Abstract
AIM To study the role of interleukin 1β-converting enzyme (ICE) in antitumor drug-induced apoptosis in tumor cells.
METHODS Morphological changes in human esophageal carcinoma Eca-109 cells after treated with 5-fluorouracil (5-FU) were observed under light and electron microscope. Expression of ICE in the tumor cells exposed to 5-FU was examined by the immunocytochemical method.
RESULTS The cells treated with 5-FU displayed disappearance of nucleoli, chromatin gathering under nuclear envelope, karyorrhexis, budding and the formation of apoptotic bodies. The expression of ICE was negative in control cells, and 5-FU could induce the ICE expression in Eca-109 cells undergoing apoptosis. The number and the staining intensity of positive cells increased with the extension of action time.
CONCLUSION 5-FU may induce apoptosis in hu-man esophageal carcinoma Eca-109 cells; ICE gene may be involved in the regulation of 5-FU-induced apoptosis; and ICE protein may mediate apoptosis induced by 5-FU.
Keywords: esophageal cancinoma, cell line, apoptosis, 5-fluorouracil, interleukin 1β-con-verting enzyme
INTRODUCTION
Apoptosis is an important physiological form of cell death. It is strictly controlled by genes. It has been shown in experiments that external and internal signals of cells may start the process of apoptosis, e.g., anticancer drugs[1]. The genes involved in apop-tosis include the family of bc1-2, p53, c-myc and so on. Recent studies showed that interleukin-1β-converting enzyme (ICE) gene was a mammalian homologue of the C. elegans cell death gene ced-3[2] ant that overexpression of ICE gene could induce apoptosis in Rat-l fibroblast[3]. Thus, the expres-sion of ICE protein in human esophageal carcinoma Eca-109 cells treated with 5-fluorouracil (5-FU) was examined to investigate the role of ICE in anti-cancer drug induced apoptosis.
MATERIALS AND METHODS
Tumor cell culture
Human esophageal carcinoma Eca-109 cells were cultured in RPMI 1640 (Gibco) medium supple-mented with 10% heat-inactivated new-born calf serum 100 U/mL penicilin, and 100 μg/mL strep-tomycin, and kept in a controlled atmosphere (5% CO2) incubator at 37 °C.
Drug treatment
Exponentially growing cells were seeded in plates for 24 h and treated with 5-FU (10, 50 and 100 mg/L) for 48 h, 72 h and 96 h, respectively. The cells not treated with the drug served as control cells.
Preparation of specimens for light and electron microscopy
The tumor cells of experimental and control groups were collected, made into smears and fixed. Some smears were HE stained and observed for morpho-logical features under light microscope. some smears were stained by the immunocytochemical method in order to examine the expression of ICE protein. The cells were fixed in 25 g/L-glutaraldehyde for 1 h at room temperature, and postfixed for 1 h in 10 g/L osmium tetroxide, and dehydrated through gradient ethanol, stained and observed under elec-tron microscopy (EM) (Hitachi 600A model).
Immunocytochemical staining
Rabbit anti-human ICE (p20) multiclonal antibody (Santa Cruze) was used at 1:100. The immuno-cytochemical staining was performed using the LSAB (Dako) method. The enzyme was developed with DAB in conjuntion with hydrogen peroxide. A ne-gative control for non-specific Ig binding was em-ployed in which the first antibody was substituted with PBS. Results were judged from the number of positive cells, i.e., (-) was positive cells < 25%; (+): positive cells 25%-50%; (+ +): positive cells 51%-70%; and (+++): posi tive cells > 70%.
RESULTS
Morphological changes
Most of the floating cells were presented with nucle-ar fragmentation, nuclear disappearance, budding and the formation of apoptotic bodies. Many cells detached with trypsin-EDTA from the plates showed that chromatin was gathered under the nuclear membrane in mass or ring-shape with the disappear-ance of nucleoli. Under EM, these changes were observed more apparently. In addition, some of the tumor cells displayed budding with several circular or semicircular protrusive vesicles, some of which were detached from the main body (Figure 1). Apoptotic cells were not easily observed in the group of low co ncentration (10 mg/L), and they in-creased with higher concentration ( > 50 mg/L) and longer action time ( > 48 h).
Figure 1.
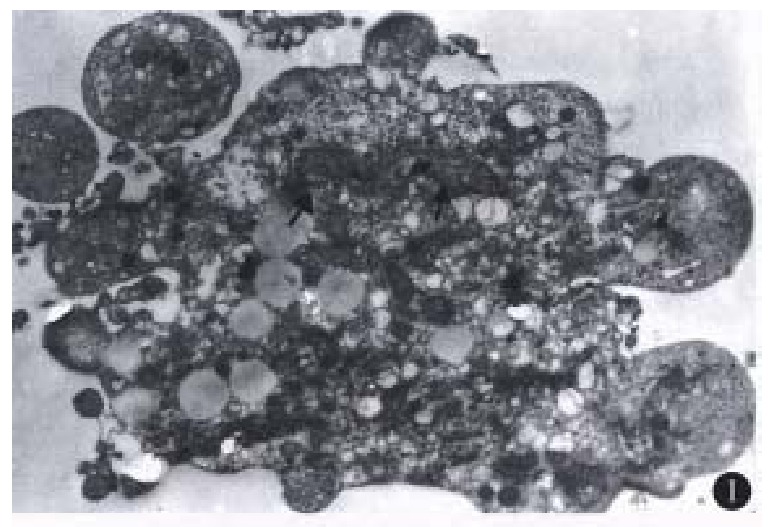
An apoptotic cell with feature of budding. Nuclear frag-ments were shown by arrows. × 6000
ICE protein expression
ICE protein was expressed negatively in control tu-mor cells, but positively in the tumor cells treated with 5-FU. The number of positive cells increased with the 5-FU exposure-time (Table 1).
