Abstract
AIM: To study the abnormal expression of β-catenin gene and its relationship with invasiveness of primary hepatocellular carcinoma among Chinese people.
METHODS: Thirty-four hepatocellular carcinoma (HCC) specimens and adjacent paracancerous tissues, 4 normal liver tissues were immunohistochemically stained to study subcellular distribution of β-catenin. Semiquantitive analysis of expression of β-catenin gene exon 3 mRNA was examined by RT-PCR and in situ hybridization. The relationship between expressions of both β-catenin protein, mRNA and clinicopathological characteristics of HCC was also analyzed.
RESULTS: Immunohistochemistry showed that all normal liver tissues and para-cancerous tissues examined displayed membranous type staining for β-catenin protein, occasionally with weak expression in the cytoplasm. While 21 cases (61.8%) of HCC examined showed accumulated type in cytoplasms or nuclei. The accumuled type Labling Index (LI) of cancer tissue and para-cancarous tissue was (59.9 ± 26.3) and (18.3 ± 9.7) respectively (P < 0.01). Higher accumulated type LI was closely related with invasiveness of HCC. Results of RT-PCR showed the β-catenin gene exon 3 mRNA Expression Index (EI) of 34 HCCs was higher than that of para-cancerous tissue and normal liver tissue. Using in situ hybridization, the signal corresponding to β-catenin gene exon 3 mRNA was particularly strong in cytoplasm of HCC when compared with those of para-cancerous and normal liver tissues. Over expression of β-catenin exon 3 was also found to be correlated with high metastatic potential of HCC.
CONCLUSION: Abnormal expression of β-catenin gene may contribute importantly to the invasiveness of HCC among Chinese people.
Keywords: hepatocellular carcinoma, wnt pathway, β-catenin gene, metastasis
INTRODUCTION
Hepatocellular carcinoma (HCC) is quite common in China. In recent years, great progresses have been made in the treatment of HCC, but the major problem is the high malignancy of HCC, that is, more than 50% of the patients receiving grossly radical treatment will suffer from recurrence within two years. So much effort has been put to investigate the molecular biological characteristics of HCC in order to lower the recurrence rate[1-20]. β-catenin is a ubiquitous intracellular protein which is important in both intercellular adhesion and Wingless/Wnt developmental signaling transduction pathway[21]. β-catenin plays an important role in the interactions between cadherins and other transmembrane receptor proteins, such as the epidermal growth factor receptor. In addition, it is also a signaling molecule and can activate gene transcription by forming a heterodimer with the T-cell factor/lymphoid enhancer-binding factor family of DNA binging proteins[22]. Previous studies have shown that β-catenin is involved in pathways that regulate cellular differentiation and proliferation. In the absence of growth or differentiation signals, cytoplasm β-catenin is rapidly turned over under the control of the APC protein and the GSK-3β , resulting in low level of cytoplasm β-catenin level in normal cells[23,24]. The presence of a wingless-Wnt signal in normal embryonic cells stabilizes β-catenin, which accumulates in the cytoplasm, where it binds to Tcf-lymphoid enhancer factor and triggers gene transcription. Abnormal expression and/or structural abnormalities of catenins are closely associated with tumor development for human esophageal, gastric and colon cancers[25,26]. Previous study has shown that E-cadherin expression was significantly lowed and is closely related with the metastatic potential of HCC[27], and abnormal β-catenin expression has been observed by immunohistochemistry in many malignant human tumors including HCC[28], so it is our logical thoughts whether abnormality of β-catenin gene existed and what its relationship with malignancy in HCC among Chinese people is because of the close relationship between E-cadherin and β-catenin.
