Abstract
AIM: To study the inhibitory effects of VES (RRR-α-tocopheryl Succinate, VES), a derivative of natural Vitamin E, on benzo(a)pyrene(B(a)P)-induced forestomach tumor in female mice.
METHODS: The model of B(a)P-induced forestomach tumor was established according to the methods of Wattenberg with slight modifications. One hundred and eighty female mice (6 weeks old) were divided into six groups equally; negative control (Succinic acid), vehicle control (Succinate + B(a)P),positive control(B(a)P), high VES(2.5 g/kg.b.w+B(a)P), low VES (1.25 g/kg.b.w + B(a)P) ig as well as VES by ip (20 mg/kg.b.w + B(a)P). Except the negative control group, the mice were administrated with B(a)P ig. and corresponding treatments for 4 weeks to study the anti-carcinogenetic effect of VES during the initiation period. The experiment lasted 29 weeks, in which the inhibitory effects of VES both on tumor incidence and tumor size were tested.
RESULTS: The models of B(a)P-induced forestomach tumor in female mice were established successfully. Some were cauliflower-like, others looked like papilla, even a few were formed into the ulcer cavities. VES at 1.25 g/kg.b.w, 2.5 g/kg.b.w. by ig and 20 mg/kg.b.w. via ip could decrease the number of tumors per mouse (1.7 ± 0.41, 1.60 ± 0.34 and 1.1 ± 0.43), being lower than that of B(a)P group (5.4 ± 0.32, P < 0.05). The tumor incidence was inhibited by 18.2%, 23.1% and 50.0%. VES at 1.25 g/kg.b.w., 2.5 g/kg.b.w. by ig and 20 mg/kg.b.w. via ip reduced the total volume of tumors per mouse (54.8 ± 8.84, 28.4 ± 8.32 and 23.9 ± 16.05), being significantly lower than that of B(a)P group (150.2 ± 20.93, P < 0.01). The inhibitory rates were 63.5%, 81.1% and 84.1%, respectively.
CONCLUSION: VES has inhibitory effects on B(a)P-induced forestomach carcinogenesis in female mice, especially by ip and it may be a potential anti-cancer agent in vivo.
Keywords: stomach neoplasms, antineoplastic agents, vitamin E, transforming growth factors, apoptosis, benzo(a)pyrene
INTRODUCTION
RRR-α-Tocopheryl Succinate (referred to Vitamin E Succinate, VES) is a derivative of natural vitamin E (RRR-α-tocopheryl)[1]. The interest in this derivative of vitamin E is the fact that it can inhibit the proliferation of a variety of tumor cells in vitro[2]. Tumor cells responsive to VES antiproliferative effects include human monoblastic leukemia cells[3], murine B-16 melanoma cells[4], human prostatic adenocarcinoma cells[5], avian lymphoid cells[6,7], human promyelocytic cells[8], human breast cancer cells[9,10]and murine EL4 T lymphoma cells[11,12].
Although the mechanisms whereby VES inhibits the proliferation of rapidly dividing cells are not well understood, induction of cell cycle blockage[13], increasing secretion and activation of potent negative growth factors, i.e., transforming growth factor-βs and their type II- cell surface receptors [2,7,11,14], and induction of apoptosis[15-17] have been observed in VES-treated cells. Earlier studies in our laboratory using the SGC-7901 cell lines (human gastric cancer cells) as model indicated that VES has antagonistic effects on cell growth and DNA synthesis[18], and can obviously induce apoptosis of SGC-7901 cells[19,20].
To date, VES has not been extensively studied on its antitumor properties as well as its mechanism of action in vivo. Schwartz J and co-workers[21] found that injection of VES directly into the tumor-bearing buccal pouch of hamsters (twice a week for four weeks at a dose of 250 μg/injection) caused chemically induced oral epidermoid carcinomas to regress. As VES is found to be a potential chemopreventive and chemotherapeutic agent, it is of interest to ascertain the manner in which tumor growth is inhibited in VES-treated mice and to better understand the relationship between the structure and the function involved. This study characterizes the ability of VES to inhibit B(a)P-induced forestomach carcinogenesis in mice, addresses the involvement of different administration ways in this experiment, and shows that VES is capable of lowering both the tumor incidence and the total tumor size. The inhibitory effects of VES may be attributed to its intact compound.
MATERIALS AND METHODS
Materials
Reagents VES (purity > 98%) was obtained from Sigma Co. Ltd, 10% buffered formalin phosphate (10% formalin in neutral phosphate buffer) was purchased from Shanghai Chemical Co. (China), and B(a)P (purity > 98%) from Fluka Chemical Co. Ltd (Switzerland).
Animals Female Kunming mice (4-5 weeks old) were purchased from the Animal Center of the Cancer Institute in Heilongjiang Province, China. Fifteen mice were placed in a plastic cage and the animals were maintained under the following standard conditions: 22 °C ± 2 °C, 45% ± 10% relative humidity, and 12 h light/12 h dark cycles each day. All animals were fed with basic diet (purchased from the Center of Animal Experiment, Heilongjiang, China) and water.
