Abstract
This review describes the interactions between the pedunculopontine nucleus (PPN), the ventral tegmental area (VTA), and the thalamocortical system. Experiments using modulators of cholinergic receptors in the PPN clarified its role on psychostimulant-induced locomotion. PPN activation was found to be involved in the animal’s voluntary search for psychostimulants. Every PPN neuron is known to generate gamma band oscillations. Voltage-gated calcium channels are key elements in the generation and maintenance of gamma band activity of PPN neurons. Calcium channels are also key elements mediating psychostimulant-induced alterations in the thalamic targets of PPN output. Thus, the PPN is a key substrate for maintaining arousal and REM sleep, but also in modulating psychostimulant self-administration.
Keywords: Amphetamine, Cocaine, Dopamine, Nicotine, P/Q-type calcium channels, T-type calcium channels
1. Wake–sleep control by the Reticular Activating System and its influence on substance abuse
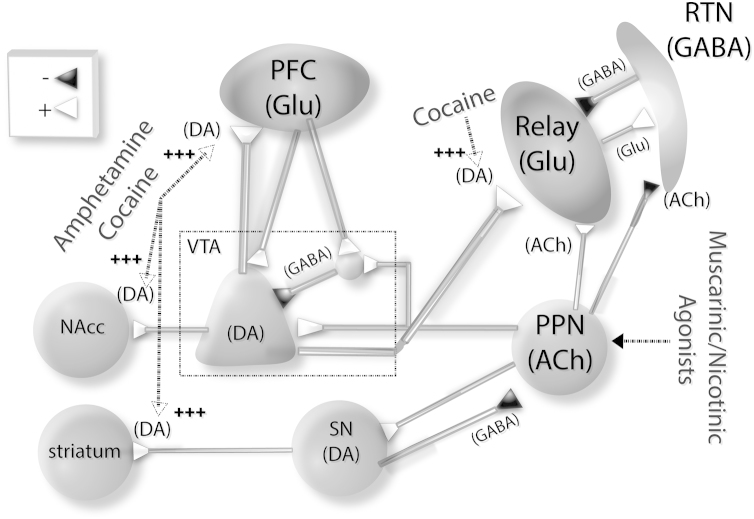
Substance abuse and the perception of withdrawal/relapse are mediated by neurobiological processes that occur when we are awake, but not when we are asleep. Furthermore, sleep disturbances (i.e., sleep deprivation) have been considered a risk for psychotimulant abuse. The Reticular Activating System (RAS) plays a central role controlling sleep homeostasis, modulating oscillatory rhythms between the thalamus and cortex that are distinguishable in the EEG during wake–sleep states [1]. The interactions between the pedunculopontine nucleus (PPN) and the thalamus are critical to its function of wake–sleep control, exerting a push–pull effect on two centers. That is, the PPN inhibits the reticular thalamic nucleus (RTN) (which decreases slow waves during sleep), and excites specific thalamic relay nuclei (which increases tonic firing during the awake state) (Fig. 1) [2], [3]. Thalamic relay neurons (which by definition send glutamatergic projections to the cortex) also receive RTN (GABAergic) afferents whose axons remain within the boundaries of the dorsal thalamic nucleus where the somata are located [4]. Thalamic relay neurons are bushy and, depending on the size of the soma, project to different layers of the cortex [5], [6]. RTN neurons, on the other hand, have axons that collateralize within the nucleus and also project to dorsal thalamic nuclei, but not to the cortex; they have long dendrites, whose secondary and tertiary branches possess vesicle-containing appendages that form synapses on the dendrites of other reticular neurons [7]. The cells of the RTN are electrically coupled via gap junctions, providing a coherent recurrent inhibitory signal to thalamic relay cells, causing activation of T-type calcium channels responsible for slow waves during sleep [8]. Therefore, cholinergic afferents from the PPN to the RTN are inhibitory (pull away from slow wave sleep) [9], but excitatory to thalamic relay neurons (push towards waking). This prevents the bursting mediated by the activation of T-type calcium channels by RTN inhibition [10], and tonically depolarizes thalamic relay neurons, thus inducing a global disinhibition of thalamocortical activity. That is, when the PPN is activated, slow wave sleep is reduced and arousal is increased.
Fig. 1.