MATERIALS AND METHODS
Tissue
Thirty-four HCC specimens and adjacent para-cancerous tissues, four normal liver tissues obtained from patients who underwent surgery in Liver Cancer Institute, Zhongshan Hospital, Shanghai Medical University were analyzed. The tissues were each cut into three parts: one was fixed in formalin, and then embedded in paraffin. Paraffin sections were stained with HE for histological examination of HCC and were also used for immunohistochemistry. One was immediately frozen by liquid nitrogen and stored at -80 °C, which was to be used for DNA and RNA extraction. Genomic DNA was purified from all samples using standard proteinase K digestion and phenol/chloroform extraction. Total RNA was extracted using a Trizol reagent (Promega) according to the protocol recommended by the manufacturer. And the last was rinsed in cold PBS, placed in OCT compound, and immediately frozen in liquid nitrogen, which was to be used for in situ hybridization.
Immunohistochemical staining
Immunohistochemical analysis was carried out with the avidin-biotin complex immunoperoxidase technique as described previously[29]. As the primary antibody, polyclonal human anti-β-catenin antibody (Sigma) was used at 500 × dilution. As the secondary antibody, biotinylated anti-rabbit IgG (Dako) was used at 100 × dilution. Staining was performed using avidin-biotin reagents, 3, 3’-diaminobenzidine, and hydrogen peroxide. As a negative control, duplicate sections were immunostained without exposure to the primary antibodies. All cases were divided into two groups according to immunostaining pattern. Cases with a membrancous staining pattern similar to that in normal hepatic cell were classified as membraneous or normal and cases with marked cytoplasmic and nuclear staining in addition to the membranous staining were defined as accumulated or abnormal. Cells from five randomized views were counted and the cell labeling index (LI) was arbitrarily defined as: (positive cells counted/all cells counted) × 100.
RT-PCR
Primers for PCR were designed to amplify the consensus sequence for GSK-3 β phosphorylation in exon 3 of β-catenin gene, based on the published cDNA sequence of human β-catenin gene. To verify the validity of amplification, the primers were designed within the region of exon 3 of β-catenin gene, and the amplification was performed by direct PCR and RT-PCR respectively. Primers, F: AAAGCGGCTGTTA-GTCACTGG R: GACTTGGGAGGTATCCACATCC. PCR: PCR mixture, containing 100 pM of primer A and B each, deoxyribonucleotide triphosphates at 200 μmol·L-1 each, 1.5 mmol·L-1 MgCl2, 2 U Taq polymerase (Promega) and 2 μL DNA template was adjusted to 50 μL by adding double distilled water. Then the mixture was overlaid with 50 μL mineral oil and subjected to amplification for 40 cycles. Each cycle consisted of 95 °C for 60 s, 55 °C for 45 s, 72 °C for 45 s. RT-PCR: Total RNAs were reverse-transcribed to obtain the cDNA that was going to be amplified. PCR was also performed under the above same condition except for adding 1 μL cDNA to the PCR mixture. A 450 bp fragment of β-actin mRNA was also amplified by RT-PCR as the internal control. The PCR products were identified first onto 20 g·L-1 agrose gel and photographed. The photos of RT-PCR were scanned by optical density scanner (Shimadzu C-9000) and the gene expression index (EI) was arbitrarily defined as density Lum of β-catenin/density Lum of β-actin.
