Abstract
AIM: To study the pathogenicity of hepatitis G virus (HGV) and observe the genesis and pathological process of hepatitis G.
METHODS: HGV-RNA in serum was detected by RT-PCR assay. The immunohistochemical assays of liver tissue were performed with HGV monocoloned antibody (McAb) expressed from the region of HGV NS5 nucleic acid sequence. The clinical and pathological data of 52 patients with hepatitis G were discussed. In animal experiment, the Chinese Rhesus monkeys were infected with the serum of a patient with HGV infection. And the dynamic changes in serology and liver histology of animals were observed.
RESULTS: One hundred and fifty-four patients with HGV-RNA positive were selected from 1552 patients with various kinds of hepatitis. Of 154 patients with HGV infection, 52 were infected with HGV only, which accounted for 33.8% (52/154) and 102 with positive HGV-RNA were super-infected with other hepatitis viruses, which accounted for 66.2% (102/154). The clinical and pathological observation showed that the acute and chronic hepatitis could be induced by HGV. The slight abnormality of transaminases ALT and AST in serum of monkeys lasted nearly 12 months and histological results showed a series of pathological changes.
CONCLUSION: HGV is a hepatotropic virus and has pathogenicty.
Keywords: hepatitis virus G, pathogenicity
INTRODUCTION
Hepatitis G virus (HGV/GBV) is a new type of hepatitis virus which was first identified by Simons and Linnen between 1995 and 1996[1,2]. It has been shown that HGV is a single-stranded, positive chain DNA virus which has world-wide distribution, and spread by blood circulation. HGV infection is mostly mixed with infections caused by other types of hepatitis viruses. The patients with single hepatitis G infection were rare. It makes the investigation difficult. Currently, the pathogenicity of hepatitis G virus is controversial. We report the results of our study below.
MATERIALS AND METHODS
Patients
One hundred and fifty-four patients with hepatitis G infection were collected from 1552 patients with different types of hepatitis admitted consecutively to our hospital during recent 4 years. The serum level of alanine trasaminase (ALT) in all investigated patients was twice that of the normal subjects. Fifty-two of these cases were simply HGV infected and 102 were super-infected with other types of hepatitis virus. The liver biopsies were performed in 42 of the 52 patients with single HGV infection.
Etiological assays for serum specimens
Serum specimens were tested for HGV-RNA by retro-polymerase chain reaction (RT-PCR), for HBV-M, anti-HAV IgM, anti-HEV IgG, anti-HCV IgG by enzyme-linked immunosorbent assay (ELISA), for HBV-DNA by PCR, and for HCV-RNA by RT-PCR. Reagents were obtained from Shanghai Meihua Company, DA-AN Genotype-Diagnostic Center of Zhongshan Medical University, Medical Institute of Nanjing Military Area, respectively. The primers of HGV were obtained from the Institute of Microbiology and Epidemiology of Chinese Military Academy of Medical Sciences.
Histological and immunohistochemical assays
The biopsied liver tissues from the patients were sent to 3 pathologists for histological examinations under light and electron microscopy to determine the degree of necrosis of the liver cells, inflammatory cellular infiltration and fibroplastic proliferation. As for immunohistological examinations, monoclonal antibodies (McAb) were obtained from HGV NS5 gene antigen, the labeled antigen and antibody were presented by the Institute of Microbiology and Epidemiology of Chinese Military Academy of Medical Sciences. The histochemical reagents with HCVAg (NS3) and HBsAg were obtained from Beijing Zhongshan Biotechnical Limited Company and Fuzhou Maixin Biotechnical Company Limited, respectively. The technical procedures were followed according to the requirements for use.
RESULTS
The relationship between HG and the other different types of viral hepatitis
One hundred and fifty-four cases of HG in our study were collected from 1552 cases of different types of viral hepatitis and evidence of an HGV infection were confirmed by positive HGV-RNA twice in all 154 cases. The relationship between them is shown Table 1.
Table 1.
The relationship between HG and the other viral hepatitis
| Group | No. of cases | No. of positive HGV-RNA | % |
| HNA-E | 583 | 52 | 8.9 |
| HA | 38 | 2 | 5.3 |
| HB | 713 | 69 | 9.1 |
| HC | 125 | 18 | 14.4 |
| HB + HC | 17 | 5 | 29.4 |
| HA + HB | 22 | 2 | 13.6 |
| HB + HE | 20 | 1 | 5.0 |
| The others * | 7 | 0 | 0 |
| Total | 1552 | 154 | 9.9 |
*The others include: HA + HC 2, HA + HE 1, HC + HE 3 and HA + HB + HE 1.
