INTRODUCTION
The mechanism that DNA hypomethylation leads to activation of oncogene and occurrence of malignant neoplasm is being increasingly recognized by researchers. Normal DNA methylation plays important role in stabilizing the phenotype of cell. DNA methylation status reduction and/or pattern alteration are related to activation and abnormally high expression of some oncogenes and cellular malignancy[1-6]. c-fms oncogene encodes for colony stimulating factor 1 receptor (CSF-1R)[7], c-fms/CSF-1R was highlyexpressed in hepatocellular carcinoma (HCC) tissue, but the mechanism remained obscure[8,9]. In this study, restrictive endonucleases Hpa II/Msp I digestion and Southern blot were used to study methylation status alteration of c-fms oncogene in HCC tissue and matching circum-cancer liver tissue, meanwhile the relationship between the alteration and clinical pathology of HCC was investigated. The gist of this study was to clarify the mechanism leading to c-fms oncogene high expression in hepatocellular carcinogenesis.
MATERIALS AND METHODS
Subjects
Thirty HCC patients were verified with pathological examination (25 males and 5 females, age range 32-76 years, mean 55 years). Fresh hepatic tissue of HCC focus and circum-tissues 2 cm from HCC focus were cut off, normal control was from HBV negative hepatic tissue of renal transplantation donor. All the tissues were washed with physiological saline and put in freezing tube, and then they were put in liquid nitrogen for storage.
Main reagents
Restrictive endonucleases Hpa II/Msp I were purchased from Japan TaKaRa Bio-Company. DIG random labeling and detection kit were purchased from Boster Bio-Company. Nylon transfer membranes used for Southern blot were purchased from Shanghai Bio-Company. c-fms plasmid was kindly provided by academician Gu Jianren in the National Laboratory for Oncogene and Related Genes, Shanghai Cancer Institute.
METHODS
DNA extraction
About 0.5 g hepatic tissue was triturated in mortar, and a dissolvent (10 mmol/L Tris-Cl pH8.0, 10 mmol/L EDTA pH8.0, 0.5% SDS) 500 μL was added. The preparation was digested with proteinase K and RNase, then it was extracted twice with saturated hydroxybenzene and once with chloroformisoamylalcohol (chloroform:isoamylalcohol = 24:1), and at last 2.5 volumes of absolute alcohol and 0.1 volume of 3 mol/L sodium acetate (pH5.2) were added to precipitate the DNA. The extracted DNA was dissolved in TE buffer (pH8.0), and DNA concentration was detersmined with an ultraviolet spectrophotometer.
Probe labeling
Plasmid extraction, restrictive endonucleases digestion, reclamation and purification were performed as the methods recorded in Molecular Cloning. The probe was labeled with DIG random labeling and detection kit.
Southern blot
Genome DNA 10 μg was digested with restrictive endonucleases Hpa II and Msp I 50 U each for 12 hours respectively. Digested DNA was examined with 0.8% agarose electrophoresis. The buffer used for electrophoresis was 0.5 × TBE. Electrophoresis was ended when 2 kb marker shifted to the middle of the gel. Photo was taken under ultraviolet ray transmission. The gel was immersed in 0.25 mol/L HCl for 10 minutes, washed with distilled water for 20-30 minutes, and then immersed in denaturalizing liquid twice for 15 minutes each time. The gel was again washed twice with distilled water, and then it was immersed into neutralizing liquid twice for 15 minutes each time. A piece of nylon membrane of the same size as the gel was cut, it was immersed into 2 × SSC for 20 minutes. Transfer was performed for 18 hours at room temperature by the capillary transfer method. Then the nylon membrane was taken out and washed several times with 2 × SSC. After this, it was fixed for 20 minutes under long wave ultraviolet radiation. The transferred nylon membrane was sealed in a plastic bag and then pre-hybridizing reagent was perfused into the bag. The bag was put in a 42 °C water bath for 2 hours, and then pre-hybridizing reagent was decanted off. The bag was sealed, after the hybridizing reagent containing the DIG-labeled probe had been put in. The bag was put into a 42 °C water bath and hybridized for 20 hours. After hybridization the nylon membrane was taken out, and showed coloration according to the instruction of the DIG random labeling and detection kit.
Pathological examination and classification
The tumors were variously classified into unifocal or multifocal, and massive or nodular (a main tumor mass with satellites). The size and inner-condition of the tumors were recorded. The histological grading was based on Edmondson standard I-IV scale.
