Abstract
AIM: To study the colon innervation of trisomy 16 mouse, an animal model for Down’s syndrome, and the expression of protein gene product 9.5 (PGP 9.5) in the stenosed segment of colon in Hirschsprungs disease (HD).
METHODS: Trisomy 16 mouse breeding; cytogenetic analysis of trisomy 16 mice; and PGP 9.5 immunohistochemistry of colons of trisomy 16 mice and HD were carried out.
RESULTS: Compared with their normal littermates, the nervous system of colon in trisomy 16 mice was abnormally developed. There existed developmental delay of muscular plexuses of colon, no submucosal plexus was found in the colon, and there was 5 mm aganglionic bowel aparting from the anus in trisomy 16 mice. The mesentery nerve fibers were as well developed as shown in their normal littermates. Abundant proliferation of PGP 9.5 positive nerve fibers was evealed in the stenosed segment of HD colon.
CONCLUSION: Trisomy 16 mice could serve as an animal model for Hirschsprung’s disease for aganglionic bowel in the distal part of colon. Abundant proliferation of PGP 9.5 positive fibers resulted from extrinsic nerve compensation, since no ganglionic cells were observed in the stenosed segment of the colon in HD. HD has a genetic tendency.
Keywords: Hirschsprungs disease, colon, down syndrome, immunohistochemistry, nervous system, trisomy 16 mouse
INTRODUCTION
The most significant pathophysiological change of Hirschsprung’s disease is its characteristic abnormal enteric nervous development of colon which resulted in the regional enterospasm and narrowed lumen, loss of intestinal peristalsis and relaxing reflex of the internal enteric sphincter[1]. The physiological peristalsis of the proximal gut pushes the intestinal contents forward, leading to the second megacolon in the proximal part of the narrowed gut. Since the cause of HD is still unknown and the experiment on humans is impossible, we used protein gene product 9.5 to study the enteric nerve development of colon in trisomy 16 mice, an animal model for Down’s syndrome[2,3], and the innervation of stenosed segment of the colon in HD so as to reveal the possibility of developing the congenital megacolon in trisomy 16 mice and the genetic tendency of HD in human[4-7].
MATERIALS AND METHODS
Animal stocks and breeding[8-10]
Trisomy 16 mouse embryos examined in this study were produced by crossing NMRI-female mice provided by the Experimental Center of Medical University of Lubeck, with male mice carrying the two Robertsonian (Rb) translocation chromosomes Rb (16, 17) 8LubtwLub3 perpendicularly and Rb (11, 16) 2H. The day on which a vaginal plug was observed was considered to be the first embryonic day (ED).
Cytogenetic analysis
The pregnant female mice were sacrificed one hour after intraperitoneal injection of colchicine (0.02 mg/25-35 mg body weight) by dislocation of the neck. The embryos were collected by laparatomy. Small samples of liver were taken from each embryo for direct preparation of karyotypes, by using the standard technique of hypotonic treatment with 0.563% KCl and methanol:acetic acid fixation (3:1). Air-dried slides were observed under light microscope. Accurate ascertainment of trisomy 16 was provided by the demonstration of two Rb metecentric chromosomes and a count of 41 chromosome arms. After the defrimy of the chromosomal status, the trisomic and the normal euploid embryos of each litter could be separated (Figure 1).
Figure 1.
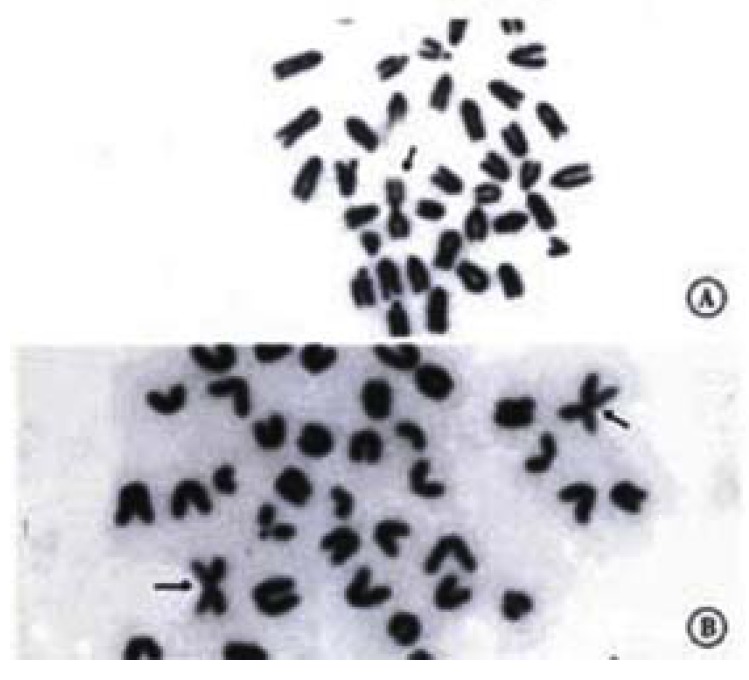
a. The chromosome of normal mice. b. The chromosome of trisomy 16 mice: two Rb metecentric chromosomes (↑) and 41 chromosome arms. × 1000
Immunohistochemical methods[11]
The guts were taken from trisomy 16 embryos and normal littermates, and fixed in a solution containing 4% paraformaldehyde and 2% picric acid in phosphate-buffered saline (PBS) for approximately 2 hours at room temperature. Fifteen pieces of narrowed HD guts were obtained from the patients aged between 18 months and 9 years. Six segments of normal colons served as controls. The diagnosis of HD was based on the case history, clinical manifestations, examination of barium enema and pressure measurement of rectum and anal. All the cases were confirmed postoperatively by pathological examinations.