There were 52 patients with the simple HG infection (33.8%), and 103 of HG super-infected with other type of HV. Most of the cases were complicated with HCV and/or HBV infection (66.2%).
The clinical and pathological features of HGV infection
Of 52 cases of HG, the biopsies were performed in 42. The clinical and/or pathological diagnosis of 154 cases is shown in Table 2.
Table 2.
The clinical and/or pathological diagnosis of 154 cases
| Group | No.of cases | Acute hepatitis | Chronic hepatitis | Severe | Cirrhosis hepatitis |
| Sigle HG | 52 | 2 | 49 | 1 | |
| Super-infected HG | 102 | 80 | 4 | 18 |
It is difficult to identify what damages of the liver are predominant clinically and pathologically in the patients with HG super-infected with other HV infection. We had intensively observed the clinical manifestations and pathological lesions in single-infected cases of HG so as to better clarify the pathogenicity of HGV. Of the 52 cases, only 2 cases were acute hepatitis (confirmed by clinical and pathological evidence). Most of single-infected patients with HG had chronic hepatitis.
Case 1 A 46-year-old female with a history of surgery in 1988 was hospitalized with endometriosis. During the operation, blood transfusion was taken. Fifty days after transfusion, she felt weak and had a poor appetite, abnormal liver functions with ALT 180 U/L. After treatment with biphenyl dimethyl dicaboxylate, the ALT level was lowered. But her liver function was continued to be abnormal (ALT: 66-120, AST: 57-108 U/L) for 9 years. Serum examinations of pathogens performed in many large hospitals were negative. She was admitted to our hospital on Sept. 27, 1997. Laboratory examinations showed ALT 106 U/L, AST-192 U/L, normal level of TSB, A/G 1.17, and r-GP 22%. Serum tests for HA-E were negative. The test for HGV-RNA was positive. Liver biopsy was taken on October 18. Histological examinations showed hepatocyte swelling, acidophilic degeneration, piecemeal necrosis, obvious fibroplastic proliferation in the portal area and P-P bridging hepatic necrosis. The pathologic diagnosis showed chronic hepatitis: G 2-3, S 1-2. CT-displayed splenomegaly and occupied lesions in the liver. The patient was treated with α-Interferon at a dose of 3 million IU three times per week, for 6 months. Thirty days later, HGV-RNA became negative. Two months after cessation of the treatment, the HGV-RNA again became positive. The ALT continued to be abnormal. The patient had faint jaundice and symptoms of liver cirrhosis (Figure 1).
Figure 1.
Dynamic changes of serum ALT, AST and HGV-RNA in patient Hu.
Case 2 An 8-year-old girl was admitted on July 9, 1998 because of abdominal distension. Splenomegaly and a small amount of ascites were displayed by B super-sound examination. Repeated examinations showed positive HGV-RNA, ALT 60 U, AST 120 U/L, A/G 0.98, and r-GP 28%. CT showed uneven liver density with small nodular lesions, splenomegaly extended in an area of 8 costae. The clinical diagnosis was chronic hepatitis G infected from liver cirrhosis (Figure 2).
Figure 2.
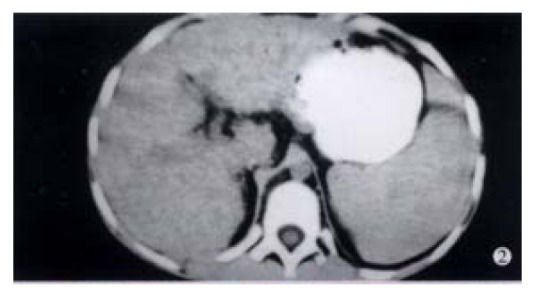
CT picture displays uneven liver density, small nodular changes and splenomegaly extended in an area of eight costae. EM × 15000
In our group, there were 2 patients with acute hepatitis G having following histological findings: cloudy hepatocyte swelling, partial vacuolation (Figure 3), punctate necrosis of liver cells, focal lymphocyte infiltration, and accidophilic degeneration of partial liver cells. In patients with chronic hepatitis G, the histological examinations of the liver tissue showed extended portal area, a moderate degree of lymphocyte infiltration, and piecemeal necrosis (Figure 4). A tendency of P-P bridge necrosis in the portal area could be found. In a case of acute hepatitits G, the electron micrographs showed shrinkage of liver cells with zigzag edges, extension of rough surfaced endoplasmic reticulum in hepatocytes, and proliferation of collagen fibrils extended into the cytoplasm in the damaged hepatocytes (Figure 5). In immunohistochemical assays, brown-yellow granules were found in the cytoplasm of hepatocytes stained by specific HGV McAb in either acute hepatitis G or chronic hepatitis G patients. The positive-stained granules were also seen in the nuclei of a few hepatocytes (Figure 6). Negative results were obtained by histochemical analysis with anti-HBs McAb and anti-HCV (McAb) expressed from HCV N3. All these results suggested that HGV could cause a series of histological damages of the liver.