Schematic diagram showing psychostimulants/neuromodulators of PPN and thalamocortical circuits. Projections from PPN to key dopaminergic nuclei underlying psychostimulant effects. The PPN projects to the ventral tegmental area (VTA) and substantia nigra (SN). Dopaminergic VTA neurons in turn project to the medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc). Amphetamines/cocaine (two stimulants that exert their effects by drastically increasing extracellular DA concentration) self-administration can be modulated by PPN efferents to VTA and SN. Cholinergic modulation by the PPN can modulate VTA and thalamic nuclei, exciting glutamatergic relay neurons while inhibiting GABAergic reticular thalamic neurons. Upper left box: (+) represents excitatory, glutamatergic or cholinergic synapses, and (−) GABAergic, or cholinergic inhibitory synapses.
Altered thalamocortical dynamics are the basis for several types of neurological and neuropsychiatric conditions named thalamocortical dysrhythmia syndrome [11], [12]. Abnormal activity of relay neurons has been related to an increase of low frequency oscillatory activity due to protracted activation of the low threshold voltage-activated (LVA) T-type calcium currents (CaV3 mediated), and in turn relayed to the cortical mantle resulting in a mistiming between sensory and arousal inputs. In summary, an enhancement of low frequency thalamocortical activity during awake states would underlie aberrant sensory processing [13].
The ventral tegmental area (VTA) is a key neural substrate involved in the modulation of psychostimulant abuse, and its output is essential for the rewarding effects of addictive drugs [14]. The VTA receives cholinergic RAS input from the PPN, which modulates the high frequency states of waking and rapid eye movement (REM) sleep (Fig. 1) [1]. Cholinergic efferents from the PPN to the VTA are part of a loop that includes the medial prefrontal cortex (mPFC) [15]. This loop is composed of mPFC glutamatergic efferents to dopaminergic and GABAergic neurons in the VTA and to the nucleus accumbens (NAcc) through a polysynaptic circuit that includes the PPN. In addition, the VTA sends dopaminergic and GABAergic efferents to the NAcc. Activation of the PPN thus increases VTA dopaminergic output, and increases extracellular dopamine (DA) levels in the NAcc and mPFC [16], which suggests that the PPN in part regulates the reward and motivational functions of the VTA [17].
Higher glutamatergic efferent activation from the mPFC would in turn reduce VTA dopaminergic output through its direct activation of local GABAergic interneurons within the VTA. Recent optogenetic experiments confirmed that PPN-VTA pathway stimulation can elicit psychostimulant-like behavior in the absence of any drug administration [18]. Since midbrain dopaminergic neurons originating in the VTA and substantia nigra pars compacta (SNc) have been previously described as the neural substrates underlying individual vulnerability to psychostimulant addiction [19], [20], [21], elucidating the functional modulation of the VTA and SNc by the PPN is a key to understanding how drug reinforcing, craving, and psychomotor-stimulant effects are modulated by a wake-promoting nuclei such as the PPN.
2. Drugs of abuse as modulators of calcium channels in the PPN and its targets
Psychostimulants—like thalamic relay neurons, about 40% of PPN cells have T-type calcium channels that mediate low threshold spikes (LTS) [1]. However, the most common calcium channels in the PPN are high threshold voltage-dependent calcium channels. Our group found that every PPN neuron has N- and/or P/Q-type calcium channels [22], which mediate beta/gamma band intrinsic membrane oscillations [23]. PPN calcium channels, in particular the P/Q-type, are modulated by muscarinic M2 receptors, providing a physiologically relevant fine-tuning of beta/gamma band oscillations in this nucleus [23]. P/Q-type (Cav2.1) calcium channels are widely distributed in the CNS [24], [25], and play a central role in the physiology of PPN [26], [27], [28], as well as its thalamocortical targets [13], [29]. High threshold voltage-dependent calcium channels, and P/Q-type channels in particular, have been described in presynaptic terminals mediating synaptic transmission in both glutamatergic pyramidal and inhibitory interneurons [24], [25]. Using two-photon imaging, P/Q-type channels have been located in the dendritic compartments of thalamocortical neurons [29]. In PPN neurons, P/Q- and N-type channels have also been associated with distal dendritic compartments [27], [28]. These calcium channels are critical to the induction and maintenance of high frequency oscillation states like waking and REM sleep [22], [23], [27], [28]. The study of the effects of psychostimulants on these channels is critical to determining the role of the PPN in psychostimulant abuse.