Whole mount preparations of the gut were dissected out under stereomicroscopic control[12-14], and freezing sections were made at a thickness of 12 μm, then washed in 0.01 M PBS and 50% ethanol for 2 hours at room temperature. Forimmunohistochemistry, peroxidase-anti-peroxidase (PAP) was used in the present experiment.
The specimens were treated with 10% normal goat serum (Dakopatts × 907) for 30 min, then incubated in a primary antiserum protein gene product 9.5 (PGP 9.5) (Ultraclone RA 95101, UK) overnight at room temperature, diluted to 1:400 in a solution containing 10% normal goat serum, 0.01% sodium azide and 0.05% thimerosal in PBS. After three washes in PBS for 30 min, the specimens were incubated in goat anti-rabbit IgG (Dakopatts Z421) overnight at room temperature as the secondary antiserum, and diluted to 1:100 in the above solution. The specimens were then exposed in rabbit PAP (rabbit antibody to horseradish peroxidase and horseradish peroxidase) overnight at room temperature and diluted to 1:100 in a solution containing 0.1% bovine serum albumin and 0.05% thimerosal in PBS pH7.4. 4 Cl1-naphtol or DAB was used as the chromogen for the peroxidase reaction. Control included the omission of the primary antiserum. PGP 9.5 is a cytoplastic protein composed of 212 amino acids with molecular weight of 2.45 kb. This protein only exists in the neurons and neuroendocrine cells. PGP 9.5 is a specific protein with an excellent localizing function for the neurons[15-19].
RESULTS
Development of trisomy 16 mice and their normal littermates
Owing to the abnormality of their chromosomes, the trisomy 16 mice were characterized by their small size, generalized edema and a failure of closed eyelids after embryonic day 16 (ED16). Both the small size and lid-gap defect were attributed to the generalized growth retardation and developmental delay, leading death at the time of birth in most mice. The trisomy 16 mice in the present study were chosen from the gestational age between ED13 and ED18. The survival rate of the trisomy 16 mice was lower with the gestational age. The lowest survival rate of trisomy 16 mice was observed at the time of their birth (Figure 2).
Figure 2.
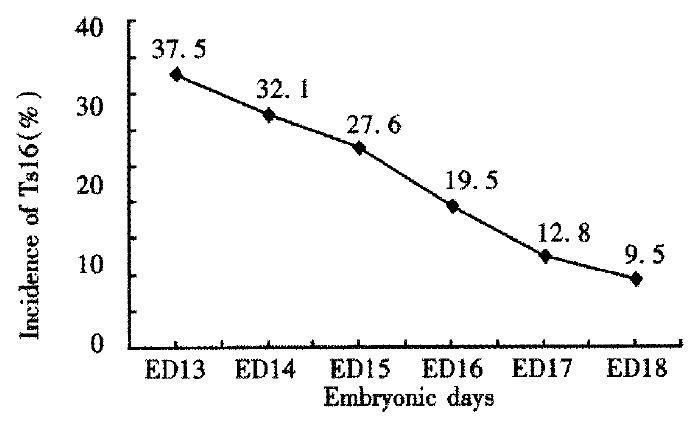
The survival rate of trisomy 16 mice with different embryonic days (ED).
Development of colon innervation in trisomy 16 mice and their normal littermates
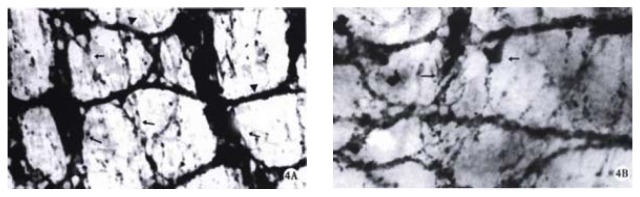
The nerve development of colon in normal littermates is a continuous process. Based on the morphology and staining degree of the PGP 9.5 positive reactions, the nerve development of colon could be divided into three stages: primary stage (ED14), juvenile stage (ED15 and ED16) and mature stage (ED17 and ED18). At ED14, scantly-distributed PGP 9.5 positive neurons were observed with light staining and round nucleus in the center of the neurons. At ED15, the distribution density of neurons increased greatly with some nervous processes, through which the neurons were connected with each other (Figure 3A). At ED16, muscular plexuses appeared with gathered neurons to form the light-stained ganglion. But the number of neurons in the ganglion was less and sparely arranged. At ED17 and ED18, the precursor cells of muscular plexuses migrated into the submucosal layer to form submucosal plexuses. The arrangement of nervous fibers in submucosal plexus was irregular, accompanied by some small-sized and sparely-distributed ganglions, while muscular plexuses were composed of primary, secondary and tertiary strands displaying a nerve meshwork with regular wide meshes and numerous large ganglia. The muscular and submucosal plexuses were further developed with much stronger PGP 9.5 staining at ED18 (Figure 4A). The increasing number of large-sized ganglions was observed with stronger immunostaining and arranged along the primary nerve strands. Numerous fine nerve strands in the gut villi were observed at ED18 (Figure 5).