Table 1.
ICE expression in Eca-109 cells treated with 5-FU
| Doses (mg/L) |
Degrees of ICE expression |
||
| 48 h | 72 h | 96 h | |
| 0 | - | - | - |
| 50 | + | ++ | +++ |
| 100 | ++ | ++ | •+++ |
Although individual control cells were sometimes stained brown, they were small in number and positive granules existed only near the cellular mem-brane. Brown granules in the cells treated with 5-FU were distributed all over the cytoplasm and cel-lular membrane. Most of apoptotic cells expressed ICE protein, however, a few cells with obvious features of apoptosis did not expresse ICE. In addition, some cells without features of apoptosis expressed ICE protein (Figure 2).
Figure 2.
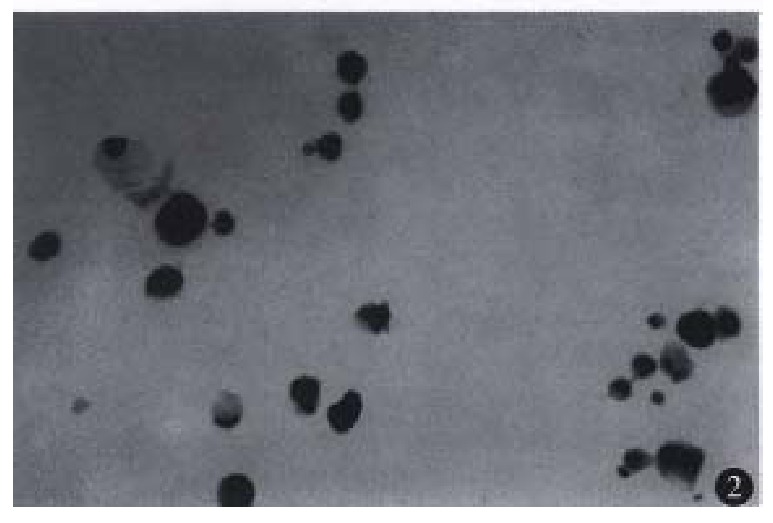
ICE protein expression in esophageal carcinoma cells after exposure to 5-FU. LSAB × 400
DISCUSSION
5-FU may induce apoptosis in human esophageal carcinoma Eca-109 cells. 5-FU is one of the com-monly used antitumor drugs. One of the principal mechanisms of action of 5-FU is inhibition of the enzyme thymidylate synthase (TS) and affecting DNA synthase. In recent years, it has been shown that 5-FU may induce apopt osis in fibroblast and a-cute leukaemic T-lymphocytes[4,5]. The present study indicated that 5-FU may induce apoptosis in human esophageal carcinoma Eca-109 cells. Chro-matin gathering under nuclear, the disappearance of nucleoli and the formation of ring-shaped nucleus may be the early changes of apoptosis. Apoptotic cells became visible late at 24 h and apparent by 48h. It may be related to the characteristic action of time-dependence.
ICE may mediate apoptosis induced by 5-FU. Ced-3 is known as one of the essential genes for cells to undergo apoptosis in C. elegans. ICE gene, a ho-mologue of the ced-3, was recently identified as in-ducer of apoptosis in Rat-1 fibroblasts[3]. Kondo et al[6] reported that cisplatin increased the mRNA ex-pression of ICE and induced apoptosis in malignant glioma cells. In our study 5-FU increased the ex-pression of ICE protein in Eca-109 cells undergoing apoptosis, suggesting that ICE protein may play a role in apoptosis induced by 5-FU. The expression of ICE protein was also detectable in some cells without features of apoptosis. From the phe-nomenon that the expression of ICE protein appeared at the early stage of apoptosis even before development of apoptosis, we inferred that ICE protein may be involved in the development of apoptosis. The induction of ICE does not necessarily trigger cell death, but the manifestation of cells which were seriously injured. If the injury can not be repaired, ICE may activate its downstream target molecules such as apopain, one member of the ICE family, and in the end, target proteins necessary for the existence of cells are cleaved and apoptosis be-comes irreversible. The reason why negative expres-sion of ICE protein was observed in some cells with typical features of apoptosis is not clear. The anti-body used in this study was specific to ICEp20 sub-unit. There is no cross-reaction between ICEp20 and ICEp10 subunits. Other member(s) of ICE family may be also involved in apoptosis. It is known that at least six members of the ICE family are expressed in mammalian cells. However, whether ICE acts di-rectly or through its action product interleukin-1β is not fully understood. The recent studies found that ICE may directly cleave actin which makes up cy-toskelet on and that alteration in actin was implica-ted in the formation of plasma membrane budding and disintegration of cells[7].
Footnotes
Edited by Jing-Yun Ma
References
- 1.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 3.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 4.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 5.Huschtscha LI, Bartier WA, Ross CE, Tattersall MH. Characteristics of cancer cell death after exposure to cytotoxic drugs in vitro. Br J Cancer. 1996;73:54–60. doi: 10.1038/bjc.1996.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo S, Barna BP, Morimura T, Takeuchi J, Yuan J, Akbasak A, Barnett GH. Interleukin-1 beta-converting enzyme mediates cisplatin-induced apoptosis in malignant glioma cells. Cancer Res. 1995;55:6166–6171. [PubMed] [Google Scholar]
- 7.Kayalar C, Ord T, Testa MP, Zhong LT, Bredesen DE. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci USA. 1996;93:2234–2238. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]


