Abstract
AIM: To assess the relationship between gastric acid output (GAO) and both pattern of gastroesophageal reflux (GER) and esophageal lesions, and to evaluate the role of GAO and other potential pathogenetic factors in the development of esophagitis.
METHODS: Gastric acid secretory testing and 24-h intraesophageal pH monitoring were performed in 31 patients with esophagitis and concomitant duodenal ulcer (E + DU) and compared with those of 72 patients with esophagitis (E) alone.
RESULTS: The GAO in patients with E + DU was significantly higher than in patients with E (P < 0.05). There was no significant difference between the two groups of patients as to endoscopicl findings and parameters of GER (P > 0.05). A multiple regression analysis with stepwise deletion showed that the presence of hiatal hernia (HH), GER in upright position and age appeared to correlate significantly with the presence of esophagitis.
CONCLUSIONS: No parallel relationship between GAO and severity of GER or esophageal lesions exists in patients with E + DU, and that GAO is not a major pathogenetic factor in GER disease.
Keywords: esophagitis/etiology, gastric acid/secretion, duodenal ulcer/etiology, gastroesophageal reflux/etiology, hydrogen-ion concentration, multivariate analysis
INTRODUCTION
It is generally agreed that gastroesophageal reflux (GER) may be multifactorial in its pathogenesis. Over the past decade, many investigators have focused their attention on the role of lower esophageal sphincter (LES), hiatal hernia (HH), esophageal mucosal sensitivity, and esophageal or gastric emptying in the development of GER disease. Some studies have shown that LES, hiatal hernia, esophageal emptying and esophageal mucosal sensitivity are, to a certain extent, involved in the pathogenesis of GER[1-10]. However, although gastric acid is believed to be an important factor in the development of GER disease, it is still unclear whether the severity of GER and esophageal lesions is necessarily related to increased gastric acid output. Moreover, little is known which factors are crucial in the pathogenesis of GER disease. Therefore, the aims of this study are: to assess the relationship between gastric acid output (GAO) and both pattern of GER and severity of esophageal lesions in patients with esophagitis and concomitant duodenal ulcer (E + DU), in comparison with patients with esophagitis only (E); and to evaluate the role of GAO and other potential pathogenetic factors in the development of reflux esophagitis by multiple regression analysis with stepwise deletion.
PATIENTS AND METHODS
Patients
Two groups of patients were enrolled in this study. Of these patients, 31 (27 men, 4 women; mean age of 44 years) had E + DU and 72 (53 men, 19 women; mean age of 42 years) had esophagitis only. All patients had symptoms of GER disease, i.e., chronic heartburn and regurgitation, and/or up-per abdominal pain, for a median duration of 55.4 months (range from 3 to 120 months). None of the patients had taken H2-receptor blockers or H+/K+-ATPase inhibitors for 2 weeks before 24-h pH-monitoring and gastric acid secretory testing.
Methods
All patients underwent upper GI endoscopy, followed by 24 h intraesophageal pH monitoring and acid secretory testing over a 2-week period. Endoscopy was performed by the same gastroenterologist in all patients. The degree of esophagitis was assessed endoscopically by using the criteria of Savary and Miller[11] (grades I to IV). Of these patients, only few presented grades II and III esophagitis, and therefore grades I and II, and III and IV, respectively, were grouped together.
The 24-h intraesophageal pH monitoring was carried out by a routine method used in our laboratory[12,13]. A glass pH-electrode with an incorporated potassium chloride reference (Ingold electrode, No. 440) was introduced via the nasoesophageal route and positioned with the tip 5 cm above the gastroesophageal junction, identified with the pHª²meter. Esophageal pH values were recorded with a solid-state recorder (Autronicord CM 18).
Analysis of pH recording was made on a computer with a dedicated program. The parameters recorded included the frequency and duration of GER in upright position (day-time) and supine position (night-time), and GER frequency exceeding 5 min. GER was defined as abnormal if total reflux duration amounted to 7% during the 24-h monitoring[14].