Figure 3.
a. Myenteric plexus of normal littermates at ED 15. The nerve meshworks had regular meshes with many neurons (↑). × 400 b. The colon of trisomy 16 mice contained some neurons (arrow) with different staining and size at ED15. × 400
Figure 4.
At ED 18, the myenteric plexuses of trisomy 16 mice and their normal littermates in whole mount preparation. × 250 A. The developed myenteric plexus with primary (long arrow), secondary (arrow) and tertiary (arrowhead) strands were displayed in the colon of normal littermates. The primary strands were composed of broad fiber bundles and numerous ganglia with longitudinally arranged meshes. B. The myenteric plexus of trisomy 16 mice was poor with less ganglia containing some neurons (arrow) at different staining.
Figure 5.
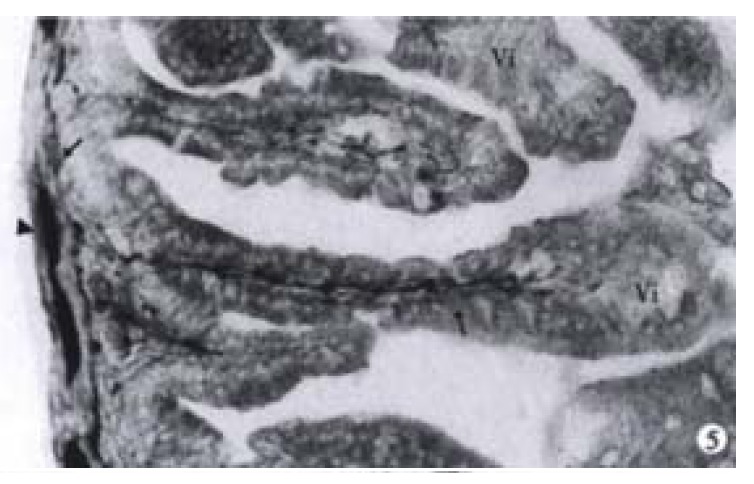
At ED 18, the nerve fibers (long arrow) stretching from the enteric plexuses reached the gut villi (Vi). The myenteric (arrowhead) and submucosal (arrow) plexuses were clear. × 160
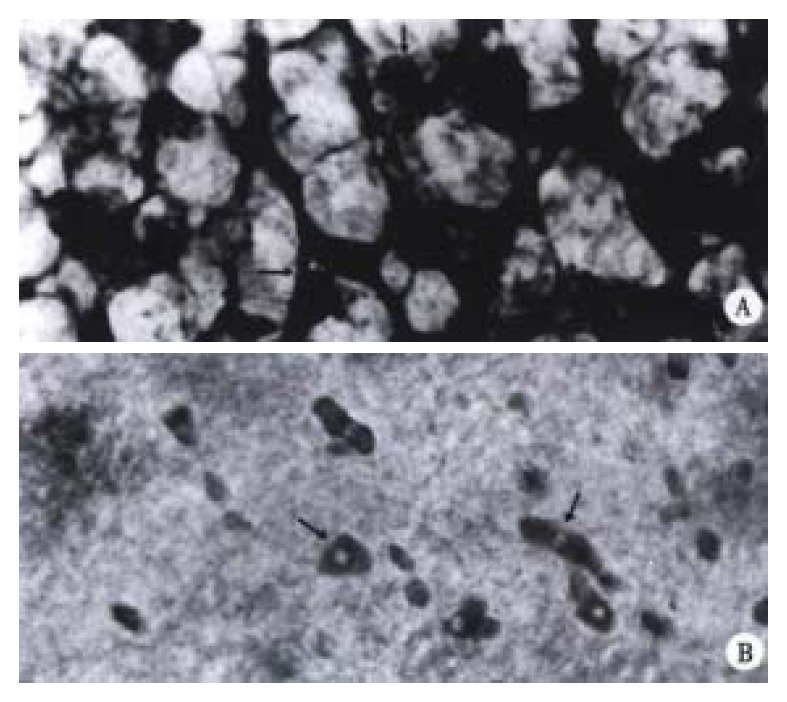
When compared with the normal littermates, the developmental delay of the colon- innervation was observed in trisomy 16 mice. Some neurons appeared with a scattered distribution and light staining at ED15 (Figure 3B) and the neurons were found with some nerve processes by which irregular nervous meshwork formed at ED16. In the colon of trisomy 16 mice at ED17 and ED18, only one plexus was visible, i.e., the myenteric plexus with a few small-sized ganglions (Figure 4B). From ED14 to ED18 there was no submucosal layer in colon, and 5 mm aganglionic bowel was found at the distal end of the colon in trisomy 16 mice (Figure 6), which was revealed as a characteristic morphological evidence of the development of congenital megacolon in trisomy 16 mice. This important finding was constantly verified in all the experimental trisomy 16 mice.