RESULTS
Southern blot of HCC tumor tissue and circum-cancer liver tissue
Hpa II and Msp I are isoschizomers. Both can digest CCGG sequence, but when the sequence is methylated into C-mCGG, only Msp I can digest while Hpa II can not. Methylation status of the gene may thus be determined by relying on such a difference. The data in this study showed that after digestion with Hpa II, hybridizing bands of less than 6.5 kb in size were observed in 36.7% (11/30) of HCC tissue and 13.3% (4/30) of circum-cancer liver tissue, which resembled those of the hybridizing bands digested with Msp I. The results indicated that the methylation status of c-fms oncogene in the tissue was decreased (Figure 1, Figure 2).
Figure 1.
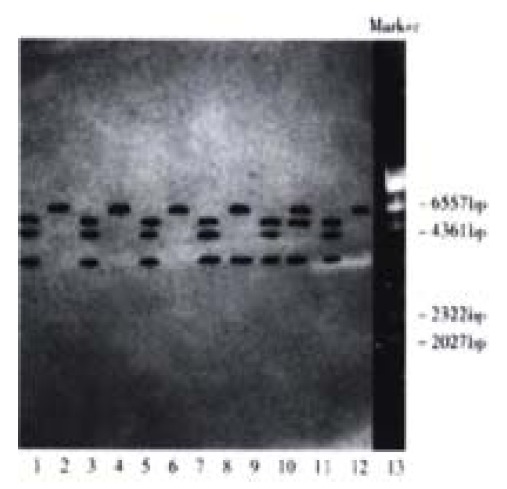
Southern blot of HCC tissue genome DNA digested with Hpa II/Msp I. Lane 1, 3, 5, 7, 9, 11 Msp I digestion; Lane 2, 4, 6, 8, 10, 12 Hpa II digestion; Lane 13 λ DNA/Hind III markers; Lane 1, 2 normal control hepatic tissue; Lane 3-12 HCC tissue; Lane 8, 10 hybridizing bands of less than 6.5 kb in size appeared after Hpa II digestion
Figure 2.
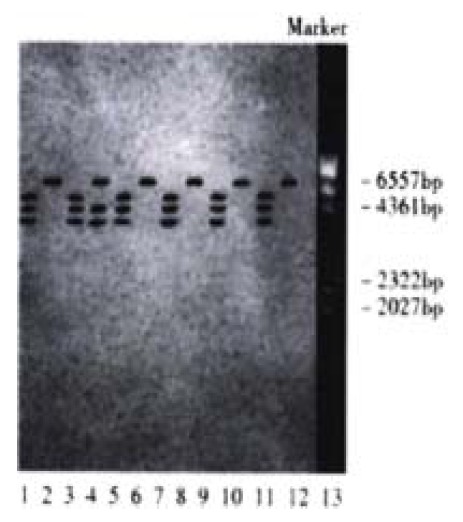
Southern blot of circumª²cancer liver tissue genome DNA digested with Hpa II/Msp I. Lane 1, 3, 5, 7, 9, 11 Msp I digestion; Lane 2, 4, 6, 8, 10, 12 Hpa II digestion; Lane 13 λ DNA/Hind III markers; Lane 1, 2 normal control hepatic tissue; Lane 3-12 circum-cancer liver tissue; Lane 4 hybridizing bands of less than 6.5 kb in size appeared after Hpa II digestion
Methylation status comparison of c-fms oncogene in HCC tissue and circum-cancer liver tissue
Hypomethylation rate of c-fms oncogene in HCC tissue and circum-cancer liver tissue was compared, the difference was determined, the result is shown in Table 1.
Table 1.
Methylation status of c-fms oncogene (number of patients)
| Methylation status | HCC tissue | Circum-cancer liver tissue |
| Normal methylation | 19 | 26 |
| Hypomethylation | 11 | 4 |
Note: χ2 = 4.36, P < 0.05, hypomethylation rate of c-fms oncogene in HCC tissue was higher than that in circum-cancer liver tissue.
Relationship between hypomethylation of c-fms oncogene and clinical pathology
The relationship between hypomethylation of c-fms oncogene in hepatic tissue and the sex, age and pathological Edmondson scale of patients was analysed; the results are shown in Table 2.
Table 2.