In situ hybridization
Cryostat sections (6 μm) were obtained, dried for 2 h at RT, and delipidated in chloroform for 5 min. Sections were fixed in 40 g·L-1 paraformaldehyde/PBS for 7 min, rinsed in PBS for 3 min, rinsed twice in 2 × SSC for 5 min, and prehybridized at 42 °C for 60 min in 4 × SSC/100 g·L-1 dextran sulfate/1 × Denhardt’s solution/2 mM EDTA/500 g·L-1 deionized formamide/500 mg·L-1 salmon sperm DNA. Hybridization was for 16 h in 100 μL of prehybridization solution and 20 g·L-1 digoxin labeled oligonucleotides (TGTTCC-CACTCATACAGGACTTGGGAGGTATCCACATCCTCTTCCTCAGGA). After hybridization, sections were rinsed twice in 2 × SSC for 5 min at 37 °C, 3 times for 5 min each in 60 g·L-1 formamide and 0.2 × SSC at 37 °C and twice for 5 min each in 2 × SSC at RT. Sections were then rinsed in 100 mol·L-1 Tris·HCl, pH 7.5/150 mol·L-1 NaCl for 5 min, and treated with the same solution saturated with blocking mix for 30 min, and then reacted with a 1:2000 dilution of alkaline phosphatase-conjugated sheep antidigoxigenin Fab fragments (750 × 103·L-1) in the same solution. They were rinsed twice in 100 mol·L-1 Tris HCl, pH7.5 and 150 mol·L-1 NaCl for 5 min each, then in 100 mol·L-1 Tris·HCl, pH9.5/100 mol·L-1 NaCl/50 mol·L-1 MgCl2 for 10 min, and then reacted with 0.18 g·L-1 5-bromo-4-chloro-3-indolyl phosphate, 0.34 g·L-1 nitroblue tetrazolium, and 240 mg·L-1 levamisole (Sigma) in the same solution for 6 h in the dark at RT. The reaction was stopped with 10 mol·L-1 Tris·HCl (pH8.0) and 1 mol·L-1 EDTA. Sections were counterstained in nuclear methyl green, mounted with aqueous solution, and the final results of average density area and density l μm of 500 signal positive cells were analyzed by a multifunctional true digital system (MTDS) using a computer. Albumin oligonucleotide probe and hybridization solution without probe were used as positive and negative control respectively.
RESULT
Immunohistochemical analysis
Immunostaining with polyclonal antibody was performed to evaluate the significance of β-catenin accumulation in HCC. Immunohistochemistry showed that all normal liver tissues and para-cancerous tissues examined showed membranous type, occasionally with weak expression of β-catenin in the cytoplasm, but no β-catenin accumulation in nuclei was found. While for HCC, 21 cases (61.8%) showed accumulated type (Figure 1). The LI of accumulated type for tumor tissue and paracancerous tissue were 59.9 ± 26.3 and 18.3 ± 9.7 respectively (P < 0.01), while the LI of membraneous type for tumor tissue and paracancerous tissue were 24.6 ± 8.5 and 91.8 ± 10.6 respectively (P < 0.01, Table 1). When LI of accumulated type was analyzed according to the clinicopathological characteristics of HCC, close relationship could be seen with capsule, portal vein tumor thrombus, pathological grade, intrahepatic metastasis (Table 2) and postoperative recurrence (Figure 2).
Figure 1.

Immunohistochemistry of β-catenin. A: In normal liver tissue, the staining was mainly positive on the cellular membrane (arrowpoint), with very weak cytoplasmic staining. × 200 B: Para-cancerous cirrhotic liver tissue showed membrane staining (arrowpoint) like normal liver tissue. C,D: For HCC, cytoplasmic and nuclear staining was dominant (arrowpoints), whereas membrane staining was rare. × 200
Table 1.
Labeling index for β-catenin accumulated type and membraneous type in HCC and para-cancerous tissues (n = 34, x- ± s)
| Tissue | Membraneous | Accumulated |
| HCC | 59.9 ± 26.3 | 24.6 ± 8.5 |
| Para-cancerous tissue | 18.3 ± 9.7b | 91.8 ± 10.6b |
P < 0.01 vs HCC.
Table 2.