Figure 6.
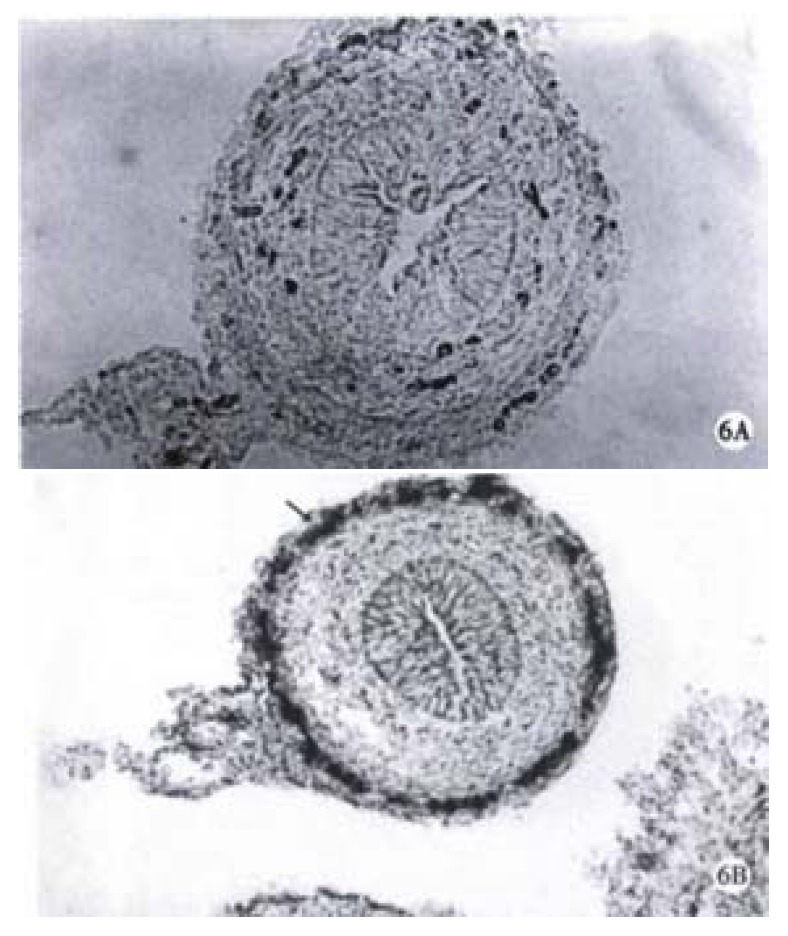
Aganglionic segment of trisomy 16 mouse colon. × 160 B. The colon of normal littermate with ganglia (arrow). × 100
The exogenous innervation of colon in trisomy 16 mice and their littermates
As early as ED14, PGP 9.5 positive nerve fibers occurred in the mesentery of both trisomy 16 mice and their normal littermates, which gradually increased with the embryonic development. At ED17 and ED18, rich mesenteric nerve fibers were detected. In all whole mounts of PGP 9.5 nerve fibers in mesentery, they were observed regularly running in parallel and close to the blood vessels and innervated the colon in both trisomy 16 mice and their normal littermates (Figure 7). Thus, the arcades of the mesenteric vessels were clearly delineated by their immunostained perivascular plexuses.
Figure 7.
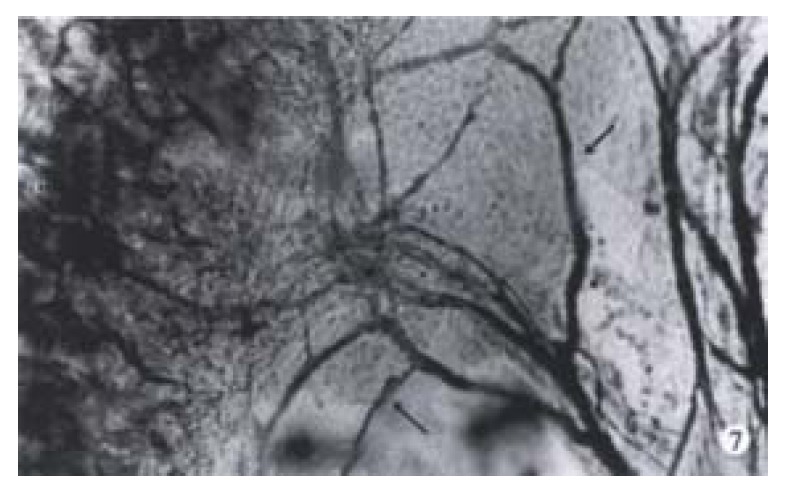
The myenteric nervous fibers (arrow) were detected in the colon (C) mesentery of trisomy 16 mice. × 160
The distribution of PGP 9.5 positive nerve fibers in the stenosed segment of HD and normal colons
The ganglions of the muscular plexus and submucosal plexus from the normal colon all revealed highly positive reaction to PGP 9.5 (Figure 8). The number of ganglion in muscular plexus was greater than that in the submucosal ones. The neurons presented a dark brown color and gathered as a mass. The adjacent neurons extended processes, by which they were connected with each other. PGP 9.5 positive nerve fibers were distributed in all intestinal layers. The richest part was in the circular muscular layer and parallel to the muscular fibers. In the proper mucous membrane, nerve network was formed around the intestinal glands. In the submucosal layer, the nerve fibers were arranged around the vessels. But in the stenosed segment of HD colon, no ganglionic cell was observed in the muscular and submucosal plexuses. At the distal end of the stenosed segment of the colon, no nerve plexus was found. Occasionally, a few neurons existed, but with abnormal morphology, while PGP 9.5 positive nerve fibers were great in number in all intestinal layers. Big, strong, disordered and even twisted nerve tracts could be observed (Figure 9). Such disorderly arranged nerve fibers could also be found in the submucosal layer and proper mucous membrane layer of the colon. Some of the proliferated fibers were twisted along the vessels.