2.1. Amphetamines
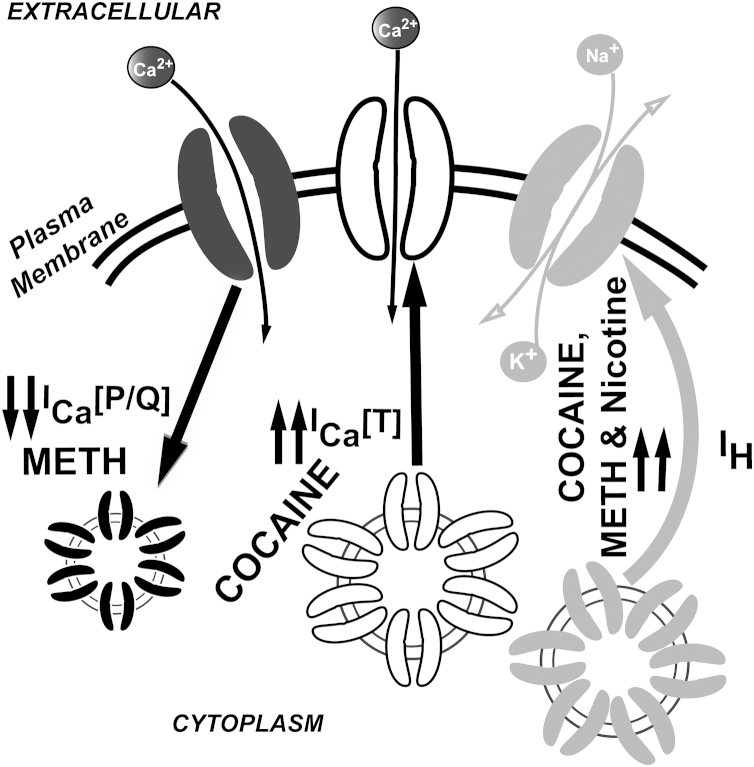
Amphetamine and methamphetamine induce increases in extracellular DA by acting in the terminals and cell bodies of midbrain DA neurons, where they induce the reverse transport of DA and prevent its uptake [30], [31]. Amphetamine is known to enhance cyclin-dependent kinase 5 (cdk5) activity [32], contributing to neuroadaptations underlying locomotor sensitization [33]. Cdk5-mediated intracellular pathways are known to down-regulate P/Q-type calcium channels [34], [35], which would mediate thalamocortical dysrhythmic interactions by unbalancing the normal P/Q-type vs T-type channels activation ratio [13]. Lowered PPN gamma band output is also to be expected after cdk5 activation, which would alter normal wake/REM sleep transitions [1]. Moreover, the effects of methamphetamine on the mPFC network has been recently found to alter calcium dynamics (by reducing calcium current density) in pyramidal neurons, while enhancing cationic hyperpolarization-activated IH current density (Fig. 2) (that could also increase low-frequency firing) [36]. Additionally, methamphetamine induced lower levels of glutamatergic synaptic transmission in the mPFC, which has been suggested to underline the mPFC hypofunctionality described in human addicts [36].
Fig. 2.
Illustration of psychostimulant/neuromodulator dependent turnover of calcium and cationic hyperpolarization-activated IH currents on PPN and thalamocortical neurons. Systemic administration of cocaine is known to up-regulate membrane expression of T-type calcium and IH cationic currents [49], [50], [51]. Systemic administration of METH reduced P/Q type calcium channel currents in medial prefrontal cortex (mPFC) [36]. In addition, nicotine upregulated IH cationic current density in PPN neurons (Garcia-Rill et al. [80]).
The basic behavioral effect of amphetamines is to induce hyperlocomotion, which can be used as a predictor of future propensity to amphetamine self-administration [37]. Acute total sleep deprivation has been described to potentiate amphetamine-induced locomotion [38]. Direct interactions between the PPN and VTA were demonstrated using excitotoxic lesions of the PPN [39]. GABAergic neuronal firing rates decrease during slow wave sleep and increase during REM sleep [40], suggesting a novel PPN control over VTA. More experiments are needed to further clarify this possibility as well as its possible modulation by psychostimulants.
PPN-lesioned animals showed a clear reduction in the response to a progressive ratio self-administration schedule of amphetamine [39]. Lesions of the PPN blocked the motivational (measured using a conditioned place preference paradigm) effects of amphetamine [41]. Muscarinic agonists have been shown to reduce amphetamine-induced hyperlocomotion [42], while muscarinic antagonists mediated facilitation of amphetamine-induced rotation through M2 receptors [43]. Unfortunately, all of these experiments were performed using systemic administration of muscarinic receptor modulators, making it difficult to determine the exact neural substrate of these actions in the brain (i.e., it is possible that muscarinic receptor modulation might affect the activity of other cholinergic nuclei such as the nucleus basalis that projects to cortex and to the RTN) [44].