Relationship between labeling index of β-catenin accumu-lated type, expression index of β-catenin mRNA and clinicopatho-logical characteristics of HCC
| n | LI of β-catenin accumulated type | EI of β-catenin mRNA | |
| Male | 31 | 58.4 ± 14.2 | 0.8 ± 0.2 |
| Female | 3 | 54.1 ± 15.3 | 0.9 ± 0.1 |
| AFP ≤ 20 ng/mL | 9 | 49.3 ± 17.2 | 0.8 ± 0.1 |
| AFP > 20 ng/mL | 25 | 54.3 ± 13.7 | 0.8 ± 0.1 |
| Tumor size | |||
| ≤ 5 cm | 15 | 58.7 ± 20.4 | 0.8 ± 0.2 |
| 5 cm~10 cm | 7 | 54.4 ± 21.3 | 0.8 ± 0.2 |
| > 10 cm | 12 | 55.9 ± 17.9 | 0.8 ± 0.1 |
| Capsule | |||
| Complete | 15 | 72.2 ± 23.4 | 0.7 ± 0.1 |
| Incomplete | 19 | 44.4 ± 21.1b | 0.9 ± 0.1a |
| Intrahepatic Metastasis Yes | 14 | 77.2 ± 25.5 | 0.9 ± 0.2 |
| Intrahepatic Metastasis No | 20 | 41.3 ± 19.6b | 0.7 ± 0.1a |
| Portal vein thrombus Yes | 19 | 79.8 ± 14.9 | 0.9 ± 0.2 |
| Portal vein thrombus No | 15 | 52.8 ± 25.9a | 0.6 ± 0.2a |
| Edmondson’s Grade II | 19 | 39.7 ± 20.0 | 0.7 ± 0.4 |
| Edmondson’s Grade III | 15 | 75.9 ± 18.7b | 0.8 ± 0.2 |
| Cirrhotic nodule ≤ 0.5 cm | 23 | 54.3 ± 12.5 | 0.8 ± 0.2 |
| Cirrhotic nodule >0.5 cm | 11 | 62.2 ± 16.6 | 0.8 ± 0.1 |
P < 0.05,
P < 0.01.
Figure 2.

Labeling index (LI) of β-catenin. Recurrent patient (n = 15) was much higher than that of non-recurrent patient (n = 19) (84.9 ± 17.4) vs (39.1 ± 14.3).
β-catenin exon 3 mRNA expression
Since the primers were designed in such a way that the product was within β-catenin gene exon 3, direct PCR and RT-PCR were used separately to verify the amplification. Agrose gel electrophoresis showed that PCR and RT-PCR amplification products were both 132 bp, which were the same as those of normal liver tissues, para-cancerous tissues and HCC tissues. None of amplification products showed fragment that was shorter. RT-PCR results showed the β-catenin exon 3 mRNA EI were (0.77 ± 0.16) and (0.50 ± 0.05) for HCC tissues and para-cancerous tissues respectively (P < 0.05, Figure 3). In HCC, higher EI of β-catenin mRNA attempted to be seen in cancer with incomplete capsule, intrahepatic metastasis and portal vein thrombus (Table 2). Using in situ hybridization, we also found the signal corresponding to β-catenin exon 3 mRNA was particularly strong in cytoplasm of HCC when compared with those of para-cancerous tissues and normal liver tissues (Figure 4) and stronger signal of β-catenin mRNA was also closely related to incomplete capsule, intrahepatic metastasis and portal vein thrombus.
Figure 3.
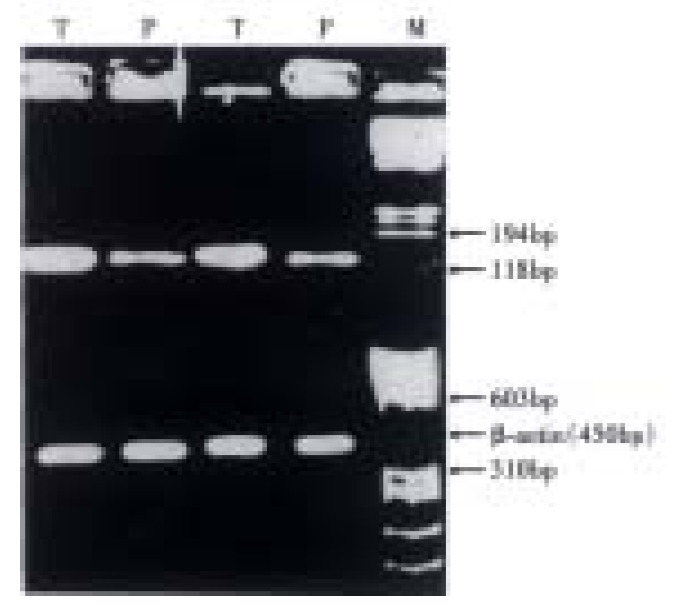
β-catenin mRNA expression index (EI). HCC was higher vs para-cancerous tissue (P < 0.05). P: para-cancerous tissue; T: HCC; M: nucleic acid molecular mass marker.