Figure 8.
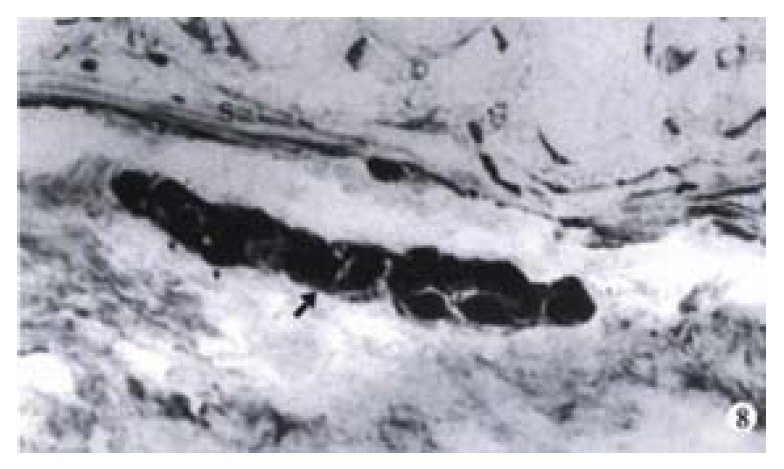
The submucosal ganglia (arrow) of normal colon of human. S: submucosal muscular layer. × 100
Figure 9.
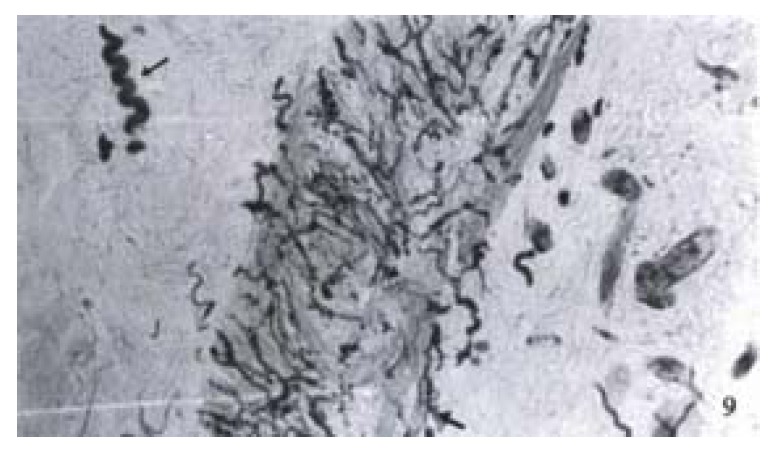
The aganglionic colon of Hirschprung’s disease. Abundant proliferation of nerve fibers (large arrow) was found in the stenosed segment of colon. A wave-like nerve fiber (arrow). × 100
DISCUSSION
The incidence of HD is 1/5000 due to the developmental defect of enteric nervous system[20-23]. Recently, HD incidence is increasing, especially the genetic HD and Down’s syndrome with congenital megacolon[24-29], which greatly aroused researchers’ interest in investigating the causes. The present study was based on PGP 9.5 as primary antibody to reveal the innervation of the narrowed gut in HD and the nerve development of the colon in trisomy 16 mice.
