Abstract
AIM: To offer a more simple method with a high sensitivity and specificity for detection of hepatoma cells in peripheral blood of the patients with HCC.
METHODS: Improved nested RT-PCR method was used to detect the expression of AFP mRNA in nuclear cells separated from peripheral venous blood.
RESULTS: AFP mRNA contained in ten hepatoma cells was detected from 2 mL peripheral blood.
CONCLUSION: The improved nested RT-PCR assay for AFP mRNA expressed in cancer cells in peripheral blood might be a valuable method for clinical diagnosis of HCC.
Keywords: liver neoplasms; carcinoma, hepatocellular; RNA, messenger/blood; alpha-feto-proteins polymerase chain reaction; neoplasm metastasis
INTRODUCTION
The metastatic potential of hepatocellular carcinoma (HCC) is not only directly correlated with prognosis of the patients with HCC, but also extremely valuable for selection of adequate therapies for HCC. Detection of circulating hepatoma cells in peripheral blood might be an indicator of metastatic potential of hepatocellular carcinoma. Human α-Fe-toprotein (AFP) is a well-known marker for hepatoma cells. Although the serum AFP level could be one of the useful biochemical indicators for diagnosis of HCC, the conventional measurements for serum AFP concentration is not so sensitive to identify an extremely small amount of cancer cells in peripheral blood. Based on the nested RT-PCR method for detecting AFP mRNA in the circulation reported recently[1,2], we merged the reverse transcription reaction and the first PCR into a single step, and then combined it with nested PCR. By this way, AFP mRNA contained in 10 human hepatoblastoma cells (HepG-2) could be detected in 2 mL peripheral venous blood. Being highly sensitive and specific, the improved method requires relatively a small amount of blood sample, and less time to obtain similar satisfactory results.
MATERIALS AND METHODS
Materials
HepG-2 was derived from the Institute of Virology, Chinese Academy of Preventive Medical Sciences. The AFP primers[3] purified by HPLC were obtained from Cybersyn, BJ. The external primers were: 5’ACTGAATCCAGAACACTGCATAG3’ and 5’TGCAGTCAATGCATCTTTCACCA3’. The internal primers were: 5’TGGAATAGCTTCCATATTGGATTC3’ and 5’AAGTGGCTTCTTGAACAAACTGG3’. Taq DNA polymerase and AMV reverse transcriptase were purchased from Sino-American Corp. and Promega Inc., respectively.
Methods
Separation of nuclear cells from peripheral blood. Fresh healthy peripheral venous blood was collected into a sterilized tube containing 0.5% EDTA. One thousand HepG-2 cells were mixed with 2 mL healthy blood and serial dilution of cancer cells was then prepared. Each dilution sample contained 1000, 100, 10 and 1 HepG-2 cells per 2 mL of blood, respectively. Nuclear cells were separated from peripheral venous blood using routine method by Ficoll isolation.
Extraction of total RNA from nuclear cells. Total RNA of nuclear cells separated from whole blood was extracted using single-step method by acid guanidinium thiocyanate extraction[4].
Reverse transcription and the first PCR. A total amount of 50 μL reaction solution contained 5 μL 10 × PCR buffer, 0.2 mmol/L of each dNTPs, 20 pmol-50 pmol of each external primers, 50 U RNAase inhibitor, 30 U AMV reverse transcriptase, 3 U Taq DNA polymerase and RNA template. The mixture solution was incubated at 42 °C for 30 min, and followed by initial denaturation at 94 °C for 2 min. The PCR procedure was carried out as follows: denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 40 s, and the thermal cycles were repeated 32 times.
Nested PCR. Reaction mixture of 50 μL contained 5 μL of 10 × PCR buffer, 0.2 mmol/L of each dNTPs, 20 pmol-50 pmol of each internal primers, 3 U Taq DNA polymerase and 10 μL of the first PCR product. The PCR procedure was: denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 40 s; and the cycles were repeated 32 times as well.
Gel electrophoresis. The final amplification product was electrophoresed on 2% agrose gel and stained with ethidium bromide for the specific band of 101 bp.
Internal standard control assay. Total RNA extracted from HepG-2 cells was quantified and diluted serially with DEPC-treated water, which was used as the internal standard control of this assay. Each of internal standard control samples contained 5, 1, 0.5, 0.05, 0.005, 0.0005 and 0.00005 μg of RNA template, respectively. The diluted samples were applied to nested RT-PCR amplification following the same procedure described above.
RESULTS
The amplification template used in this method was total RNA derived from HepG-2 cells. To seek a rapid and simple detecting method for AFP mRNA expression in hepatoma cells, we merged the reverse transcription reaction and the first PCR amplification into a single step and then combined with nested PCR. Gel electrophoresis analysis showed that specific bands (101 bp) of AFP mRNA were not observed in nuclear cells from healthy peripheral venous blood. When 1000, 100, 10 HepG-2 cells were added into 2 mL blood, specific bands of 101 bp were demonstrated in all RNA samples from nuclear cells in each of diluted samples. However, RNA from the diluted blood containing 1 HepG-2 cells was in-sufficient for detection (Figure 1).
Figure 1.