Figure 4.
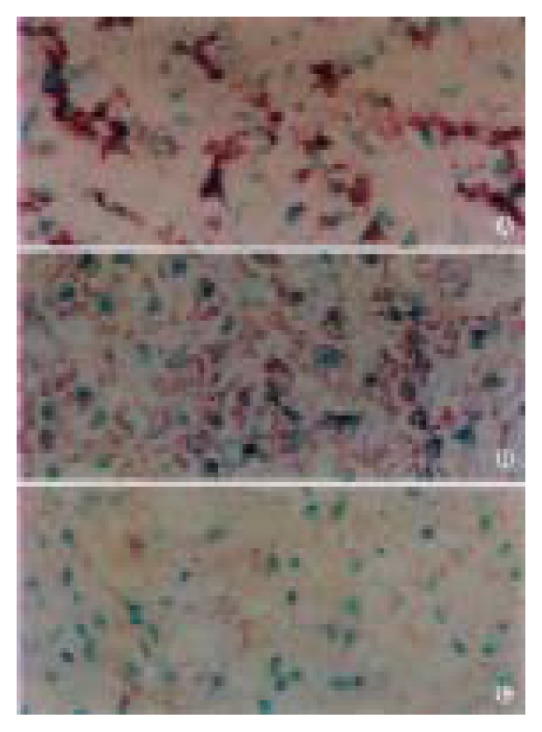
In situ hybridization of β-catenin gene mRNA. Stronger in HCC (A) vs para-cancerous cirrhotic liver tissue and (B) normal liver tissue (C).
DISCUSSION
Previous studies have shown that activation of the wnt pathway results in up-regulation of cytoplasmic β-catenin and its translocation to the nucleus, presumably via the binding of β-catenin to T-cell factor/lymphoid-enhancing factor family members[25,26,30]. Thus, as a first assessment, we examined the subcellular localization of β-catenin in 34 HCC specimens with the result that 61.8% of HCC specimens showed to be accumulated type, suggesting cytoplasmic stabilization of the protein. This showed that activation of Wnt pathway maybe of importance in the carcinogenesis of HCC among Chinese people. Although either β-catenin mutations involving the GSK-3 β phophorylation sites or inactivation of APC and some other factors are related to activation of the Wnt pathway in colon cancer and melanomas[31,32], loss of heterozygosity at the APC locus on chromosome 5 has been detected only at low frequency in human HCC, indicating that inactivation of APC may be infrequent[33]. So mutation of exon 3 of β-catenin gene is probably one of the most important factors activating Wnt pathway and thus causing β-catenin protein accumulated in the cytoplasms in HCC.
Although some studies have been made to investigate β-catenin mutation and abnormal Wnt pathway in HCC[34-41], no previous results have been reported concerning about the relationship between expression abnormality of β-catenin and clinocopathological features of HCC. Furthermore, research reports about the relationship between β-catenin abnormal expression and clinicopathological features of tumors such as colon cancer[42,43], melanoma[44,45], breast carcinoma[46,47], gastric carcinoma[48,49], and lung carcinoma[50,51] are rather various and some of the results were even totally contradictory. That is partly due to most of the previous immunohistochemical studies on β-catenin did not differentiate between membrane-associated type and intracellular accumulated type. Most tumors showed reduced β-catenin in the cytoskeletal fraction but increased β-catenin in the cytosolic fraction and truncated β-catenin protein which was encoded by mutational β-catenin gene was found bound weakly to β-catenin monoclonal antibody when compared with non-truncated β-catenin[52]. This is the reason why we chose polyclonal antibody instead of monoclonal antibody in our study. In this study we aimed to determine which type of expression abnormalities for β-catenin correlate with clinicopathological features and postoperative recurrence in HCC. Our results demonstrated that although great difference existed between cancer tissue and non-cancer tissue, we failed to show the LI of membraneous type to be correlated with the invasiveness of HCC (data not shown here). But, the LI of accumulated type was discovered closely related with the invasive characteristics of HCC, higher EI would predict high ability of invasiveness of HCC and thus a worse prognosis. This was different from another article about gastric carcinoma, which showed that membraneous type, instead of accumulated type, was related to the invasiveness and prognosis of the tumor[47].