Enough evidences have been obtained from this study that trisomy 16 mice had a significant anomaly and developmental delay in colon innervation as well as 5 mm aganglionic colon at the distal end, which was the result of the chromosome abnormality. Our present experiment, found that there was a chronological sequence in the nerve development and a clear link between the gestational age and the morphological development in colon innervation of trisomy 16 mice and their normal littermates (Table 1). At ED14, the precursor cells of ectoderm migrated into the gut along the course of the vagus nerves; at ED15, nerve processes were observed in the neuron migrated gut; at ED16, the muscular plexuses and ganglions appeared in the gut; at ED17, the precursor cells in muscular layer migrated into the submucosal layer and developed into submucosal nervous plexuses; at ED18, the relatively matured muscular and submucosal plexuses occurred, the former controls the intestinal peristalsis, and the latter manages the intestinal secretions and absorption[30,31]. The lack of submucosal nerve plexuses of trisomy 16 mice accounted for the lower material absorption and poor intestinal secretions while the distal aganglionic colon could result in the neurotrophic and innervating disorders. It is now recognized that the intestinal nerve development begins from the cephalic part to the caudal end[32-41]. But HD was due to the stagnated development of the precursor cells. The earlier the stagnation of the precursor cells, the longer the aganglionic bowel. Since colon is the last segment of gut for the precursor cells to migrate into, colon is most likely to develop HD. The aganglionic colon in trisomy 16 mice strongly confirmed the above findings. The euchromosome 16 of trisomy 16 mice has the same sequence as the 21q22 genes of human euchromosome 21[8]. It is often used as an animal model for Down’s syndrome. Besides, trisomy 16 mice have constant genetic tendency and aganglionic colon, being easy to develop congenital megacolon. Epstein and associates believed that the intestinal innervation was composed of intrinsic and extrinsic ones[42]. The well-developed mesenteric nervous system of trisomy 16 mice could be regarded as a compensation for the intrinsic nerves. When there were no ganglionic cells in the intestine, the extrinsic nerve fibers would proliferate greatly. In our experiment, considerable PGP 9.5 positive nerve fibers were verified in the stenosed HD colon, which was obviously the compensative nerve proliferation to the innervation of aganglionic HD colon. Since trisomy 16 mice had severe cardiovascular abnormalities, multi-organ deformities[43-45] and could hardly survive after delivery, so in the present study, the distal colon end of trisomy 16 mice showed no such extrinsic compensating nerve proliferations.
Table 1.
The development of the colon and mensenteric innervation of trisomy 16 mice and their normal littermates in chronological sequence
|
ED13 |
ED14 |
ED15 |
ED16 |
ED17 |
ED18 |
|||||||
| N | T | N | T | N | T | N | T | N | T | N | T | |
| PM | - | - | + | - | ++ | + | ++ | + | +++ | ++ | +++ | ++ |
| PS | - | - | - | - | - | - | + | - | ++ | - | +++ | - |
| MF | - | - | + | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
N: Normal littermates; T: Trisomy 16 mice. PM: Myenteric plexus; PS: Submucosal plexus; MF: Mesentery nervour fiber; -: No neurons and immunoreactive nerve fibers detected; +: A few scattering neurons and no enteric plexus or sparse immunoreactive fibers detected; +: Lowly developed enteric plexus or moderate immunoreactive fibers detected; +++: Moderately developed enteric plexus or abundant immunoreactive fibers detected; ++++: Fully developed enteric plexus or very abundant immunoreactive fibers detected.
Among all clinically verified HD cases, 20% were caused by the lack of genetic factors[46]. Trisomy 16 mice, with its constant chromosome abnormality, is a reliable animal model for studying the genetic tendency of HD. The regional microcircumstance was closely related to the changes of the genetic inheritance. Tennyson and associates have found two striking abnormalities, i.e., an overgrowth of the muscularis mucosa, particularly in the outer longitudinal layer and an extensive thickening of the basal lamina around smooth muscle cell.
These excessive accumulation of basal lamina material and muscularis mucosa interfered with normal migration of precursor cells[47-51]. Payette and associates[52] thought that the excessive accumulation of components of basal lamina material in the aganglionic region of the lethal spotted mutant mouse stopped precursor cells from migrating into the terminal bowel of lethal spotted mutant mice. Our study revealed that 5 mm aganglionic bowel in length aparting from anus in trisomy 16 mice, might be caused by the change of regional micro-circumstance due to the abnormal chromosome, finally resulting in the nerve defect.
Footnotes
Supported by the Grants of Analysis and Measurement of Zhejiang Province and Education Committee of Zhejiang Province.
Edited by Ma JY
References
- 1.Li JC, Mi KH, Busch LC, Liu WG, Kühnel W. The expression of protein gene product 9 . 5 in trisomy 16 mouse colon and Hirschsprung's disease. Zhongguo Bingli Shengli Zazhi. 1999;15:1030–1033. [Google Scholar]