Figure 3.
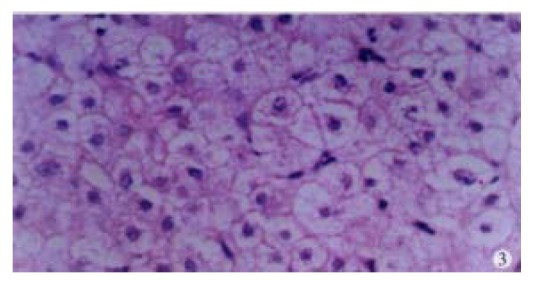
Histological changes of the liver showing cloudy swelling of hepatocytes.
Figure 4.
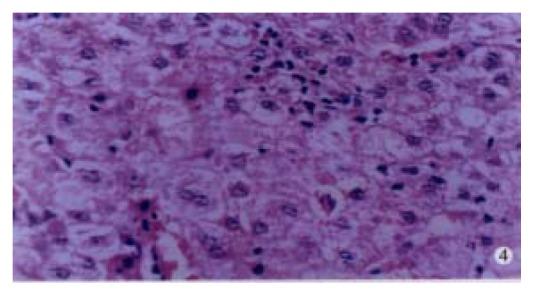
Histological changes of the liver in a patient with acute single-infected hepatitis G showing punctate necrosis, focal lymphocyte infiltration, and acidophilic degeneration of hepatocytes.
Figure 5.
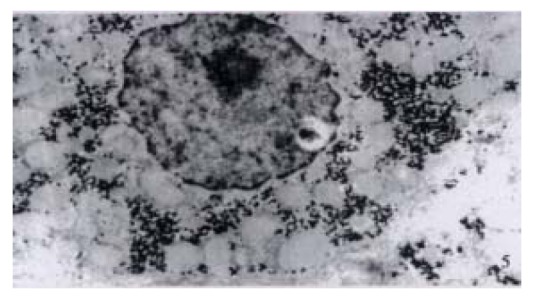
Ultrastructure of the liver tissues in a patient with acute simple HGV infection showing shirinkage of liver cells with zigzag edges, extension of rough surfaced endoplastic reticulum of hepatocytes, proliferation of collagen fibrils extended into the cytoplasm of hepatocytes. EM × 15000
Figure 6.