2.2. Cocaine
The rewarding properties and abuse potential of cocaine is derived in part from elevated levels of DA neurotransmission in limbic circuits [45], [46]. Cocaine can also increase serotonin (5-HT) neurotransmission via inhibition of its re-uptake [47]. Individual vulnerability to cocaine self-administration has been associated with changes in neuronal intrinsic properties, in particular, with higher action potential frequency and bursting of VTA neurons, and to a lesser extent SNc neurons. In addition, animals that manifested higher self-administration rates also exhibited higher locomotor responses to a novel environment prior to psychostimulant-administration [48], strongly suggesting that the basal modulation of VTA neurons by the PPN can be considered critical to psychostimulant abuse liability.
While Schmidt et al. [49] showed that enhanced glutamatergic transmission in the PPN promoted cocaine priming-induced reinstatement of cocaine seeking, others [50] used the infusion of a diphtheria toxin conjugated to urotensin-II into the PPN (which resulted in the loss of >95% of PPN cholinergic neurons) to describe no significant alterations in cocaine self-administration. A possible explanation for the discrepancies described in recent years may be related to the strong serotonergic effects of cocaine [47]. Indeed, cocaine-mediated blocking effects of both DA and serotonin reuptake could alter the activity of PPN efferents to VTA, as well as the degree of activation of serotonergic afferents from the raphe nuclei to the PPN. Such a possibility has been recently proposed to explain the differential actions of cocaine and methylphenidate on GABAergic transmission in mouse ventrobasal thalamic nucleus [51]. A cocaine-mediated enhancement in GABA release has been described to induce thalamocortical dysrhythmic interactions in mice due to the over activation of T-type calcium channels expressed in thalamocortical neurons (Fig. 2) [52]. Indeed, pre-treatment with T-type channel blockers was shown to prevent the effects of cocaine on the thalamocortical system, suggesting a new role mediating GABA release of the T-type channels located in the presynaptic terminals of parvalbumin-rich reticular thalamic neurons [53]. After three-day systemic treatment with cocaine, both T-type and P/Q-type calcium channels were over expressed [51], suggesting a compensatory mechanism by which thalamocortical neurons attempt to cope with the deleterious effects of cocaine.
Acute total sleep deprivation potentiated cocaine-induced hyperlocomotion in mice [54]. Therefore, PPN activation is important in the modulation of cocaine self-administration, although the mechanisms underlying such an effect need further characterization.
2.3. Modafinil, a non-addictive psychostimulant, deserves separate discussion
We found that the prescription drug modafinil, which is used for the treatment of narcolepsy and daytime sleepiness, prevented the neurotoxic effects of methamphetamine on striatal circuits [55], [56]. Modafinil acts by increasing electrical coupling especially in GABAergic neurons [58], [59], which decreases input resistance in GABAergic cells throughout the brain, but particularly in the PPN. Such an effect would disinhibit glutamatergic and dopaminergic interactions between PPN and VTA nuclei. In addition, modafinil can counteract methamphetamine-mediated impairments in mPFC function [57], [60], which is a key area regulating cognition and normal sleep physiology [58], [61]. More studies are still needed in order to address the possible role of calcium channels in the apparent cognitive-enhancing profile of modafinil for the treatment of deficits mediated by methamphetamine.
Modafinil is known to mediate its effect on thalamocortical neurons through a CaMKII-dependent intracellular pathway [59] that has been described to enhance P/Q-type channel function [62]. These mechanisms might explain why modafinil-mediated over activation of the CaMKII intracellular pathways can lead to enhance walking time.
2.4. Nicotine
Nicotinic receptor agonists induced a depolarization early in development (~day 12) that switched across days 15–17 until they elicited hyperpolarization by day 21 in the rat PPN [63]. Nicotine has also been found to have an inhibitory effect on the PPN, initially reducing arousal. This may explain the initial anxiolytic effects of cigarette smoke [64], [65]. Nicotine in the fetus may have long-term consequences on RAS activity. Nicotine from cigarette smoke can cross the placenta, exposing the fetuses from smoking mothers to higher nicotine concentrations in both amniotic fluid and umbilical vein than the ones in maternal vein serum [66]. Toxicological effects of perinatal cigarette smoke exposure include lower birth weight [67], higher rate of spontaneous abortion [68], and increased incidence of sudden infant death syndrome [69]. Maternal smoking during pregnancy can lead to increased aggression [70], and problems with sustained attention and impulsivity in adolescent offspring [71]. Children of smoking mothers are at increased risk behavior problems [72], [73], behavioral disorders [74], [75], drug abuse [70], and for high rates of violent and persistent criminal offenses [70], [76].