Relationship between hypomethylation of c-fms oncogene and clinical pathology
| Item | Total number |
HCC tissue |
Circum-cancer liver tissue |
|||
| Number | % | Number | % | |||
| Sex | Male | 25 | 10 | 41.0 | 3 | 12.0 |
| Female | 5 | 1 | 20.0 | 1 | 20.0 | |
| Age(yrs) | < 60 | 22 | 7 | 31.8 | 2 | 9.1 |
| ≥ 60 | 8 | 4 | 50.0 | 2 | 25.0 | |
| Edmondson | I-II | 14 | 2 | 14.3 | 1 | 7.1 |
| scale | III-IV | 16 | 9 | 56.3b | 3 | 18.8 |
P < 0.05, vs Edmondson I-II.
Chi-square test showed that there was no significant relationship between hypomethylation of c-fms oncogene and sex and age of patients (P > 0.05). There was significant relationship between Edmondson scale and hypomethylation of c-fms oncogene in HCC tissue (χ2 = 5.66, P < 0.05), while there was no significant relationship between Edmondson scale and hypomethylation of c-fms oncogene in circum-cancer liver tissue(P > 0.05).
DISCUSSION
In the process of cellular carcinogenesis, genetic and epigenetic mechanism contributes to abnormal expression of gene. The genetic mechanism involves the mutation and chromosome rearrangement, with which genetic products of abnormal molecular structures can be produced[10-19]. The epigenetic mechanism mainly refers to an altered methylation status of 5’-cytosine in the DNA sequence, which leads to an abnormal expression of gene in quantity, but no change in DNA sequence and genetic product[20-24]. In the process of cellular carcinogenesis, the methyl groups in CpG islands of DNA are lost gradually. Although the accurate mechanism between DNA hypomethylation and cellular carcinogenesis remains obscure, some data showed that DNA hypomethylation could affect chromatin condensation in the middle stage, which led to a change and/or rupture of chromosome[25-31]. DNA hypomethylation is a kind of molecular structure leading to abnormal expression of gene; it facilitates a high expression of gene[32]. Highly specific DNA methylation types exist in human genomes, and are reflected in genetic characteristics of DNA, such as transcription, duplication, recombination, transposition and mutation. One biological function of human genome DNA methylation is to keep promoter of gene in silent status for long. 5’ methyl-cytosine in special DNA sequence can mediate the specific combination and interaction between DNA and some functional proteins. As the interactions locate in the core region which regulates biological function of gene, sequence-specific methylation of DNA can affect cellular function[33-39]. Furthermore, DNA methylation depends on combining special proteins, and it directly or indirectly induced conformational alterations of DNA. DNA methylation is a kind of modificatory mode of cellular DNA at transcription level. In human genome, CpG islands in house keeping genes including a great number of oncogenes are in the form of methylation. Transcription and expression of the methylated genes especially oncogenes are limited. Decline of DNA methylation status at special sites of oncogene induces abnormal expression of the gene[28,40-43].
c-fms oncogene locates at 5q 33.3 of human chromosome and encodes CSF 1R, which has tyrosine kinase activity[44]. c-fms/CSF-1R is highly expressed in hepatocellular carcinogenesis, and its expressing level in HCC tissue is higher than that in circum-cancer liver tissue. Through the mechanism of signal transduction, CSF-1R stimulates the growth and development of HCC[45-50]. The whole length of c-fms oncogene is 43 kb, in which there are 49 CCGG sites. The data in this study indicated that the hypomethylation rate of c-fms oncogene in HCC tissue was higher than that in circum-cancer liver tissue, while no hypomethylation of c-fms oncogene in normal hepatic tissue was observed. So hypomethylation is an important activating mechanism of c-fms oncogene in hepatocellular carcinogenesis. The data also showed that the hypomethylation rate of c-fms oncogene in Edmondson III-IV HCC was higher than that in Edmondson I-II HCC. All the evidence indicated that hypomethylation of c-fms oncogene might be an important late genetic incident in hepatocellular carcinogenesis. HCC with hypomethylation of c-fms oncogene may show the characteristics of higher malignancy and rapid development, and provide a clinical guide in the selection of surgery, radiotherapy and chemotherapy, and also in judging the prognosis of patients.
c-fms/CSF-1R is abnormally high-expressed in hepatocellular carcinogenesis at a late stage, meanwhile hypomethylation of c-fms oncogene occurs, and the hypomethylation rate of c-fms oncogene in HCC tissue is higher than that in the circum-cancer liver tissue. These data indicate that hypomethylation of c-fms oncogene is a kind of molecular mechanism leading to abnormally high CSF-1R expression and promoting the occurrence and development of HCC. So blocking the process of hypomethylation of c-fms oncogene, consequently decreasing a high expression of CSF-1R may have great clinical significance in decreasing malignant phenotype of HCC and improving prognosis of patients.
