Abstract
AIM: To investigate the anti-HBV effect of oxymatrine (oxy) in vivo.
METHODS: HBV transgenic mice were produced by micro-injection of a 4.2 kb fragment containing the complete HBV genomes. Expression level of HBsAg and HBcAg in the transgenic mice liver was determined by immunohistochemical assay.
RESULTS: Four groups (6 mice in each group) were injected intraperitoneally with oxy at the dosage of 100, 200, and 300 mg/kg or with saline once a day for 30 d. Both HBsAg and HBcAg were positive in livers of all the six mice in the control group (injected with saline), and were positive in livers of two mice in 100 mg/kg group and 300 mg/kg group. In 200 mg/kg group, HBsAg and HBcAg were negative in livers of all the six mice. Based on the results, 200 mg/kg is the ideal dosage to explore the effect of oxy at different time points. According to the oxy treatment time, mice were divided into four groups: 10 d, 20 d, 30 d and 60 d (4 mice in each group). Each mouse underwent liver biopsy two weeks before the treatment of oxy. Down-regulation of HBsAg and HBcAg appeared after treatment of oxymatrine for 10 d and 20 d, Dane-like particles disappeared after the treatment of oxy for 20 d under electron microscopy, however, the expression level of HBsAg and HBcAg returned to normal 60 d later after oxy treatment.
CONCLUSION: oxymatrine can reduce the contents of HBsAg and HBcAg in transgenic mice liver,longer treatment time and larger dosage do not yield better effects.
Keywords: hepatitis B virus, antiviral agents, oxymatrine, hepatitis B surface antigens, hepatitis B core antigens, immunohistochemistry
INTRODUCTION
There are about 30 million patients suffering from chronic hepatitis B in China. Patients with active liver disease carry a high risk of developing cirrhosis and hepatocellular carcinoma. At present, the most effective medicine is IFN-α, but it is too expensive for most patients. Even in the patients treated by IFN-α, the sera negative-convertion rate of HBeAg and HBVDNA is about 40%[1-3]. Previous works in our department by Cai X et al[4] showed that the sera negative-convertion rates of HBeAg in patients with chronic active hepatitis B treated with oxymatrine was 61%. To investigate the inhibiting mechanism of oxymatrine (oxy) on HBV replication, transgenic mice were employed as animal model to observe the changes of HBsAg and HBcAg expression in liver tissues.
MATERIALS AND METHODS
Materials
Matrine injection is the product of Ningxia Pharmaceutical Company Ltd., containing 98% oxymatrine. The restriction enzymes were purchased from PROMEGA. Taq enzyme purchased from SANGON. Immunohistochemical kits were purchased from DAKO. Primers of PCR were designed by ourselves and synthesized by GIBCO. 32P labeled kit was the product of PROMEGA. Other reagents were purchased from WASON, HUAMEI and so on.
Methods
Preparation of animals HBV transgenic mice (official designation: ICR-TgN HBV adr1.2 SMMU) were produced by micro-injection of a 4.2 kb fragment containing the complete HBV genome (adr subtype)[5]. Structural analysis of the transgene revealed that at least one complete uninterrupted HBV genome was present. HBsAg and HBeAg were not detectable in the sera of the mice, but can be detected in livers by immunohistochemical assay (ABC), which was used to determine the expression level of HBV.
PCR and Southern-blot analysis Total tail genome DNA was analyzed by PCR using HBV-specific primers 5’CCCAATGGAACACTCACC [sense], 5’AGGAACCACTGAACAAATGGC [antisense]), generating a 380 bp fragment. Twenty mililiter of PCR products were analyzed by electrophoresis on a 1% agarose gel in the presence of 0.5 mg of ethidium bromide per mililiter. DNA bands were visualized by UV fluorescence. Southern-blot analysis was performed on total genomic DNA by agarose gel electrophoresis of 30 mg restricted genomic DNA. Samples added on nylon filters were hybridized with HBV specific 32P labeled DNA probes.
Histological analysis and electron microscopy were carried out as routine methods. The expression of HBsAg and HBcAg in liver tissues were assessed by immunohistochemical analysis according to Guidotti et al[6].
RESULTS
Effect of oxy at different doses on the expression of HBsAg and HBcAg Twenty-four age and sex-matched mice were divided into four groups. Each group was injected intraperitoneally with oxy at the dosage of 100, 200 and 300 mg/kg or with saline separately once a day for 30 d. Livers were harvested 2 hours after the last injection for immunohistochemical assay. Both HBsAg and HBcAg were positive in livers of all the six mice in the control group (injected with saline). In 100 mg/kg group, HBsAg and HBcAg were positive in two mice, while HBsAg and HBcAg were negative in the other four mice. In the 200 mg/kg group, both HBsAg and HBcAg were negative in all the six mice, none of the six mice had detectable HBV antigen in the livers. In the 300 mg/kg group, HBsAg and HBcAg were positive in two mice, and negative in the other four mice. No pathological changes were found in the transgenic mice. Based on the results, we considered that 200 mg/kg is the ideal dosage of oxy for further study.