Behavioral deficits related to arousal and attentional problems in humans have been identified in rats exposed to nicotine prenatally. These animal models show deficits in attention and memory in maze performance [77], [78], learning [77], and operant behaviors [79]. We studied the effects of prenatal exposure to cigarette smoke on the physiology of PPN cells postnatally [80]. We found that PPN neurons exhibited lower resting membrane potential and lower action potential threshold (tending to increase PPN firing), both of which could be related to an increase in hyperpolarization-activated IH current (that could also increase low-frequency firing). IH current enhancement has been also described for cocaine-mediated effects on thalamocortical neurons (Fig. 2) [52]. IH and T-type currents have been described to mediate low frequency oscillatory activity in the thalamaocortical system [52]. We speculate that hyperpolarization-activated cyclic nucleotide-gated proteins may be over activated by prenatal cigarette smoke exposure, and that a possible down regulation of IH proteins may be responsible for the enhancement in arousal as well as for the attentional deficits [72], as well as impulse control problems (exaggerated fight-or-flight responses) reported in the children of mothers who smoked during pregnancy [71], [73].
3. Conclusion
Drug abuse is a major global health problem. Although some people that have suffered from psychostimulant abuse are able to recover, relapse occur in almost half of the cases. Also, addicts to drugs of abuse have shown persistent neuropathological changes that may be related to the profound dysfunction present in addict populations [81]. Thus, psychostimulant addiction is treated like a chronic illness, requiring repeated episodes of therapy. The symptoms of relapse are similar to those in depression and anxiety, which may require pharmacological therapy in addition to cognitive therapy and support groups. These functions all involve arousal and, therefore, the RAS. For example, impaired sleep is present during withdrawal, and insomnia-like symptoms are present in heroin, cocaine, methamphetamine, and addicted users of other drugs [82]. Basically, disturbed sleep predicts relapse to alcohol and psychoactive drug abuse [83]. Just as with psychiatric and neurological disorders, rebalancing the wake–sleep homeostatic system is key to treatment response and, in fact, signals successful alleviation of symptoms. In addition, future work characterizing the role of psychostimulants on voltage-gated calcium channels in the RAS and target areas may shed some light on their mechanisms and their possible treatment.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH awards P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by Grants from FONCYT Agencia Nacional de Promoción Científica y Tecnológica; BID1728 OC.AR. PICT 2007-1009; PICT 2008-2019, PICT-2012-1769 and UBACYT 2014-2017#20120130101305BA (to Dr. Urbano); and by Grants from CONICET-PIP 2011-2013-11420100100072 and from FONCYT Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-0924 (to Dr. Bisagno).
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Garcia-Rill E. Academic Press, Elsevier; 2015. The RAS and Drug Abuse in Reticular activating system in Waking and the Reticular Activating System Health and Disease; pp. 277–290. [Google Scholar]
- 2.Hallanger A.E., Levey A.I., Lee H.J., Rye D.B., Wainer B.H. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J. Comp. Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M., Pare D., Parent A., Smith Y. Projections of cholinergic and noncholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M., McCarley R.W. Springer; New York: 2005. Brain Control of Wakefulness and Sleep. [Google Scholar]
- 5.Steriade M., Datta S., Pare D., Oakson G., Curro Dossi R.C. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steriade M., Jones E.G., McCormick D.A. Elsevier; Amsterdam: 1997. Thalamus, Volume I: Organization and Function. [Google Scholar]
- 7.Deschenes M., Madariaga-Domich A., Steriade M. Dendrodendritic synapses in cat reticularis thalami nucleus, a structural basis for thalamic spindle synchronization. Brain Res. 1985;334:169–171. doi: 10.1016/0006-8993(85)90580-3. [DOI] [PubMed] [Google Scholar]
- 8.Landisman C.E., Long M.A., Beierlein M. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Ari Y., Dingledine R., Kanazawa I., Kelly J.S. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. J. Physiol. (Lond.) 1976;261:647–671. doi: 10.1113/jphysiol.1976.sp011579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bista P., Meuth S.G., Kanyshkova T., Cerina M., Pawlowsk M., Ehling P., Landgraf P., Borsotto M., Heurteaux C., Pape H.C., Baukrowitz T., Budde T. Identification of the muscarinic pathway underlying cessation of sleep-related burst activity in rat thalamocortical relay neurons. Pflugers Arch. 2012;463:89–102. doi: 10.1007/s00424-011-1056-9. [DOI] [PubMed] [Google Scholar]