Basal acid secretory analysis was performed in the absence of any antisecretory medication for 2 weeks before the study, in accordance with Raufman et al[15]. In brief, after a nasogastric tube was introduced into and positioned in the gastric antrum, the gastric contents were emptied by aspiration. Four consecutive 15-min samples of gastric secretion were obtained by continuous aspiration, and the samples were titrated with 0.01 N NaOH to pH7.0. Fasting basal acid output (BAO) was expressed as milliequivalents of acid per hour (mEq/h). After a BAO was obtained, the patients underwent stimulated acid secretory testing. Six μg/kg of pentagastrin were injected intramuscularly. Sum of the four highest consecutive 15-min samples was represented as a maximal acid output (MAO), and that of the two highest consecutive 15-min period within 2 h of receiving the stimulant was represented as a peak acid output (PAO). At the same time, basal gastrin level in the serum was measured. Normal values for BAO, MAO, PAO and basal gastrin in our laboratory are as follows: BAO, 0.37 mEq/h ± 0.27 mEq/h; MAO, 2.05 mEq/h ± 1.07 mEq/h; PAO, 3.36 mEq/h ± 1.19 mEq/h; and basal gastrin, 37.8 ng/L ± 2.84ng/L.
Chisquare test and Student’s t test were used to evaluate the data of GI endoscopy and gastric acid secretion, respectively. Statistical evaluation was made using the Mann-Whitney U test for the parameters of 24 h intraesophageal pH monitoring. Multiple regression analysis with stepwise deletion was used to evaluate role of some potential pathogenetic factors in the development of reflux esophagitis, which were rated in order of importance. The methods and steps are as follows:
a. The formula of multiple regression: y = b0 + b1X1 + b2X2 + … + bkXk where y and b0 represent a dependent variable and a constant factor, X1, X2, …, Xk represent independent variables, and b1, b2, …, bk are the standard partial regression coefficient of independent variables[16].
b. Dependent variable and independent variables. In this study, the dependent and independent variables were selected on the basis of the hypothesis that the pathogenesis of the reflux esophagitis was multifactorial, and GER, gastric acid, hiatal hernia, etc. may be all involved, to a certain extent, in the pathogenesis of esophagitis. Accordingly, esophagitis was defined as the depen-dent variable, and its numerical values assigned to different grades of esophagitis were: 1 (Grade I or II) and 2 (Grade III or IV). Ten variables such as age, HH, GER, gastric acid, etc. were de fined as the independent variables. Table 1 gives in detail these independent variables and their definition.
Table 1.
Independent variables
| Variables | Items | Definition |
| X1 | Age | Years |
| X2 | Hiatal hernia | 0 (no) |
| 1 (yes) | ||
| X3 | Smoking cigarette | 0 (< 1.2 cig./week) |
| 1 (< 10 cig./day) | ||
| 2 (> 10 cig./day) | ||
| X4 | Alcohol consumption | 0 (< once/month) |
| 1 (once/week) | ||
| 2 (> once a week) | ||
| X5 | GER in upright position | Percentage time |
| X6 | GER in supine position | Percentage time |
| X7 | BAO | mEq/h |
| X8 | MAO | mEq/h |
| X9 | PAO | mEq/h |
| X10 | Gastrin | μg/L |
c. Multiple regression with stepwise deletion. This analysis was done by means of a statistical package (Statpak 3.1, Northwest Analytical, Inc. Portland, Oregon, U.S.A.). Coefficients of all in-dependent variables were calculated by the multiple regression equation. The variables with very small or negative coefficients were dropped because they were shown to give very weak joint contribution to the dependent variable y. The independent variables with weaker contribution to dependent variable were further deleted from small to large value by backward regression analysis. After above procedures were repeated, the independent variables with weaker contribution to the dependent variable were removed in a step-by-step fashion. In the last step, the remaining independent variables ranked from small to large values.
d. Percentage of contribution. In order to compare the contribution of each independent variable to dependent variable in a concise way, we developed a formula to calculate a percentage contribution of individual independent variable, which was deduced from the equation of multiple regression: y = b0 + b1X1 + b2X2 + …+ bkXk When value of the constant factor b0 is assumed as 0, namely, b0 = 0, the multiple regression equation becomes y = b1X1 + b2X2 + … + bkXk, and percentage contribution of individual independent variable to dependent variable y can be calculated by the following formula: (Mean value of individual variable × its coefficient)/(Sum of such products for all independent variables) × 100% The larger the percentage value, the more important the corresponding variables in joint contribu-tion .