The effect of oxy at different time on the expression of HBsAg and HBcAg
Mice were divided into four groups according to the oxy treatment time, 10 d (group 1), 20 d (group 2), 30 d and 60 d (group 3 and 4). In each group, 4 mice were randomly entered. Each mouse underwent liver biopsy two weeks before the treatment of oxy (200 mg/kg). The liver samples before and after oxy treatment were collected, and immunohistochemical analysis was performed to determine the expression level of HBsAg and HBcAg. All the samples contained HBsAg and HBcAg positive cells, and the positive and negative cells were counted in 5 randomly selected high field vision, and χ2 test was made to compare the HBV expression level before and after oxy treatment. In group 1, the number of HBsAg and HBcAg positive cells was significantly lower than before treatment of oxy in all the four mice livers. In group 2, the similar results were observed (Figure 1 and Figure 2), and Dane-like particles could be found in the livers before oxy treatment under electron microscope and such particles could not be found after the treatment of oxy for 20 d (Figure 3). In group 3, the expression of HBV was decreased only in two of four mice. No difference was observed on the expression of HBsAg and HBcAg between the two liver samples harvested before and after the treatment of oxy in group 4.
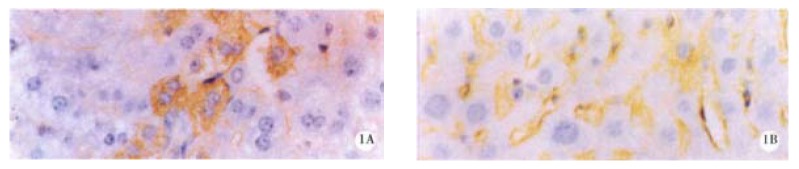
Figure 1.
HBsAg in liver of the mice before (A) and after (B) treatment with 200 mg/kg oxy for 20 d.
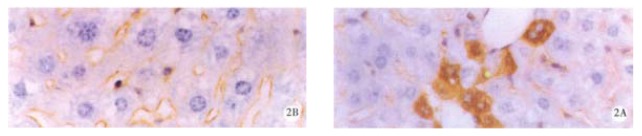
Figure 2.
HBcAg in liver of the mice before (A) and after (B) treatment with 200 mg/kg oxy for 20 d.
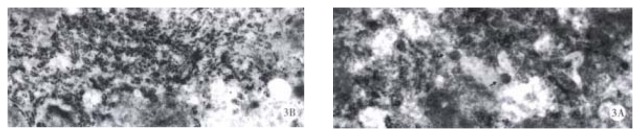
Figure 3.
Dane’s like particles can be seen in the liver of untreated mice (A) and disappeared after treatment with 200 mg/kg oxy for 20 d (B).
DISCUSSION
As a traditional Chinese medicine, Sophra Flavescens Ait has been used for the treatment of many diseases for thousands of years. Its extract, oxy, has long been extensively used in China. It is reported that oxy has a lot of pharmacological functions which can be divided into four classes: ① Anti-bacterial and anti-parasitic actions. It has been reported that oxy can cure acute dysentery, Trichomonas vaginalis and Giardia lamblia infection[7-9], but the mechanism is still unclear. ② Regulating immune reaction. Oxy can stimulate immune response at a low concentration while inhibiting immune response at a high concentration[10]. Recently, more researchers have paid attentions to the immune inhibitory effect of oxy. It has been reported that oxy has many functions such as anti-inflammation,anti-hypersensitivereactions,inhibiting histamine releasing[11-14]. The mechanism may be related to the changes of cAMP in the cell[15] and inhibiting production of cytokine[16]. ③ Inducing production of cytochrome P450. Oxy can increase the content of P450 in the rat liver significantly after treatment of oxy at the dosage of 200 mg/kg for 4d[17]. ④ Anti-virus actions. Liu JX reported that oxy could inhibit coxsackie virus B3 in vivo and in vitro [18,19]. Cai X found that the sera negative convertion rates of HBVDNA and HBeAg were 61.9% and 61.0% in chronic active hepatitis treated by oxy, while such rates in the therapy of IFN-α were 57.9% and 55.3%[4]. In our study, the content of HBV antigen in livers of transgenic mice decreased significantly after treatment of oxy for 10 and 20 d. Dane-like particles disappeared in the liver of transgenic mice after oxy treatment for 20 d. However, HBV expression level returned to normal after treatment by oxy for 60 d. We concluded that oxy can be used as an effective drug in managing HBV infection. There are two features of oxy on HBV: ① HBsAg and HBcAg was down regulated at the same time, ② longer time and larger dose did not yield better effect.