- 11.Llinás R.R., Ribary U., Jeanmonod D., Kronberg E., Mitra P.P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llinás R.R., Urbano F.J., Leznik E., Ramírez R.R., van Marle H.J. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Llinas R.R., Choi S., Urbano F.J., Hee-Sup S. γ-Band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc. Natl. Acad. Sci. USA. 2007;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to the drugs of abuse and pathology. Br. J. Pharmacol. 2008;1:438–445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floresco S.B., West A.R., Ash B., Moore H., Grace A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 17.Good C.H., Lupica C.R. Properties of distinct ventral tegmental area synapses activated via pedunculopontine or ventral tegmental area stimulation in vitro. J. Physiol. (Lond.) 2009;587:1233–1247. doi: 10.1113/jphysiol.2008.164194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammel S., Lim B.K., Ran C., Huang K.W., Betley M.J., Tye K.M., Deisseroth K., Malenka R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob G.F. Circuits, drugs, and drug addiction. Adv. Pharmacol. 1998;42:978–982. doi: 10.1016/s1054-3589(08)60910-2. [DOI] [PubMed] [Google Scholar]
- 20.White F.J., Kalivas P.W. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 21.Wise R.A. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 22.Luster B., D׳Onofrio S., Urbano F., Garcia-Rill E. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol. Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kezunovic N., Hyde J., Goitia B., Bisagno V., Urbano F.J., Garcia-Rill E. Muscarinic modulation of high frequency oscillations in pedunculopontine neurons. Front. Neurol. 2013;4(176):1–13. doi: 10.3389/fneur.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillman D., Chen S., Aung T.T., Cherksey B., Sugimori M., Llinas R.R. Localization of P-type calcium channels in the central nervous system. Proc. Natl. Acad. Sci. USA. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchitel O.D., Protti D.A., Sanchez V., Cherkesey B.D., Sugimori M., Llinas R.R. P-type voltage dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc. Natl. Acad. Sci. USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kezunovic N., Urbano F.J., Simon C., Hyde J., Smith K., Garcia-Rill E. Mechanism behind gamma band activity in pedunculopontine nucleus (PPN) Eur. J. Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Rill E., Kezunovic N., Hyde J., Beck P., Urbano F.J. Coherence and frequency in the reticular activating system (RAS) Sleep Med. Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbano F.J., Kezunovic N., Hyde J., Simon C., Beck P., Garcia-Rill E. Gamma band activity in the reticular activating system (RAS) Front. Neurol. Sleep Chronobiol. 2012;3(6):1–16. doi: 10.3389/fneur.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedroarena C., Llinas R.R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc. Natl. Acad. Sci. USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiden L.S., Sabol K.E., Ricaurte G.A. Amphetamine: effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993;32:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 31.Sulzer D., Chen T.K., Lau Y.Y., Kristensen H., Rayport S., Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mlewski E.C., Krapacher F.A., Ferreras S., Paglini G. Transient enhanced expression of Cdk5 activator p25 after acute and chronic d-amphetamine administration. Ann. N. Y. Acad. Sci. 2008;1139:89–102. doi: 10.1196/annals.1432.039. [DOI] [PubMed] [Google Scholar]
- 33.Singer B.F., Neugebauer N.M., Forneris J., Rodvelt K.R., Li D., Bubula N., Vezina P. Locomotor conditioning byamphetamine requires cyclin-dependent kinase 5 signaling in the nucleus accumbens. Neuropharmacology. 2014;85:243–252. doi: 10.1016/j.neuropharm.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomizawa K., Ohta J., Matsushita M., Moriwaki A., Li S.T.S.T., Takei K., Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J. Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karila L., Weinstein A., Aubin H.J., Benyamina A., Reynaud M., Batki S.L. Pharmacological approaches to methamphetamine dependence: a focused review. Br. J. Clin. Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González B., Rivero-Echeto C., Muñiz J.A., Cadet J.L., García-Rill E., Urbano F.J., Bisagno V. Methamphetamine blunts Ca2+ currents and excitatory synaptic transmission through D1/5 receptor-mediated mechanisms in the mouse medial prefrontal cortex. Addict. Biol. 2015 doi: 10.1111/adb.12249. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierre P.J., Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- 38.Saito L.P., Fukushiro D.F., Hollais A.W., Mári-Kawamoto E., Costa J.M., Berro L.F., Aramini T.C., Wuo-Silva R., Andersen M.L., Tufik S., Frussa-Filho R. Acute total sleep deprivation potentiates amphetamine-induced locomotor-stimulant effects and behavioral sensitization in mice. Pharmacol. Biochem. Behav. 