RESULTS
All patients underwent GI endoscopy, and the endoscopicl results in two groups of patients are listed in Table 2. There was no statistical difference between the two groups of patients as to the endoscopicl findings.
Table 2.
Endoscopicl findings in two groups of patients
|
Esophagitis |
HH (%) | Ulcer (%) | ||
| I-II (%) | III-IV (%) | |||
| E + DU (n = 31) | 74.6 | 25.4 | 49.0 | 100 |
| E (n = 72) | 79.1 | 20.9 | 55.6 | 0 |
| P value | > 0.05 | > 0.05 | > 0.05 | |
E + DU = patient with esophagitis and duodenal ulcer E = patient with esophagitis only
Figure 1 gives the parameters of gastric acid secretion in both groups of patients. There was no significant difference between the two groups of patients in the values of serum gastrin (P > 0.05). The parameters of 24 h intraesophageal pH monitoring in patients with E + DU and those with esophagitis are shown in Table 3. There was no statistical difference between the two groups as to the parameters of GER (P > 0.05).
Figure 1.
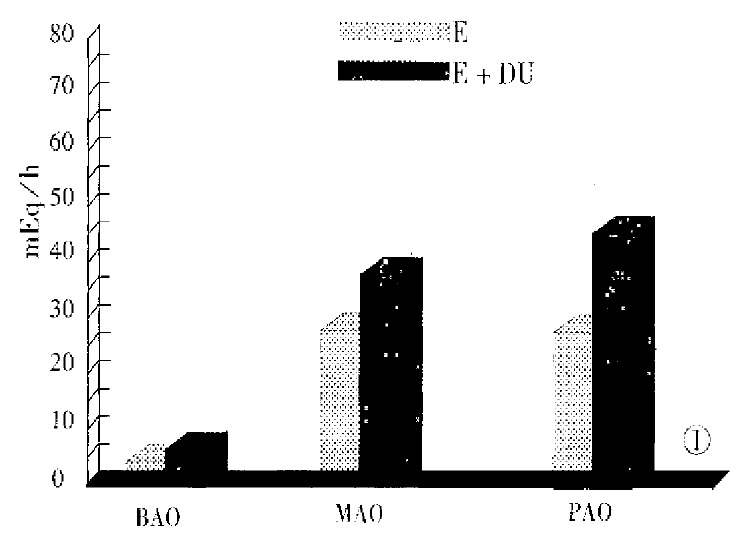
Gastric acid output in two groups of patients.
Table 3.
Parameters of 24h intraesophageal pH-monitoring
| GER-up* (% time) | GER-sup (% time) | Episode > 5 min (No.) | |
| E + DU | 15.94 ± 5.91 | 13.98 ± 14.20 | 7.85 ± 3.67 |
| E | 15.96 ± 14.58 | 14.33 ± 21.20 | 8.28 ± 7.10 |
| P value | > 0.05 | > 0.05 | > 0.05 |
*GER-up = GER in upright position GER-sup = GER in supine position
Figure 2 shows the results of multiple regression of dependent variable y (esophagitis) on 10 independent variables. In patients with E + DU, four rounds of regression with stepwise deletion analysis were performed. The first round of regression ended with deletion of independent variables X3 (smoking cigarette), X6 (GER in supine position), X7 (BAO) and X9 (PAO) because they showed very weak joint contribution or negative values. The remaining variables underwent a second round of regression with stepwise deletion, and 2 variables, i.e. X4 (alcohol consumption) and X10 (gastrin) were deleted. In the third round of regression, X8 (MAO) was deleted. The fourth round of regression on the remaining variables was completed, showing the coefficients of variables in order of importance: X2 (HH), 0.5696282; X5 (GER in upright position), 1.027288E-02; and X1 (age), 8.406402E-04. Similarly, in patients with E, the in-dependent variables deleted in the first two rounds of regression were: smoking cigarette, alcohol consumption and gastrin; and age, GER in supine position, BAO and PAO. The third round ended with the following results: X5 (GER in upright position), 1.156702E-02; X8 (MAO), 9.72133E-04; and X2 (HH), 4.629944E-02 (Table 4).