Our results strongly suggested that oxy can significantly inhibit the expression of HBV antigen in transgenic mice and the replication of HBV as well[20]. But how oxy give play to its effect can not be concluded from our experiments. However, based on the previous researches, it seems that oxy may act by two ways: ① oxy acts as an immune reaction regulator: Since HBV transgenic mice were first found by Chisari in 1985[21], in vivo study of HBV has become convenient and objective. Different lineage of HBV transgenic mice has also been found in our country[5,22]. A serial studies by Chisari et al[23-28] have shown that certain soluble products of the immune response, especially IFN-γ, TNF-α, IFN-α, IL-2 and IL-12 could suppress the steady-state content of HBV messenger RNA in the hepatocytes of transgenic mice. Furthermore, these effects were found to be mediated by a post-transcriptional mechanism that selectively accelerates the degradation of cytoplasmic HBV mRNA[27]. The same events were set in motion when HBsAg-specific CTL secreted IFN-γ and induced TNF-α after antigen recognition[23,29]. The interhepatic nucleocapsid particles and replicative intermediates were also eliminated during unrelated virus infection[30,31] or during hepatocellular regeneration after partial hepatectomy[32]. Oxy is a strong immune regulator, Wang HX has reported that oxy can inhibit the competence of LAK cell killing P815 cell by about 70%-80%[33], and Shang HS reported that oxy has the same effect of marcophage on P815 cell[34], which proved that oxy may be an agonist of IL-2. Thus, IL-2 could not be the mediator of oxy inhibiting HBV. Whether other cytokines may be the mediator remains unclear. ② Oxy acts as an inducer of cytochrome P450. HBV antigen is exogenous proteins in mice hepatocytes. mRNA of HBV in hepatocytes may be degraded by cytochrome P450. Therefore, oxy can induce the production and enhance the activity of cytochrome P450[17], hence accelerating the degradation of HBV mRNA and inhibiting HBV replication. Further study is needed.
Oxy is a broad-spectrum anti virus drug, at least to HBV and coxsackie B virus 3 so far. This may give us new hope for the treatment of chronic hepatitis HBV infection including other viral infection such as HCV and HIV infection.
Cirrhosis is a servere consequence of chronic HBV infection and preventing the development of cirrhosis is very difficult[35]. Gan LW et al[36] reported that oxy can inhibit the liver fibrosis induced by CCl4 in rats. Oxy can not only down-regulate HBV expression but also inhibit the liver fibrosis. Based on the two points, we concluded that oxy can be used as an effective drug in managing HBV infection. However, the exact mechanism of oxy inhibiting expression of HBV and liver fibrosis has not yet been fully understood. Further studies both basicaly and clinically are needed.
Footnotes
Supported by Projects of the Science Development Foundation of Shanghai, No.994919033 and Tackling Key Problems in Science and Technology from the State Science and Technology Ministry, TJ99-LA01.
Edited by Ma JY
References
- 1.Hoofnagle JH, Peters M, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Hallahan C, Park Y, Meschievitz C, Jones EA. Randomized, controlled trial of recombinant human alpha-interferon in patients with chronic hepatitis B. Gastroenterology. 1988;95:1318–1325. doi: 10.1016/0016-5085(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Wang YL, Shi L. Clinical analysis of the efficacy of inter-feron alpha treatment of hepatitis. World J Gastroenterol. 1998;4(Suppl 2):85–86. [Google Scholar]
- 3.Shi JJ, Miao F, Liu FL. Therapeutic effect of medicinal herbs and western drugs on hepatitis B virus. World J Gastroenterol. 1998;4(Suppl 2):61–62. [Google Scholar]
- 4.Cai X, Wang GJ, Qu Y, Fan CH, Zhang RQ, Xu WS. Clinical effi-cacy of kurorinone in the treatment of chronic hepatitis B. Dier Junyi Daxue Xuebao. 1997;18:47–49. [Google Scholar]
- 5.Hu WJ, Dai DS, Li JX, Wang XM, Sun W, Hao GR, Hu YP. The generation of hepatitis B virus transgenic mice homozygote. Dier Junyi Daxue Xuebao. 1999;20:598–600. [Google Scholar]
- 6.Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang ZW. Report of 60 case acute dysentery treated by matrine. Zhonghua Neike Zazhi. 1960;8:357–358. [Google Scholar]
- 8.Sun DX. Chinese medicine matrine can cure Trichomonas vaginalis. Zhonghua Fuchanke Zazhi. 1958;6:544. [Google Scholar]
