13]. Our results showed that CCl4 obviously decreased GSH content in the hepatocytes, but PD could partly reverse it. This suggested that the nature of PD protecting-SH com-pounds (such as GSH ) from CCl4 injury may be the third mechanism of its hepatoprotection.
It is interesting that PD of 10-5 mol/L was more ef-fective than that of 10-4 mol/L, at the same time, the hepatoprotective action of PD was in dose dependence at concentrations of 10-7 mol/ L-10-5 mol/ L. Its mechanisms of action need to be further studied.
Footnotes
Supported by the Bureau of TCM Administration of Guangdong Province, No.96033.
Edited by Jing-Yun Ma
References
- 1.Ouyang CG. Chemical compounds in Polygonum cuspidatum. Chin Trad Herbal Drugs. 1987;18:44–45. [Google Scholar]
- 2.Zhang PW, Yu CL, Wang YZ, Luo SF, Sun LS, Li RS. Influence of 3,4',5-trihydroxystibene-3-beta-mono-D-glucoside on vascular endothelial epoprostenol and platelet aggregation. Zhongguo Yaoli Xuebao. 1995;16:265–268. [PubMed] [Google Scholar]
- 3.Zhang PW, Shan CW, Zhang J, Yu CL. Effects of polydatin on human hemody-namics and cholesterol. J Med Coll PLA. 1995;15:47–48. [Google Scholar]
- 4.Wang YZ, Luo SF, Zhang PW, Yu CL. Reducing effect of 3,4',5-trihydroxystibene-3-beta-mono-D-glucoside on arterial thrombosis induced by vascular endothelial injury. Zhongguo Yaoli Xuebao. 1995;16:159–162. [PubMed] [Google Scholar]
- 5.Abbert WN, ZX mo. Protective effects of polydatin, an active compound from polygonum cuspidatum, on cerebral ischemic damage in rats. Chin Pharmaco Bull. 1996;12:126–129. [Google Scholar]
- 6.Jin WJ, Chen SY, Qian ZX, Shi XH. Effects of polydatin IV on inhibiting respi-ratory burst of PMNS and scavenging oxygen free radicals. Chin Pharmaco Bull. 1993;9:355–357. [Google Scholar]
- 7.Wang D, Tang Y. Quantitative determination of polydatin in Polygonum cuspi-datum. Chin Trad Herbal Drugs. 1987;18:16–18. [Google Scholar]
- 8.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 9.Hase K, Kasimu R, Basnet P, Kadota S, Namba T. Preventive effect of lithospermate B from Salvia miltiorhiza on experimental hepatitis induced by carbon tetrachloride or D-galactosamine/lipopolysaccharide. Planta Med. 1997;63:22–26. doi: 10.1055/s-2006-957596. [DOI] [PubMed] [Google Scholar]
- 10.Zheng YT, Ben KL. Use of MTT assay for the determination of cell viability and proliferation. J Immuno (CHN) 1992;8:266–269. [Google Scholar]
- 11.Huang ZS, Wang ZW, Zhong SQ. Protective effects of emodin on CCl4-induced injury of primary cultured rat hepatocytes. Chin J Integr Med. 1998:(in press). [Google Scholar]
- 12.Tribble DL, Aw TY, Jones DP. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology. 1987;7:377–386. doi: 10.1002/hep.1840070227. [DOI] [PubMed] [Google Scholar]
- 13.Zhu B, Liu GT. Cytotoxic effect of hydrogen peroxide on primary cultured rat hepatocytes and its mechanisms. Chin J Pharm Toxic. 1996;10:260–266. [Google Scholar]