Figure 2.
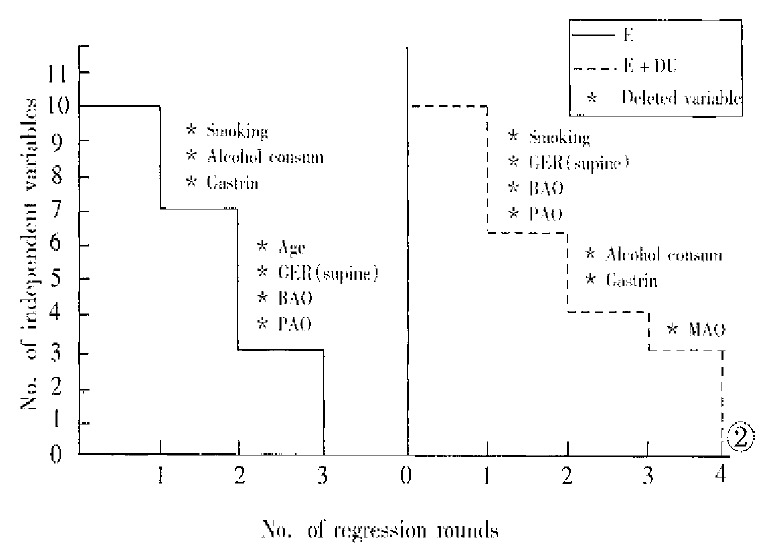
Flow chart of multiple regression analysis with stepwise deletion.
Table 4.
Contribution of independent variable to dependent variable
| Variables |
Coefficients (b) |
X value (mean) | Contribution (%) | |
| 1st RR | Last RR | |||
| E + DU | ||||
| X2 (HH) | 0.3943586 | 0.5696282 | 0.36363 | 52.39 |
| X5 (GER-up) | 4.044132E-02 | 1.027288E-02 | 14.65455 | 38.08 |
| X1 (age) | 2-869943E-02 | 8.406402E-04 | 44.81818 | 9.53 |
| E | ||||
| X5 (GER-up) | 2.735608E-02 | 1.156702E-02 | 19.21875 | 80.56 |
| X8 (MAO) | 3.405805E-02 | 9.72133E-04 | 28.34812 | 9.99 |
| X2 (HH) | 0.1626756 | 4.629944E-02 | 0.562600 | 9.45 |
RR = round of regression GER up = GER in upright position
In patients with E + DU, the percentage contributions of independent variables X2, X5 and X1 to y were 52.39%, 38.08% and 9.53%, respectively. Similarly, the percentage contributions of variables X5, X8 and X2 in patients with E to y were 80.56%, 9.99% and 9.54%, respectively.
DISCUSSION
Gastric acid secretion is considered to be an important pathogenetic factor in the development of GER disease. In several studies, a basal acid output higher than normal has been found in patients with reflux esophagitis[17-19]. Collen et al[20] have demonstrated that GER patients who did not respond to standard ulce rhealing doses of H2-blocker showed gastric acid hypersecretion. These results stress the point that gastric acid hypersecretion is a crucial factor for GER disease and for the resistance of GER patients to H2-blockers. However, our results showed that although the gastric acid output in patients with E + DU was significantly higher than in patients with E, there was no significant difference between two groups of patients as to severity of esophageal lesions and patterns of GER. These results suggest that no parallel relationship between GAO and severity of GER or esophageal lesions exists in patients with E + DU.
One approach to investigate the reason why the increased gastric acid output is not accompanied by aggravation of both GER patterns and severity of esophageal lesions is to assess quantitatively the role of the various potential pathogenetic factor involved, which may not only influence esophagitis but also interact. Therefore, multiple regression analysis with stepwise deletion is needed. According to this method, the value of any regression coefficient depends on all the other variables included in the regression. With stepwise deletion, the standard partial regression coefficient can be used as a measure of relative importance, the X being ranked in order of the size of their coefficients.