- 2.Miyabara S. Animal model for congenital anomalies induced by chromosome aberrations. Cong Anom. 1990;30:49–68. [Google Scholar]
- 3.Epstein CJ, Cox DR, Epstein LB. Mouse trisomy 16: an animal model of human trisomy 21 (Down syndrome) Ann N Y Acad Sci. 1985;450:157–168. doi: 10.1111/j.1749-6632.1985.tb21490.x. [DOI] [PubMed] [Google Scholar]
- 4.Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kääriäinen H. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 5.Pelet A, Attie T, Goulet O, Eng C, Ponder BA, Munnich A, Lyonnet S. De-novo mutations of the RET proto-oncogene in Hirschsprung's disease. Lancet. 1994;344:1769–1770. doi: 10.1016/s0140-6736(94)92908-4. [DOI] [PubMed] [Google Scholar]
- 6.Martucciello G, Favre A, Takahashi M, Jasonni V. Immunohistochemical localization of RET protein in Hirschsprung's disease. J Pediatr Surg. 1995;30:433–436. doi: 10.1016/0022-3468(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 7.Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fékété C, Ponder BA, Munnich A. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR, Epstein CJ. Comparative gene mapping of human chromosome 21 and mouse chromosome 16. Ann N Y Acad Sci. 1985;450:169–177. doi: 10.1111/j.1749-6632.1985.tb21491.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman DM, Bayney RM, Li YW, Khosrovi H, Berger CN, Epstein CJ, Mobley WC. Dysregulation of gene expression in mouse trisomy 16, an animal model of Down syndrome. EMBO J. 1992;11:619–627. doi: 10.1002/j.1460-2075.1992.tb05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyabara S, Gropp A, Winking H. Trisomy 16 in the mouse fetus associated with generalized edema and cardiovascular and urinary tract anomalies. Teratology. 1982;25:369–380. doi: 10.1002/tera.1420250314. [DOI] [PubMed] [Google Scholar]
- 11.Krammer HJ, Karahan ST, Sigge W, Kühnel W. Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg. 1994;4:274–278. doi: 10.1055/s-2008-1066117. [DOI] [PubMed] [Google Scholar]
- 12.Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kühnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181:327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- 13.Wedel T, Krammer HJ, Kühnel W, Sigge W. Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr Pathol Lab Med. 1998;18:57–70. [PubMed] [Google Scholar]
- 14.Watanabe Y, Ito F, Ando H, Seo T, Kaneko K, Harada T, Iino S. Morphological investigation of the enteric nervous system in Hirschsprung's disease and hypoganglionosis using whole-mount colon preparation. J Pediatr Surg. 1999;34:445–449. doi: 10.1016/s0022-3468(99)90496-7. [DOI] [PubMed] [Google Scholar]
- 15.Krammer HJ, Meier-Ruge W, Sigge W, Eggers R, Kühnel W. His-topathological features of neuronal intestinal dysplasia of the plexus submucosus in whole mounts revealed by immunohistochem-istry for PGP 9.5. Eur J Pediatr Surg. 1994;4:358–361. doi: 10.1055/s-2008-1066134. [DOI] [PubMed] [Google Scholar]
- 16.Jackson P, Thomson VM, Thompson RJ. A comparison of the evolutionary distribution of the two neuroendocrine markers, neurone-specific enolase and protein gene product 9.5. J Neurochem. 1985;45:185–190. doi: 10.1111/j.1471-4159.1985.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278:224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, Day IN, Thompson RJ, Polak JM. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol. 1988;69:91–104. [PMC free article] [PubMed] [Google Scholar]
- 19.Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, Yamamoto T, Yamamoto H, Ohi R, So N, Iwasaki Y. Immunocytochemical characterization of supporting cells in the enteric nervous system in Hirschsprung's disease. J Pediatr Surg. 1990;25:514–519. doi: 10.1016/0022-3468(90)90563-o. [DOI] [PubMed] [Google Scholar]
- 21.O'Kelly TJ, Davies JR, Tam PK, Brading AF, Mortensen NJ. Abnormalities of nitric-oxide-producing neurons in Hirschsprung's disease: morphology and implications. J Pediatr Surg. 1994;29:294–299; discussion 294-299. doi: 10.1016/0022-3468(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda T, Ueda M, Nakano M, Saeki M. Altered production of nerve growth factor in aganglionic intestines. J Pediatr Surg. 1994;29:288–292; discussion 292-293. doi: 10.1016/0022-3468(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 23.Neunlist M, Schemann M. Projections and neurochemical coding of myenteric neurons innervating the mucosa of the guinea pig proximal colon. Cell Tissue Res. 1997;287:119–125. doi: 10.1007/s004410050737. [DOI] [PubMed] [Google Scholar]
- 24.Hassold T, Merrill M, Adkins K, Freeman S, Sherman S. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am J Hum Genet. 1995;57:867–874. [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman SL, Petersen MB, Freeman SB, Hersey J, Pettay D, Taft L, Frantzen M, Mikkelsen M, Hassold TJ. Non-disjunction of chromosome 21 in maternal meiosis I: evidence for a maternal age-dependent mechanism involving reduced recombination. Hum Mol Genet. 1994;3:1529–1535. doi: 10.1093/hmg/3.9.1529. [DOI] [PubMed] [Google Scholar]
- 26.Zaragoza MV, Jacobs PA, James RS, Rogan P, Sherman S, Hassold T. Nondisjunction of human acrocentric chromosomes: studies of 432 trisomic fetuses and liveborns. Hum Genet. 1994;94:411–417. doi: 10.1007/BF00201603. [DOI] [PubMed] [Google Scholar]