Footnotes
Supported by the Natural Science Foundation of Guangdong Province, No 990422
Edited by Lu HM Proofread by Ma JY
References
- 1.Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, Meng X. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88:761–769. [PubMed] [Google Scholar]
- 2.Nagai H, Kim YS, Yasuda T, Ohmachi Y, Yokouchi H, Monden M, Emi M, Konishi N, Nogami M, Okumura K, et al. A novel sperm-specific hypomethylation sequence is a demethylation hotspot in human hepatocellular carcinomas. Gene. 1999;237:15–20. doi: 10.1016/s0378-1119(99)00322-4. [DOI] [PubMed] [Google Scholar]
- 3.Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn J Cancer Res. 1999;90:909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Z, Wang X, Zhao Q, Townsend CM, Evers BM. DNA methylation contributes to expression of the human neurotensin/neuromedin N gene. Am J Physiol. 1998;274:G535–G543. doi: 10.1152/ajpgi.1998.274.3.G535. [DOI] [PubMed] [Google Scholar]
- 5.Yakoob J, Fan XG, Hu GL, Zhang Z. DNA methylation and carcinogenesis in digestive neoplasms. World J Gastroenterol. 1998;4:174–177. doi: 10.3748/wjg.v4.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Lai MD, Chen J. Methylation status of p16 gene in colorectal carcinoma and normal colonic mucosa. World J Gastroenterol. 1999;5:451–454. doi: 10.3748/wjg.v5.i5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherr CJ. The fms oncogene. Biochim Biophys Acta. 1988;948:225–243. doi: 10.1016/0304-419x(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 8.Yang DH, Liu WW, Gu JR, Wan DF, Li HN, Ma AQ, Qu SM, Xu XL. Expression of cancer gene products of IGF-II, IGF-II receptors, CSF-1 receptors in primary hepatic cancer and non-cancerous liver tissue. Zhonghua Yixue Zazhi. 1992;72:476–480. [Google Scholar]
- 9.Motoo Y, Mahmoudi M, Osther K, Bollon AP. Oncogene expression in human hepatoma cells PLC/PRF/5. Biochem Biophys Res Commun. 1986;135:262–268. doi: 10.1016/0006-291x(86)90971-x. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795–1801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SM, Zhou H, Chen RC, Wang YF, Chen F, Zhang CG, Zhen Y, Yan JH, Su JH. Sequencing of p53 mutation in established human hepatocellular carcinoma cell line of HHC4 and HHC15 in nude mice. World J Gastroenterol. 1998;4:506–510. doi: 10.3748/wjg.v4.i6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins C, Kedda MA, Kew MC. Characterization of six tumor suppressor genes and microsatellite instability in hepatocellular carcinoma in southern African blacks. World J Gastroenterol. 1999;5:470–476. doi: 10.3748/wjg.v5.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, Lau WY, Lai PB, Lim BK, Huang J, et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789–795. doi: 10.1038/sj.onc.1202359. [DOI] [PubMed] [Google Scholar]
- 14.Yano M, Asahara T, Dohi K, Mizuno T, Iwamoto KS, Seyama T. Close correlation between a p53 or hMSH2 gene mutation in the tumor and survival of hepatocellular carcinoma patients. Int J Oncol. 1999;14:447–451. doi: 10.3892/ijo.14.3.447. [DOI] [PubMed] [Google Scholar]
- 15.Qin Y, Li B, Tan YS, Sun ZL, Zuo FQ, Sun ZF. Polymorphism of p16INK4a gene and rare mutation of p15INK4b gene exon2 in primary hepatocarcinoma. World J Gastroenterol. 2000;6:411–414. doi: 10.3748/wjg.v6.i3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yakicier MC, Irmak MB, Romano A, Kew M, Ozturk M. Smad2 and Smad4 gene mutations in hepatocellular carcinoma. Oncogene. 1999;18:4879–4883. doi: 10.1038/sj.onc.1202866. [DOI] [PubMed] [Google Scholar]
- 17.Park WS, Dong SM, Kim SY, Na EY, Shin MS, Pi JH, Kim BJ, Bae JH, Hong YK, Lee KS, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59:307–310. [PubMed] [Google Scholar]