In our study, ten independent variables in each patient group were evaluated by multiple regression analysis with stepwise deletion. In patients with E + DU, smoking, GER in supine position, BAO, PAO, alcohol consumption, gastrin, and MAO were deleted in a stepwise fashion because they failed to significantly affect esophagitis. Similarly, in patients with esophagitis without DU, seven variables such as smoking, alcohol consumption, gastrin, etc. were deleted by two rounds of regression. These results indicate that GER in upright position and HH are important determinants of esophagitis. Our results also demonstrate, on the contrary, that the gastric acid output is not an important pathogenetic factor responsible for GER disease.
The relationship between the development of esophagitis and the pattern of GER is also a debatable issue. Some authors have found that the development of esophagitis is related to an increased GER in supine position[21-24]. Others have argued that GER in upright position is the most important pathogenetic factor[25-28]. In our study, the result of multiple regression indicates that the GER in supine position appears to be a weak factor affecting esophagitis, whereas GER in upright position plays an important role in the pathogenesis of esophagitis in both groups of patients.
In conclusion, an increased gastric acid output in patients with E + DU does not aggravate both the pattern of GER and esophageal lesions because the gastric acid output fail to appear as a significant pathogenetic factor responsible for GER disease, whereas GER in upright position and presence of HH are significantly related to GER disease in both groups of patients.
References
- 1.Zaninotto G, DeMeester TR, Schwizer W, Johansson KE, Cheng SC. The lower esophageal sphincter in health and disease. Am J Surg. 1988;155:104–111. doi: 10.1016/s0002-9610(88)80266-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahtaridis G, Snape WJ, Cohen S. Lower esophageal sphincter pressure as an index of gastroesophageal acid reflux. Dig Dis Sci. 1981;26:993–998. doi: 10.1007/BF01314761. [DOI] [PubMed] [Google Scholar]
- 3.Little AG, DeMeester TR, Kirchner PT, O'Sullivan GC, Skinner DB. Pathogenesis of esophagitis in patients with gastroesophageal reflux. Surgery. 1980;88:101–107. [PubMed] [Google Scholar]
- 4.Berstad A, Weberg R, Frøyshov Larsen I, Hoel B, Hauer-Jensen M. Relationship of hiatus hernia to reflux oesophagitis. A prospective study of coincidence, using endoscopy. Scand J Gastroenterol. 1986;21:55–58. doi: 10.3109/00365528609034622. [DOI] [PubMed] [Google Scholar]
- 5.Kaul B, Petersen H, Myrvold HE, Grette K, Røysland P, Halvorsen T. Hiatus hernia in gastroesophageal reflux disease. Scand J Gastroenterol. 1986;21:31–34. doi: 10.3109/00365528609034617. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LF, Demeester TR, Haggitt RC. Esophageal epithelial response to gastroesophageal reflux. A quantitative study. Am J Dig Dis. 1978;23:498–509. doi: 10.1007/BF01072693. [DOI] [PubMed] [Google Scholar]
- 7.Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology. 1987;92:130–135. doi: 10.1016/0016-5085(87)90849-3. [DOI] [PubMed] [Google Scholar]
- 8.Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991;100:596–605. doi: 10.1016/0016-5085(91)80003-r. [DOI] [PubMed] [Google Scholar]
- 9.Howard PJ, Maher L, Prvde A. Symptomatic gastroesophageal reflux, abnormal oesophageal acid exposure, and mucosal acid sensitivity are three separate, though related, aspects of gastro-oesophageal reflux disease. Gut. 1991;32(1):128–132. doi: 10.1136/gut.32.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssens J, Vantrappen G, Ghillebert G. 24-hour recording of esophageal pressure and pH in patients with noncardiac chest pain. Gastroenterology. 1986;90:1978–1984. doi: 10.1016/0016-5085(86)90270-2. [DOI] [PubMed] [Google Scholar]