- 27.Zheng CJ, Byers B. Oocyte selection: a new model for the maternal-age dependence of Down syndrome. Hum Genet. 1992;90:1–6. doi: 10.1007/BF00210736. [DOI] [PubMed] [Google Scholar]
- 28.Meijers JH, van der Sanden MP, Tibboel D, van der Kamp AW, Luider TM, Molenaar JC. Colonization characteristics of enteric neural crest cells: embryological aspects of Hirschsprung's disease. J Pediatr Surg. 1992;27:811–814. doi: 10.1016/0022-3468(92)90371-d. [DOI] [PubMed] [Google Scholar]
- 29.Barone V, Weber D, Luo Y, Brancolini V, Devoto M, Romeo G. Exclusion of linkage between RET and neuronal intestinal dysplasia type B. Am J Med Genet. 1996;62:195–198. doi: 10.1002/(SICI)1096-8628(19960315)62:2<195::AID-AJMG15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.O'Kelly TJ. Nerves that say NO: a new perspective on the human rectoanal inhibitory reflex. Ann R Coll Surg Engl. 1996;78:31–38. [PMC free article] [PubMed] [Google Scholar]
- 31.Joó F. A new generation of model systems to study the blood brain barrier: the in vitro approach. Acta Physiol Hung. 1993;81:207–218. [PubMed] [Google Scholar]
- 32.Tennyson VM, Pham TD, Rothman TP, Gershon MD. Abnormalities of smooth muscle, basal laminae, and nerves in the aganglionic segments of the bowel of lethal spotted mutant mice. Anat Rec. 1986;215:267–281. doi: 10.1002/ar.1092150310. [DOI] [PubMed] [Google Scholar]
- 33.Newgreen DF, Hartley L. Extracellular matrix and adhesive molecules in the early development of the gut and its innervation in normal and spotting lethal rat embryos. Acta Anat (Basel) 1995;154:243–260. doi: 10.1159/000147776. [DOI] [PubMed] [Google Scholar]
- 34.Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- 35.Levy J. The gastrointestinal tract in Down syndrome. Prog Clin Biol Res. 1991;373:245–256. [PubMed] [Google Scholar]
- 36.Kapur RP, Yost C, Palmiter RD. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116:167–175. doi: 10.1242/dev.116.Supplement.167. [DOI] [PubMed] [Google Scholar]
- 37.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 38.Epstein ML, Poulsen KT, Thiboldeaux R. Formation of ganglia in the gut of the chick embryo. J Comp Neurol. 1991;307:189–199. doi: 10.1002/cne.903070203. [DOI] [PubMed] [Google Scholar]
- 39.Kablar B. Structural study on the appearance of innervation in the stomach of mouse and rat embryos. Tissue Cell. 1995;27:309–315. doi: 10.1016/s0040-8166(95)80051-4. [DOI] [PubMed] [Google Scholar]
- 40.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 41.Hoehner JC, Wester T, Påhlman S, Olsen L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology. 1996;110:756–767. doi: 10.1053/gast.1996.v110.pm8608885. [DOI] [PubMed] [Google Scholar]
- 42.Epstein ML, Poulsen KT, Thiboldeaux R. Formation of ganglia in the gut of the chick embryo. J Comp Neurol. 1991;307:189–199. doi: 10.1002/cne.903070203. [DOI] [PubMed] [Google Scholar]
- 43.Miyabara S, Gropp A, Winking H. Trisomy 16 in the mouse fetus associated with generalized edema and cardiovascular and urinary tract anomalies. Teratology. 1982;25:369–380. doi: 10.1002/tera.1420250314. [DOI] [PubMed] [Google Scholar]
- 44.Webb S, Brown NA, Anderson RH. Cardiac morphology at late fetal stages in the mouse with trisomy 16: consequences for different formation of the atrioventricular junction when compared to humans with trisomy 21. Cardiovasc Res. 1997;34:515–524. doi: 10.1016/s0008-6363(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig M, Busch LC, Winking H. The embryonic development of sensory organs and the skull in the trisomy 16 mouse, an animal model for Down's syndrome. Ann Anat. 1997;179:525–533. doi: 10.1016/S0940-9602(97)80010-6. [DOI] [PubMed] [Google Scholar]
- 46.Robertson K, Mason I, Hall S. Hirschsprung's disease: genetic mutations in mice and men. Gut. 1997;41:436–441. doi: 10.1136/gut.41.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buselmaier W, Bacchus C, Sterz H. Genesis and systematization of cardiovascular anomalies in murine trisomy 16. Prog Clin Biol Res. 1991;373:203–214. [PubMed] [Google Scholar]
- 48.Leffler A, Ludwig M, Schmitt O, Busch LC. Germ cell migration and early development of the gonads in the trisomy 16 mouse--an animal model for Down's syndrome. Ann Anat. 1999;181:247–252. doi: 10.1016/S0940-9602(99)80039-9. [DOI] [PubMed] [Google Scholar]
- 49.Pomeranz HD, Gershon MD. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990;137:378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- 50.Payette RF, Tennyson VM, Pham TD, Mawe GM, Pomeranz HD, Rothman TP, Gershon MD. Origin and morphology of nerve fibers in the aganglionic colon of the lethal spotted (ls/ls) mutant mouse. J Comp Neurol. 1987;257:237–252. doi: 10.1002/cne.902570209. [DOI] [PubMed] [Google Scholar]
- 51.Fekete E, Resch BA, Benedeczky I. Histochemical and ultrastructural features of the developing enteric nervous system of the human foetal small intestine. Histol Histopathol. 1995;10:127–134. [PubMed] [Google Scholar]
- 52.Payette RF, Bennett GS, Gershon MD. Neurofilament expression in vagal neural crest-derived precursors of enteric neurons. Dev Biol. 1984;105:273–287. doi: 10.1016/0012-1606(84)90285-9. [DOI] [PubMed] [Google Scholar]