Since abnormal expression of β-catenin protein can be caused by both β-catenin gene mutation and over expression, and in some HCCs, both strong membraneous type and accumulated type of staining could be observed, it is our logical thoughts to figure out whether over expression of β-catenin gene existed and what its relationship with the invasiveness of HCC was. This article is the first one to study the β-catenin gene expression in HCC at mRNA level. First we used RT-PCR to examine the expression of β-catenin gene exon 3 mRNA. Since RT-PCR was not very accurate in semi-quantitive analysis of gene expression, we chose in situ hybridization to reconfirm the results of RT-PCR. The results of them are the same, that is over expression did exist in HCC and it showed relationship with the invasiveness of HCC (data of relationship between in situ hybridization and HCC clinicopathological characteristics not shown). This could give some explanation why strong membranous and cytoplasmic distribution of β-catenin was observed on immunohistochemistry in some HCC while β-catenin gene exon 3 mutation was not observed. It was the accumulation of β-catenin, though apparently normal, that exceeded the capacity of E-cadherin combination and GSK-3 β degradation, resulting in increase and stabilization of this protein in the cytoplasm.
Although we found that LI of β-catenin accumulated type was related with HCC recurrence, we were unable to find there was such relationship between β-catenin gene EI and HCC recurrence, either by RT-PCR or in situ hybridization. This implies that the LI of β-catenin accumulated type would be of greater value in predicting recurrence of HCC. From above we can see that abnormal expression of β-catenin protein, especially the accumulated type, which is closely related to the invasiveness of HCC among Chinese people. Further study should be carried out to confirm this and investigate what the other mechanism causing abnormal expression of β-catenin gene is.
ACKNOWLEDGEMENTS
The authors are grateful to assistant professor Teng-Fang Zhu, Department of Pathology, Medical Center of Fudan University, for his technical support on in situ hybridization.
Footnotes
Supported by National Ninth Five-year Plan of Medical Sciences of China (96-906-0105).
Edited by Pan BR
References
- 1.Xu J, Mei MH, Zeng SE, Shi QF, Liu YM, Qin LL. Expressions of ICAM-1 and its mRNA in sera and tissues of patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:120–125. doi: 10.3748/wjg.v7.i1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, Taylor JA. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol Carcinog. 2001;31:68–73. doi: 10.1002/mc.1041. [DOI] [PubMed] [Google Scholar]
- 3.Sun BH, Zhang J, Wang BJ, Zhao XP, Wang YK, Yu ZQ, Yang DL, Hao LJ. Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p21(WAF1) expression and hepatic apoptosis. World J Gastroenterol. 2000;6:356–360. doi: 10.3748/wjg.v6.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol. 1999;5:111–115. doi: 10.3748/wjg.v5.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo YQ, Wu MC, Cong WM. Gene expression of hepatocyte growth factor and its receptor in HCC and nontumorous liver tissues. World J Gastroenterol. 1999;5:119–121. doi: 10.3748/wjg.v5.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao CZ, Dai YM, Yu HY, Wang JJ, Ni CR. Relationship between expression of CD44v6 and nm23-H1 and tumor invasion and metastasis in hepatocellular carcinoma. World J Gastroenterol. 1998;4:412–414. doi: 10.3748/wjg.v4.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Wu ZB, Ruan YB. Expression of nm23 gene in hepatocellular carcinoma tissue and its relation with metastasis. World J Gastroenterol. 