- 11.Savary M, Miller G. The esophagus. Handbook and atlas of endoscopy. First ed. Switzerland: Gassmann 1987 [Google Scholar]
- 12.Bianchi Porro G, Pace F. Comparison of three methods of intraesophageal pH recordings in the diagnosis of gastroesophageal reflux. Scand J Gastroenterol. 1988;23:743–750. doi: 10.3109/00365528809093943. [DOI] [PubMed] [Google Scholar]
- 13.Pace F, Sangaletti O, Bianchi Porro G. Daytime reduction of gastro-oesophageal reflux after healing of oesophagitis and its value as an indicator of favourable response to maintenance treatment. Gut. 1990;31:1025–1029. doi: 10.1136/gut.31.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser HF, Siewert JR. Investigations with the 24-hour solid-state pH metry: correlation between gastroesophageal reflux extent and reflux sequelae. Surg Gastroenterol. 1982;1(3):327–334. [Google Scholar]
- 15.Raufman JP, Collins SM, Pandol SJ, Korman LY, Collen MJ, Cornelius MJ, Feld MK, McCarthy DM, Gardner JD, Jensen RT. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 1983;84:108–113. [PubMed] [Google Scholar]
- 16.Snedecor GW, Cochran WG. Statistical methods. Iowa: The Iowa State University Press; 1979. [Google Scholar]
- 17.Collen MJ, Ciarleglio CA, Stanczak VJ. Basal acid output in patients with gastroesophageal reflux disease. Gastroenterology. 1987;92(11):1350(Abstract). [Google Scholar]
- 18.Mulholland MW, Reid BJ, Levine DS, Rubin CE. Elevated gastric acid secretion in patients with Barrett's metaplastic epithelium. Dig Dis Sci. 1989;34:1329–1334. doi: 10.1007/BF01538064. [DOI] [PubMed] [Google Scholar]
- 19.Barlow AP, DeMeester TR, Ball CS, Eypasch EP. The significance of the gastric secretory state in gastroesophageal reflux disease. Arch Surg. 1989;124:937–940. doi: 10.1001/archsurg.1989.01410080069011. [DOI] [PubMed] [Google Scholar]
- 20.Collen MJ, Lewis JH, Benjamin SB. Gastric acid hypersecretion in refractory gastroesophageal reflux disease. Gastroenterology. 1990;98:654–661. doi: 10.1016/0016-5085(90)90285-9. [DOI] [PubMed] [Google Scholar]
- 21.Demeester TR, Johnson LF, Joseph GJ, Toscano MS, Hall AW, Skinner DB. Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976;184:459–470. doi: 10.1097/00000658-197610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson D, Aldersley M, Shepherd H, Smith CL. Patterns of acid reflux in complicated oesophagitis. Gut. 1987;28:1484–1488. doi: 10.1136/gut.28.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichter I. Measurement of gastro-oesophageal acid reflux: its significance in hiatus hernia. Br J Surg. 1974;61:253–258. doi: 10.1002/bjs.1800610402. [DOI] [PubMed] [Google Scholar]
- 24.Pujol A, Grande L, Ros E, Pera C. Utility of inpatient 24-hour intraesophageal pH monitoring in diagnosis of gastroesophageal reflux. Dig Dis Sci. 1988;33:1134–1140. doi: 10.1007/BF01535790. [DOI] [PubMed] [Google Scholar]
- 25.de Caestecker JS, Blackwell JN, Pryde A, Heading RC. Daytime gastro-oesophageal reflux is important in oesophagitis. Gut. 1987;28:519–526. doi: 10.1136/gut.28.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branicki FJ, Evans DF, Jones JA, Ogilvie AL, Atkinson M, Hardcastle JD. A frequency-duration index (FDI) for the evaluation of ambulatory recordings of gastro-oesophageal reflux. Br J Surg. 1984;71:425–430. doi: 10.1002/bjs.1800710607. [DOI] [PubMed] [Google Scholar]
- 27.Rokkas T, Anggiansah A, Uzoechina E, Owen WJ, Sladen GE. The role of shorter than 24-h pH monitoring periods in the diagnosis of gastro-oesophageal reflux. Scand J Gastroenterol. 1986;21:614–620. doi: 10.3109/00365528609003108. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell JN, Heading RC. When does gastro-oesophageal reflux occur in patients with peptic oesophagitis (Abstract)? Gut. 1980;21(5):A922. [Google Scholar]