2014;117:7–16. doi: 10.1016/j.pbb.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Alderson H.L., Faulconbridge L.F., Gregory L.P., Latimer M.P., Winn P. Behavioural sensitisation to repeated d-amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience. 2003;118(2):311–315. doi: 10.1016/s0306-4522(03)00152-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee R.S., Steffensen S.C., Henriksen S.J. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J. Neurosci. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechara A., van der Kooy D. The tegmental pedunculopontine nucleus: a brainstem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J. Neurosci. 1989;9:3400–3409. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shannon H.E., Peters S.C. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J. Pharmacol. Exp. Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- 43.Hagan J.J., Tonnaer J.A.D.M., Rijk H., Broekkamp C.L.E., van Delft A.M.L. Facilitation of amphetamine-induced rotation by muscarinic antagonists is correlated with M2 receptor affinity. Brain Res. 1987;410:69–73. doi: 10.1016/s0006-8993(87)80021-5. [DOI] [PubMed] [Google Scholar]
- 44.Asanuma C. Distribution of neuromodulatory inputs in the reticular and dorsal thalami nuclei. In: Steriade M., Jones E.G., McCormick D.A., editors. Thalamus, Volume II, Experimental and Clinical Aspects. Elsevier; Amsterdam: 1997. pp. 93–154. [Google Scholar]
- 45.Wilson M.C., Bedford J.A., Buelke J., Kibbe A.H. Acute pharmacological activity of intravenous cocaine in the rhesus monkey. Psychopharmacol. Commun. 1976;2:251–261. [PubMed] [Google Scholar]
- 46.Wise R.A., Bozarth M.A. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 47.Ross S.B., Renyi A.L. Inhibition of the uptake of tritiated 5-hydroxytryptamine in brain tissue. Eur. J. Pharmacol. 1969;7:270–277. doi: 10.1016/0014-2999(69)90091-0. [DOI] [PubMed] [Google Scholar]
- 48.Grimm J.W., See R.E. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-b estradiol, and associated with abnormal vaginal cytology. Physiol. Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H.D., Famous K.R., Pierce R.C. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur. J. Neurosci. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steidl S., Wang H., Wise R.A. Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self-administration or conditioned place preference in rats. PLoS One. 2014;9:e84412. doi: 10.1371/journal.pone.0084412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goitia B., Raineri M., González L.E., Rozas J.L., Garcia-Rill E., Bisagno V., Urbano F.J. Differential effects of methylphenidate and cocaine on GABA transmission in sensory thalamic nuclei. J. Neurochem. 2013;124:602–612. doi: 10.1111/jnc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbano F.J., Bisagno V., Wikinski S.I., Uchitel O.D., Llinás R.R. Cocaine acute "binge" administration results in altered thalamocortical interactions in mice. Biol. Psychiatry. 2009;66(8):769–776. doi: 10.1016/j.biopsych.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 53.Bisagno V., Raineri M., Peskin V., Wikinski S.I., Uchitel O.D., Llinás R.R., Urbano F.J. Effects of T-type calcium channel blockers on cocaine-induced hyperlocomotion and thalamocortical GABAergic abnormalities in mice. Psychopharmacology (Berl.) 2010;212:205–214. doi: 10.1007/s00213-010-1947-z. [DOI] [PubMed] [Google Scholar]
- 54.Berro L.F., Santos R., Hollais A.W., Wuo-Silva R., Fukushiro D.F., Mári-Kawamoto E., Costa J.M., Trombin T.F., Patti C.L., Grapiglia S.B., Tufik S., Andersen M.L., Frussa-Filho R. Acute total sleep deprivation potentiates cocaine-induced hyperlocomotion in mice. Neurosci. Lett. 2014;579:130–133. doi: 10.1016/j.neulet.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 55.Raineri M., Peskin V., Goitia B., Taravini I.R., Giorgeri S., Urbano F.J., Bisagno V. Attenuated methamphetamine induced neurotoxicity by modafinil administration in mice. Synapse. 2011;65(10):1087–1098. doi: 10.1002/syn.20943. [DOI] [PubMed] [Google Scholar]