1998;4:266–267. doi: 10.3748/wjg.v4.i3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JJ, Zhou XD, Liu YK, Zhou G. Phase tissue intercellular adhesion molecule-1 expression in nude mice human liver cancer metastasis model. World J Gastroenterol. 1998;4:314–316. doi: 10.3748/wjg.v4.i4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Zhang B, Xu Y, Wang W, Shen Y, Zhang A, Xu Z. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol. 1997;123:357–360. doi: 10.1007/BF01438313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang ZY, Yu YQ, Zhou XD, Ma ZC, Yang R, Lu JZ, Lin ZY, Yang BH. Surgery of small hepatocellular carcinoma. Analysis of 144 cases. Cancer. 1989;64:536–541. doi: 10.1002/1097-0142(19890715)64:2<536::aid-cncr2820640230>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XD, Tang ZY, Yu YQ, Ma ZC, Yang BH, Lu JZ. Prognostic factors of primary liver cancer: report of 83 patients surviving 5 years or more compared with 811 patients surviving less than 5 years. J Exp Clin Cancer Res. 1991;10:81–86. [Google Scholar]
- 14.Zhou XD, Tang ZY, Yu YQ, Yang BH, Lu JZ, Lin ZY, Ma ZC, Zhang BH. Recurrence after resection of alpha-fetoprotein-positive hepatocellular carcinoma. J Cancer Res Clin Oncol. 1994;120:369–373. doi: 10.1007/BF01247463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356–1361. doi: 10.1046/j.1440-1746.2000.0150121356.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang ZY. Hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:G1–G7. doi: 10.1046/j.1440-1746.2000.02257.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu LH, Xiao WH, Liu WW. Effect of 5-Aza-2'-deoxycytidine on the P16 tumor suppressor gene in hepatocellular carcinoma cell line HepG2. World J Gastroenterol. 2001;7:131–135. doi: 10.3748/wjg.v7.i1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu MC, Yuan JM, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14:703–709. doi: 10.1155/2000/371801. [DOI] [PubMed] [Google Scholar]
- 19.Kakizoe T. Asian studies of cancer chemoprevention: latest clinical results. Eur J Cancer. 2000;36:1303–1309. doi: 10.1016/s0959-8049(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 20.Ding X, Mizokami M, Kang LY, Cao K, Orito E, Tanaka Y, Ueda R, Sasaki M. Prevalence of TT virus and GBV-C infections among patients with liver diseases and the general population in Shanghai, China. Virus Genes. 1999;19:51–58. doi: 10.1023/a:1008188623062. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 22.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 23.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 25.Pennisi E. How a growth control path takes a wrong turn to cancer. Science. 1998;281:1438–1439, 1441. doi: 10.1126/science.281.5382.1438. [DOI] [PubMed] [Google Scholar]
- 26.Peifer M. Beta-catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- 27.Huang GT, Lee HS, Chen CH, Sheu JC, Chiou LL, Chen DS. Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma. Hepatogastroenterology. 1999;46:1923–1927. [PubMed] [Google Scholar]
- 28.Endo K, Ueda T, Ueyama J, Ohta T, Terada T. Immunoreactive E-cadherin, β-catenin, β-catenin, and β-catenin proteins in hepatocellu-lar carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum Pathol. 2000;31:558–565. doi: 10.1053/hp.2000.6683. [DOI] [PubMed] [Google Scholar]
- 29.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 30.Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- 31.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 32.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 33.Piao Z, Kim H, Jeon BK, Lee WJ, Park C. Relationship between loss of heterozygosity of tumor suppressor genes and histologic differentiation in hepatocellular carcinoma. Cancer. 1997;80:865–872. [PubMed] [Google Scholar]