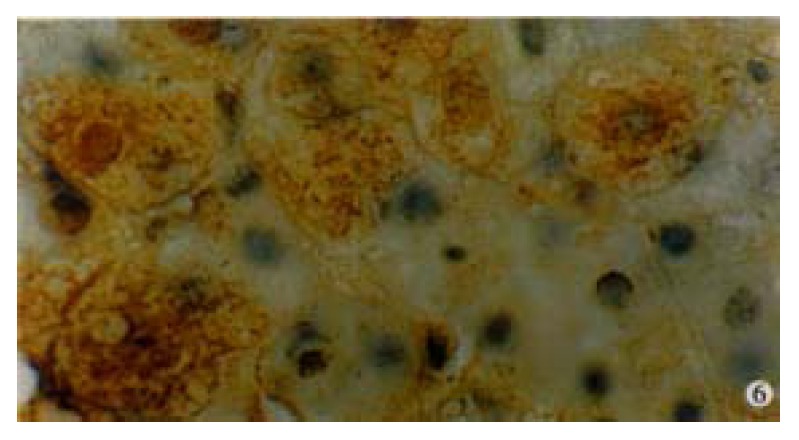
Immunohistochemical preperation stained by specific HGV McAb for HGVNS5 in a patient with single HG infection showing brown-yellow granules presented mostly in cytoplasm of hepatocytes, and partially in the nuclei. DAB staining, hematoxylin staining, BA staining. (oil) × 1000
DISCUSSION
In recent years, the pathogenicity of hepatitis G virus has caused much controversies, because of the following aspects. ① HGV infection is usually mixed with the infections caused by other hepatitis viruses. The patient with single hepatitis G infection are rare; ② A body of evidence has been limited to serologic studies without sufficient histological evidence; ③ Lack of animal modes for the study of hepatitis G. A study on hepatitis G in our hospital was started earliest in China. In 1994, at “World Chinese Symposium on Hepatopathy” our report on clinical and pathological study in chronic hepatitis produced by “non-A, non-B and non-C agents” aroused great interests[3]. In 1996, we reported the paper “Clone of partial HGV genes in Nanjing, China and analysis of its cDNA sequence”, and established the method for determination of HGV-RNA by RT-PCR. The clinical and pathological characteristics of viral hepatitis G were described[4,5]. Of the 154 cases of HG collected from 1552 cases of different kinds of hepatitis in this paper, 52 were single HGV infection and 102 were super-infected with other hepatitis viruses, which accounted for 33.8% (52/154) and 66.2% (102/154) respectively. The clinical and pathological data from the simple HG patients in this study showed that: ① the clinical symptoms of HG patients vary in degree. It was mostly- sub-clinical; ② majority of patients had no jaundice, less hepatic damage than HB and a mild to moderate elevated ALT and AST level; ③ most of the patients were chronic. Of 52 patients with HG, there were 49 chronic cases, 1 cirrhosis and only 2 acute cases in our study. One of the two patients with acute HG had jaundice (TSB 106.7 μmol/L) 30 days after blood transfusion, with the ALT level 535 U/L and AST 116 U/L; ④ histological changes were local necrosis and piecemeal necrosis in most patients, P-P bridge necrosis and a tendency of cirrhosis in a few patients. Electron micrographs of the two acute patients displayed proliferation of collagen fibrils extended into the cytoplasm of hepatocytes; ⑤ HGV antigen was generally present in cytoplasm and sometimes in the nuclei of the hepatocytes by immuohistochemical assay. We studied a Chinese Rhesus monkey intravenously attacked with the serum from a chronic hepatitis G patient only with positive HGV-RNA for one year. The dynamic changes of the serology and histology of the liver in the animal were observed before and after infection. It was found that the serological and histological changes of the animal were similar to that of the patients in our study[6]. Eighteen months after the infection, the monkey was dissected and its internal organs were taken for histological examination. The internal organs were normal except the liver tissue in which there were focal necrosis and slight piecemeal necrosis. The pathological diagnosis was confirmed to be chronic hepatitis. It is suggested that HGV would be a hepatotropic virus and had the pathogenicity to human.
ACKNOWLEGEMENTS
We would like to thank Prof. T. H. Zhang, the General Hospital of Nanjing Military Area, for his guidance in this study.
Footnotes
Supported by the Science Foundation of Jiangsu Province, No. BK97173
Edited by Ma JY
References
- 1.Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 2.Linnen J, Wages J, Zhang-Keck ZY, Fry KE, Krawczynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 3.He CL, Xu JZ, Wang XY Clinical and pathological study on chronic non-A, non-B, non-C hepatitis. Abstracts of the World Chinese Liver Disease Conference. P69. 1994, Huangshan, China [Google Scholar]
- 4.Xu J, Zhou Y, Xu J, Ju J, Wang X, Chen W, Wang H. [Clonning and sequencing of partial gene of hepatitis G virus (HGV) from Nanjing of China] Zhonghua Shiyan He Linchuang Bingbuxue Zazhi. 1997;11:27–28. [PubMed] [Google Scholar]
- 5.Xu JZ, Wang HT, Yang ZG, Le MZ, Sui YH, He CL. Clinical and pathological features of hepatitis G. Zhonghua Chuanranbing Zazhi. 1998;16:222. [Google Scholar]
- 6.Xu J, Ju J, Yang Z. [Dynamic observation of serological and histological changes of Chinese rhesus monkeys infected by hepatitis G virus] Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:71–73. [PubMed] [Google Scholar]