- 9.Chen JL, Yu SZ, Wang HJ, Yang GJ, Wang PH, Shi Y. Report of 100 case Giardia lamblia infection treated by matrine. Zhonghua Neike Zazhi. 1965;13:614. [Google Scholar]
- 10.Qian YK, Cheung HT, Richardson A. Chinese herbs (SFA, LLA)act as immunoregulator to immune cells and cytokine (IL-2,IL-3) in vitro. Zhonghua Weishengwuxue He Mianyixue Zazhi. 1988;8:312–315. [Google Scholar]
- 11.Han CL, Chen XR, Ma JJ, Lin ZB. Study of oxymatrine s pharma-codynamic effect on allergic contact dermatitis. Beijing Yike Daxue Xuebao. 1996;28:59–61. [Google Scholar]
- 12.Liao J, Zhang BH. Anti-inflammatory effects of oxymatrine. Beijing Yike Daxue Xuebao. 1988;20:313–315. [Google Scholar]
- 13.Ma JJ, Si LP, Ding Y, Lin ZB, Chen XR, Han CL, Peng QS, Fang Y. Inhibition of type I-I allergic reactions by oxymatrine. Beijing Yike Daxue Xuebao. 1991;23:445–447. [Google Scholar]
- 14.Zhang Q, Zhao FS, Hao ZM, Yin JZ, Qian YK. Study of effects and its mechanisms of oxymatrine on histamine release from perito-neal mast cells. Zhonghua Weishengwuxue He Mianyixue Zazhi. 1992;12:41–44. [Google Scholar]
- 15.Wang HX, Yu XZ, Qian YK. The effects of oxymatrine on lym-phocytes' second messengers. Zhongguo Mianyixue Zazhi. 1993;9:315–317. [Google Scholar]
- 16.Ding GF, Shang HS, Xu H, Deng YL, Long ZZ, Han GQ. The study of monokines-the measurement of IL-1 from the macrophages activated by different agents. Zhongguo Mianyixue Zazhi. 1986;2:336–339. [Google Scholar]
- 17.Yuan C, Lu SQ, Yao X. [Effects of oxymatrine on the antitumor activity and toxicity of cyclophosphamide in mice] Yaoxue Xuebao. 1987;22:245–249. [PubMed] [Google Scholar]
- 18.Liu JX, Lu DY, Yang ZM, Chen SX, Qian FR. Preliminary studies on the role of anti coxsackie group B virus of Chinese medicine sophora flavescens. Shanghai Dier Yike Daxue Xuebao. 1991;11:140–142. [Google Scholar]
- 19.Chen FX, Liu JX, Lu DY. The effects of sophora flavescens alka-loids of coxsackie virus B3 in vitro and a preliminary study of its mechanism. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1995;9:115–117. [Google Scholar]
- 20.Tang RX, Gao FG, Zeng LY, Wang YW, Wang YL. Detection of HBV DNA and its existence status in liver tissues and peripheral blood lymphocytes from chronic hepatitis B patients. World J Gastroenterol. 1999;5:359–361. doi: 10.3748/wjg.v5.i4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985;230:1157–1160. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- 22.Hu YP, Yao YC, Li JX, Wang XM, Li H, Wang ZH, Lei ZH. The cloning of 3'-truncated preS/S gene from HBV genomic DNA and its expression in transgenic mice. World J Gastroenterol. 2000;6:734–737. doi: 10.3748/wjg.v6.i5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilles PN, Fey G, Chisari FV. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992;66:3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38–40. doi: 10.3748/wjg.v5.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidotti LG, Guilhot S, Chisari FV. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1994;68:1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilhot S, Guidotti LG, Chisari FV. Interleukin-2 downregulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism. J Virol. 1993;67:7444–7449. doi: 10.1128/jvi.67.12.7444-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh VJ, Guidotti LG, Chisari FV. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidotti LG, Borrow P, Hobbs MV, Matzke B, Gresser I, Oldstone MB, Chisari FV. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guidotti LG, Matzke B, Chisari FV. Hepatitis B virus replication is cell cycle independent during liver regeneration in transgenic mice. J Virol. 1997;71:4804–4808. doi: 10.1128/jvi.71.6.4804-4808.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HX, Zhang LH, Huang Y, Qian YK. Effect of oxymatrine on LAK cell activity. Mianyixue Zazhi. 1994;10:17–19. [Google Scholar]
- 34.Shang HS, Zhao J, Chen WF. The effect of matrine on macroph-age inhibiting P815 cell proliferation. Beijing Yike Daxue Xuebao. 1986;18:127–130. [Google Scholar]
- 35.Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha(1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267–269. doi: 10.3748/wjg.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan LW, Wang GJ, Li YL. Effects of oxymatrine on liver fibrosis in rats. Dier Junyi Daxue Xuebao. 1999;20:445–448. [Google Scholar]