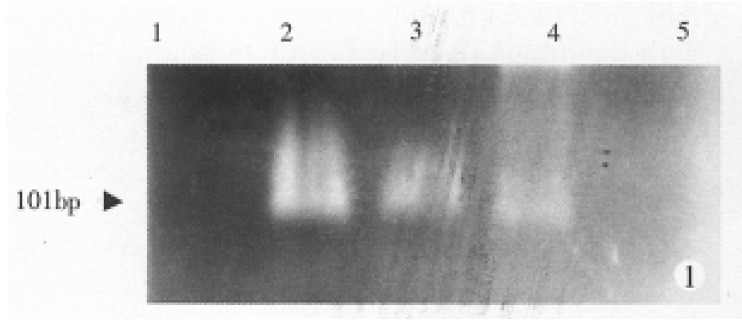
Amplification of AFP mRNA expressed in nuclear cells in peripheral blood by nested RT-PCR. Lane 1: Blood from healthy volunteers. Lanes 2-5: Titration of HepG-2 cells. Each lane includes (from left to right) 1000, 100, 10, 1 HepG-2 cells in 2 mL normal blood, respectively.
As indicated in Figure 2, internal standard control assay demonstrated that the intensity of the specific bands for AFP mRNA was significantly correlated with the amount of total RNA templates. No specific bands for AFP were detected in the sample of 5 × 10-6 μg RNA from HepG-2 cells by nested RT-PCR. With regard to negative control with absence of RNA from HepG-2 cells, it was also impossible to detect such specific bands.
Figure 2.
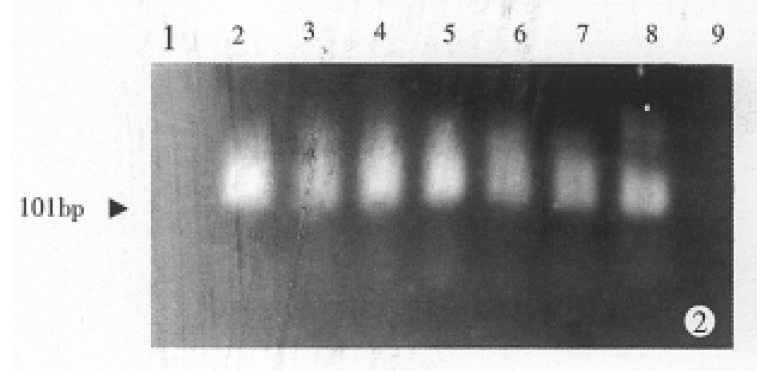
Amplification of AFP mRNA in HepG-2 cells by nested RT-PCR. Lane 1: Negative control. Lanes 2-9: Titration of total RNA extracted from HepG-2 cells. Each lane includes (from left to right) 5, 1, 0.5, 0.05, 0.005, 0.0005, 0.00005, 0.000005 μg total RNA, respectively.
DISCUSSION
Primary hepatocellular carcinoma is one of the most common malignant tumors in China, with the death rate ranking the first in the urban population and the second in rural areas of China. Prognosis of HCC is not only closely associated with early diagnosis and optimal therapy, but also with some important characteristics of hepatoma cells, such as the potential of intrahepatic metastasis, extrahepatic metastasis and recurrence. How to estimate the metastatic potential of hepatoma cells has remained a major puzzle for many years. AFP is expressed by hepatoma cells and then secreted into circulating blood, thus being regarded as a useful marker for hepatocellular carcinoma. Hepatoma with the potential of intrahepatic or extrahepatic metastasis often shed hepatoma cells into portal venous blood and peripheral blood.
It was reported that the human albumin mRNA could be used as a marker of circulating hepatocytes in HCC[5], and that AFP mRNA expression could be detected in a tiny amount of hepatoma cells in circu-lating blood of patients with HCC[1,2] by nested RT-PCR. Furthermore, they found that the level of AFP mRNA expression was significantly correlated with the prognosis of HCC. RT-PCR is a very sensitive technique for amplifying nucleic acids, which could be often used to detect tiny amounts of mRNA copies. Since nested RT-PCR utilizes a couple of internal primers to reamplify the specific PCR product, it exhibits higher sensitivity, stronger specificity and lower false positive occurrence as compared to single RT-PCR.
In this study, we attempted to provide a more simple nested RT-PCR method with high sensitivity and specificity for detecting a tiny amount of hepatoma cells in circulating blood of patients with HCC. By this method, AFP mRNA could be detect-ed when 10 HepG-2 cells were present in 2 mL peripheral blood or portal venous blood, suggesting that the sensitivity of this method is similar to that reported in literature. Thus, it might be a desirable assay for clinical evaluation of metastatic potential of hepatocellular carcinoma.
Footnotes
Project supported by the fund for scientific research of Ministry of Public Health.
References
- 1.Komeda T, Fukuda Y, Sando T, Kita R, Furukawa M, Nishida N, Amenomori M, Nakao K. Sensitive detection of circulating hepatocellular carcinoma cells in peripheral venous blood. Cancer. 1995;75:2214–2219. doi: 10.1002/1097-0142(19950501)75:9<2214::aid-cncr2820750905>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura M, Niwa Y, Hikiba Y, Okano K, Kato N, Shiina S, Shiratori Y, Omata M. Sensitive assay for detection of hepatocellular carcinoma associated gene transcription (alpha-fetoprotein mRNA) in blood. Biochem Biophys Res Commun. 1995;207:813–818. doi: 10.1006/bbrc.1995.1259. [DOI] [PubMed] [Google Scholar]
- 3.Morinaga T, Sakai M, Wegmann TG, Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci USA. 1983;80:4604–4608. doi: 10.1073/pnas.80.15.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Hillaire S, Barbu V, Boucher E, Moukhtar M, Poupon R. Albumin messenger RNA as a marker of circulating hepatocytes in hepatocellular carcinoma. Gastroenterology. 1994;106:239–242. doi: 10.1016/s0016-5085(94)95705-3. [DOI] [PubMed] [Google Scholar]