- 34.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 35.Jeng YM, Wu MZ, Mao TL, Chang MH, Hsu HC. Somatic mutations of beta-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett. 2000;152:45–51. doi: 10.1016/s0304-3835(99)00433-4. [DOI] [PubMed] [Google Scholar]
- 36.Renard CA, Fourel G, Bralet MP, Degott C, De La Coste A, Perret C, Tiollais P, Buendia MA. Hepatocellular carcinoma in WHV/N-myc2 transgenic mice: oncogenic mutations of beta-catenin and synergistic effect of p53 null alleles. Oncogene. 2000;19:2678–2686. doi: 10.1038/sj.onc.1203617. [DOI] [PubMed] [Google Scholar]
- 37.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aderca I, Taniguchi K, Dong XY, Qian CP, Nagorney DM, Burgart LJ, Smith DI, Roberts LR, Liu WG. AXIN1 and β-catenin gene mutations in human hepatocellular carcinoma. Gastroenterology. 2001;120:1849. [Google Scholar]
- 39.Fujie H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Kimura S, Koike K. Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res. 2001;20:39–51. doi: 10.1016/s1386-6346(00)00116-9. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Rashid A, Ruchelli E, Seeger C. β-catenin gene expression in hepatocellular carcinoma and hepatoblastoma. Modern Pathol. 2001;14:1172. [Google Scholar]
- 41.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghadimi BM, Behrens J, Hoffmann I, Haensch W, Birchmeier W, Schlag PM. Immunohistological analysis of E-cadherin, alpha-, beta- and gamma-catenin expression in colorectal cancer: implications for cell adhesion and signaling. Eur J Cancer. 1999;35:60–65. doi: 10.1016/s0959-8049(98)00344-x. [DOI] [PubMed] [Google Scholar]
- 43.Hiscox S, Jiang WG. Expression of E-cadherin, alpha, beta and gamma-catenin in human colorectal cancer. Anticancer Res. 1997;17:1349–1354. [PubMed] [Google Scholar]
- 44.Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XD, Hersey P. Expression of catenins and p120cas in melanocytic nevi and cutaneous melanoma: deficient alpha-catenin expression is associated with melanoma progression. Pathology. 1999;31:239–246. doi: 10.1080/003130299105052. [DOI] [PubMed] [Google Scholar]
- 46.Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur J Surg Oncol. 2001;27:31–36. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- 47.Bukholm IK, Nesland JM, Kåresen R, Jacobsen U, Børresen-Dale AL. E-cadherin and alpha-, beta-, and gamma-catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol. 1998;185:262–266. doi: 10.1002/(SICI)1096-9896(199807)185:3<262::AID-PATH97>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 48.Ramesh S, Nash J, McCulloch PG. Reduction in membranous expression of beta-catenin and increased cytoplasmic E-cadherin expression predict poor survival in gastric cancer. Br J Cancer. 1999;81:1392–1397. doi: 10.1038/sj.bjc.6693437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ougolkov A, Mai M, Takahashi Y, Omote K, Bilim V, Shimizu A, Minamoto T. Altered expression of beta-catenin and c-erbB-2 in early gastric cancer. J Exp Clin Cancer Res. 2000;19:349–355. [PubMed] [Google Scholar]
- 50.Toyoyama H, Nuruki K, Ogawa H, Yanagi M, Matsumoto H, Nishijima H, Shimotakahara T, Aikou T, Ozawa M. The reduced expression of e-cadherin, alpha-catenin and gamma-catenin but not beta-catenin in human lung cancer. Oncol Rep. 1999;6:81–85. doi: 10.3892/or.6.1.81. [DOI] [PubMed] [Google Scholar]
- 51.Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–4796. [PubMed] [Google Scholar]
- 52.Hugh TJ, Dillon SA, O'Dowd G, Getty B, Pignatelli M, Poston GJ, Kinsella AR. beta-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. 1999;82:504–511. doi: 10.1002/(sici)1097-0215(19990812)82:4<504::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]