- 18.Luo D, Liu QF, Gove C, Naomov N, Su JJ, Williams R. Analysis of N-ras gene mutation and p53 gene expression in human hepatocellular carcinomas. World J Gastroenterol. 1998;4:97–99. doi: 10.3748/wjg.v4.i2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng XM, Peng WW, Yao JL. Codon 249 mutations of p53 gene in development of hepatocellular carcinoma. World J Gastroenterol. 1998;4:125–127. doi: 10.3748/wjg.v4.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai DN, Xie YF, Bian L, Yao X. Study on p16 gene aberrant methylation of colon cancer cell lines. Shijie Huaren Xiaohua Zazhi. 1999;7:676–678. [Google Scholar]
- 21.Kitamura T, Watanabe S, Sato N. Liver regeneration, liver cancers and cyclins. J Gastroenterol Hepatol. 1998;13 Suppl:S96–S99. [PubMed] [Google Scholar]
- 22.Koyama M, Nagai H, Bando K, Ito M, Moriyama Y, Emi M. Localization of a target region of allelic loss to a 1-cM interval on chromosome 16p.13.13 in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90:951–956. doi: 10.1111/j.1349-7006.1999.tb00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KS, Lee YI. Biallelic expression of the H19 and IGF2 genes in hepatocellular carcinoma. Cancer Lett. 1997;119:143–148. doi: 10.1016/s0304-3835(97)00264-4. [DOI] [PubMed] [Google Scholar]
- 24.Hada H, Koide N, Morita T, Shiraha H, Shinji T, Nakamura M, Ujike K, Takayama N, Oka T, Hanafusa T, et al. Promoter-independent loss of mRNA and protein of the Rb gene in a human hepatocellular carcinoma. Hepatogastroenterology. 1996;43:1185–1189. [PubMed] [Google Scholar]
- 25.Robertson KD, Jones PA. DNA methylation: past, present and future directions. Carcinogenesis. 2000;21:461–467. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 26.Rizwana R, Hahn PJ. CpG methylation reduces genomic instability. J Cell Sci. 1999;112(Pt 24):4513–4519. doi: 10.1242/jcs.112.24.4513. [DOI] [PubMed] [Google Scholar]
- 27.Qu GZ, Grundy PE, Narayan A, Ehrlich M. Frequent hypomethylation in Wilms tumors of pericentromeric DNA in chromosomes 1 and 16. Cancer Genet Cytogenet. 1999;109:34–39. doi: 10.1016/s0165-4608(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 28.Qu G, Dubeau L, Narayan A, Yu MC, Ehrlich M. Satellite DNA hypomethylation vs. overall genomic hypomethylation in ovarian epithelial tumors of different malignant potential. Mutat Res. 1999;423:91–101. doi: 10.1016/s0027-5107(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 29.Jeddeloh JA, Stokes TL, Richards EJ. Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat Genet. 1999;22:94–97. doi: 10.1038/8803. [DOI] [PubMed] [Google Scholar]
- 30.Dao D, Walsh CP, Yuan L, Gorelov D, Feng L, Hensle T, Nisen P, Yamashiro DJ, Bestor TH, Tycko B. Multipoint analysis of human chromosome 11p15/mouse distal chromosome 7: inclusion of H19/IGF2 in the minimal WT2 region, gene specificity of H19 silencing in Wilms' tumorigenesis and methylation hyper-dependence of H19 imprinting. Hum Mol Genet. 1999;8:1337–1352. doi: 10.1093/hmg/8.7.1337. [DOI] [PubMed] [Google Scholar]
- 31.Goto T, Christians E, Monk M. Expression of an Xist promoter-luciferase construct during spermatogenesis and in preimplantation embryos: regulation by DNA methylation. Mol Reprod Dev. 1998;49:356–367. doi: 10.1002/(SICI)1098-2795(199804)49:4<356::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Wachsman JT. DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutat Res. 1997;375:1–8. doi: 10.1016/s0027-5107(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 33.Lu S, Davies PJ. Regulation of the expression of the tissue transglutaminase gene by DNA methylation. Proc Natl Acad Sci USA. 1997;94:4692–4697. doi: 10.1073/pnas.94.9.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, Gartler SM. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci USA. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovarík A, Van Houdt H, Holý A, Depicker A. Drug-induced hypomethylation of a posttranscriptionally silenced transgene locus of tobacco leads to partial release of silencing. FEBS Lett. 2000;467:47–51. doi: 10.1016/s0014-5793(00)01077-2. [DOI] [PubMed] [Google Scholar]