- 56.Raineri M., Gonzalez B., Goitia B., Garcia-Rill E., Krasnova I.N., Cadet J.L., Urbano F.J., Bisagno V. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7(10):e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raineri M., González B., Rivero-Echeto C., Muñiz J.A., Gutiérrez M.L., Ghanem C.I., Cadet J.L., García-Rill E., Urbano F.J., Bisagno V. Differential effects of environment-induced changes in body temperature on modafinil׳s actions against methamphetamine-induced striatal toxicity in mice. Neurotox. Res. 2014;27(1):71–83. doi: 10.1007/s12640-014-9493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Rill E., Heister D.S., Ye M., Charlesworth A., Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbano F.J., Leznik E., Llinas R.R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc. Natl. Acad. Sci. USA. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González B., Raineri M., Cadet J.L., García-Rill E., Urbano F.J., Bisagno V. Modafinil improves methamphetamine-induced object recognition deficits and restores prefrontal cortex ERK signaling in mice. Neuropharmacology. 2014;87:188–197. doi: 10.1016/j.neuropharm.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edgar C.J., Pace-Schott E.F., Wesnes K.A. Approaches to measuring the effects of wake-promoting drugs: a focus on cognitive function. Hum. Psychopharmacol. 2009;24(5):371–389. doi: 10.1002/hup.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X., Lautermilch N.J., Watari H., Westenbroek R.E., Scheuer T., Catterall W.A. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc. Nat. Acad. Sci. USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Good C.H., Bay K.D., Buchanan R., Skinner R.D., Garcia-Rill E. Muscarinic and nicotinic responses in the developing pedunculopontine nucleus (PPN) Brain Res. 2007;1129:147–155. doi: 10.1016/j.brainres.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 64.Benowitz N.L. Pharmacology of nicotine: addiction and therapeutics. Ann. Rev. Pharmacol. Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- 65.Simosky J.K., Stevens K.E., Freedman R. Nicotinic agonists and psychosis. Curr. Drug Targets— CNS Neurol. Disord. 2002;1:177–190. doi: 10.2174/1568007024606168. [DOI] [PubMed] [Google Scholar]
- 66.Luck W., Nau H., Hansen R., Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 67.Eskanazi B., Prehn A.W., Cristianson R.E. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am. J. Public Health. 1995;85:395–398. doi: 10.2105/ajph.85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kline J., Stein Z.A., Susser M., Warburton D. Smoking: a risk factor for spontaneous abortion. New Engl. J. Med. 1997;297:793–796. doi: 10.1056/NEJM197710132971501. [DOI] [PubMed] [Google Scholar]
- 69.Bulterys M. High incidence of sudden infant death syndrome among northern Indians and Alaska natives compared with southwestern Indians: possible role of smoking. J. Com. Health. 1990;15:185–194. doi: 10.1007/BF01350256. [DOI] [PubMed] [Google Scholar]
- 70.Weissman M.M., Warner V., Wickramaratne P.J., Kandel D.B. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 71.Fried P.A., O’Connell C.M., Watkinson B. 60 and 72-month follow-up of children prenatally exposed to marijuana, cigarettes and alcohol: cognitive and language assessments. J. Dev. Behav. Pediatr. 1992;13:383–391. [PubMed] [Google Scholar]
- 72.Milberger S., Biederman J., Farraone S.V., Chen L., Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Wasserman G.A., Liu X., Pine D.S., Graziano J.H. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicol. Teratol. 2001;23(1):13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 74.Fergusson D.M., Woodward L.J., Horwood L.J. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch. Gen. Psychiat. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- 75.Wakschlag L.S., Lahey B.B., Loeber R., Green S.M., Gordon R.A., Leventhal B.L. Maternal smoking during pregnancy and the risk of conduct disorders in boys. Arch. Gen. Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- 76.Rasanen P., Hakko H., Isohanni M., Hodgins S., Jarvelin M.R., Tiihonen J. Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the Northern Finland 1966 Birth Cohort. Am. J. Psychiatry. 1966;156:857–862. doi: 10.1176/ajp.156.6.857. [DOI] [PubMed] [Google Scholar]
- 77.Levin E.D., Briggs S.J., Christopher N.C., Rose J.E. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol. Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- 78.Sorenson C.A., Raskin L.A., Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol. Biochem. Behav. 1991;40:991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- 79.Martin J.C., Becker R.F.T. The effects of maternal nicotine absorption or hypoxic episodes upon appetitive behavior of rat offspring. Dev. Psychobiol. 1971;4:133–147. doi: 10.1002/dev.420040205. [DOI] [PubMed] [Google Scholar]
- 80.Garcia-Rill E., Buchanan R., McKeon K., Skinner R.D., Wallace T. Smoking during pregnancy: postnatal effects on arousal and attentional brain systems. NeuroTox. 2007;28:915–923. doi: 10.1016/j.neuro.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cadet J.L., Bisagno V., Milroy C.M. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasler B.P., Soehner A.M., Clark D.B. Circadian rhythms and risk for substance use disorders in adolescence. Curr. Opin. Psychiatry. 2014;27(6):460–466. doi: 10.1097/YCO.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brower K.J., Perron B.E. Sleep disturbances as a universal risk factor for relapse in addictions to psychoactive substances. Med. Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]