- 36.Schmutte C, Fishel R. Genomic instability: first step to carcinogenesis. Anticancer Res. 1999;19:4665–4696. [PubMed] [Google Scholar]
- 37.Cho M, Grabmaier K, Kitahori Y, Hiasa Y, Nakagawa Y, Uemura H, Hirao Y, Ohnishi T, Yoshikawa K, Ooesterwijk E. Activation of the MN/CA9 gene is associated with hypomethylation in human renal cell carcinoma cell lines. Mol Carcinog. 2000;27:184–189. [PubMed] [Google Scholar]
- 38.Felgner J, Heidorn K, Körbächer D, Frahm SO, Parwaresch R. Cell lineage specificity in G-CSF receptor gene methylation. Leukemia. 1999;13:530–534. doi: 10.1038/sj.leu.2401386. [DOI] [PubMed] [Google Scholar]
- 39.Florl AR, Löwer R, Schmitz-Dräger BJ, Schulz WA. DNA methylation and expression of LINE-1 and HERV-K provirus sequences in urothelial and renal cell carcinomas. Br J Cancer. 1999;80:1312–1321. doi: 10.1038/sj.bjc.6690524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones PL, Wolffe AP. Relationships between chromatin organization and DNA methylation in determining gene expression. Semin Cancer Biol. 1999;9:339–347. doi: 10.1006/scbi.1999.0134. [DOI] [PubMed] [Google Scholar]
- 41.Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Hum Mol Genet. 1999;8:2317–2323. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- 42.Devereux TR, Horikawa I, Anna CH, Annab LA, Afshari CA, Barrett JC. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 1999;59:6087–6090. [PubMed] [Google Scholar]
- 43.Torres L, Avila MA, Carretero MV, Latasa MU, Caballería J, López-Rodas G, Boukaba A, Lu SC, Franco L, Mato JM. Liver-specific methionine adenosyltransferase MAT1A gene expression is associated with a specific pattern of promoter methylation and histone acetylation: implications for MAT1A silencing during transformation. FASEB J. 2000;14:95–102. doi: 10.1096/fasebj.14.1.95. [DOI] [PubMed] [Google Scholar]
- 44.Cui J, Yang DH. CSF-1 receptor/c-fms and liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:696–697. [Google Scholar]
- 45.Hamilton JA. CSF-1 signal transduction. J Leukoc Biol. 1997;62:145–155. doi: 10.1002/jlb.62.2.145. [DOI] [PubMed] [Google Scholar]
- 46.Choudhury GG, Sylvia VL, Pfeifer A, Wang LM, Smith EA, Sakaguchi AY. Human colony stimulating factor-1 receptor activates the C-raf-1 proto-oncogene kinase. Biochem Biophys Res Commun. 1990;172:154–159. doi: 10.1016/s0006-291x(05)80186-x. [DOI] [PubMed] [Google Scholar]
- 47.Roussel MF. Signal transduction by the macrophage-colony-stimulating factor receptor (CSF-1R) J Cell Sci Suppl. 1994;18:105–108. doi: 10.1242/jcs.1994.supplement_18.15. [DOI] [PubMed] [Google Scholar]
- 48.Flick MB, Sapi E, Perrotta PL, Maher MG, Halaban R, Carter D, Kacinski BM. Recognition of activated CSF-1 receptor in breast carcinomas by a tyrosine 723 phosphospecific antibody. Oncogene. 1997;14:2553–2561. doi: 10.1038/sj.onc.1201092. [DOI] [PubMed] [Google Scholar]
- 49.Hatch WC, Ganju RK, Hiregowdara D, Avraham S, Groopman JE. The related adhesion focal tyrosine kinase (RAFTK) is tyrosine phosphorylated and participates in colony-stimulating factor-1/macrophage colony-stimulating factor signaling in monocyte-macrophages. Blood. 1998;91:3967–3973. [PubMed] [Google Scholar]
- 50.Rohrschneider LR, Bourette RP, Lioubin MN, Algate PA, Myles GM, Carlberg K. Growth and differentiation signals regulated by the M-CSF receptor. Mol Reprod Dev. 1997;46:96–103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]