Methods
B(a)P-induced forestomach tumorgenesis Female Kunming mice (6 weeks old) were divided into six groups, each group was composed of 30 mice. Succinic acid, B(a)P and VES were dissolved in corn oil. In group 1 (negative control) and group 2 (vehicle control), the mice were intubated with 1 g/(kg b.w.) succinic acid 4 times per week for 4 weeks. In all groups except group 1, the mice were intubated with 1 mg per mouse B(a)P twice a week for 4 weeks. The mice in group 3 (positive control) were given nothing except B(a)P. Groups 4 and 5 were given 2. 5 g/(kg b.w.) or 1.25 g/(kg b.w.) VES by the same way 4 times/week for 4 weeks. Group 6 was given 20 mg/(kg b.w.) VES via ip twice a week for 4 weeks. The mice were then sacrificed at week 11, 16 and 29 after the first administration of B(a)P. Buffered formalin-phosphate (10%) was immediately injected into the stomach by intubation into the mouth so that the stomach was distended and fixed. Each stomach was removed and placed on a plastic sheet and the number of tumors in each forestomach was counted. The samples were stored in 10% buffered formalin-phosphate for histological examination.
Tumor volume All tumors were examined with the aid of a magnifying lens, and tumor size was measured. As described previously[22], tumor volume was determined by measuring the three dimension size of all tumors using the average of the three measurements to calculate radium. Tumor volume was calculated with the formula: volume = 4/3πR3
Histological examination of tumors Tumors found by visual examinations were further confirmed histologically. The stomach samples were excised, fixed in 10% buffered formalin-phosphate, embedded in paraffin and processed for histologic slides with hematoxylin and eosin (HE) staining. Slides were read blindly by a pathologist and the tumors were classified according to the pathological principle.
Statistical analysis
The significance of data was determined by t test.
RESULTS
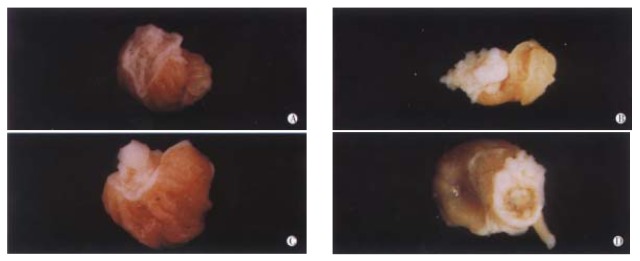
Establishment of B(a)P-induced forestomach tumor model On the basis of previous method with some slight modifications, the model of B(a)P-induced forestomach tumor was successfully established. Treatment of Kunming mice with 1 mg/mouse of B(a)P by ig twice a week for 4 weeks mainly resulted in four forms of tumors (Figure 1). Some bigger ones appeared cauliflower-like, the moderate tumors looked like papilloma, and the smaller ones were usually grain-like. A few tumors had broken into ulcer-like cavities.
Figure 1.
The model of B(a)P-induced forestomach tumor. A. Normal mucosa of forestomach; B. Cauliflower-like tumor; C. Papilla-like tumor; D. Ulcer cavities-like tumor.
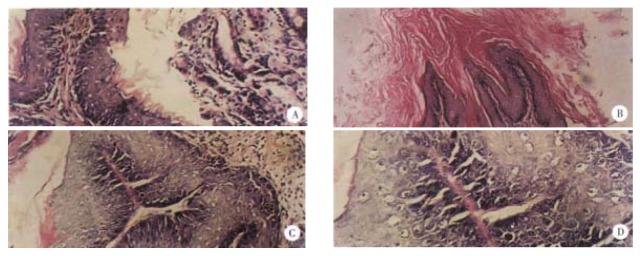
The dynamic processes of tumorigenesis in pathology at the week 11, 16 and finally 29 were observed. Before the treatment with B(a)P, the thickness of normal gastric mucosa was uniform with all characterizstics of gastric mucosa in previous studies[23-25]. The pathological changes at week 11 after the first administration of B(a)P were mainly the hyperplasia of the gastric mucosa, just as what we said “precancerous lesions”[26], and only a few papillomas could be seen. Under this condition, the thickness of epidermins was not uniform, some positions were very thin, while others were very thick. The nuclei of hyperplastic neoplastic cells were enlarged, round or ovoid with prominent necleoli. At week 16, the dominant features were papillomas, being more serious than those at week 11, and the proportion of squamous cell carcinomas in the positive group (B(a)P) was 20%, which was more malignant than before. Tumor cells proliferated lumpy into the connective tissues and were polyhedral, with abundant cytoplasms and large nuclei, in which nucleolus was prominent and some were polynucleolar. The desmosomes were clear, the mitotic figures and a few keratinized cells could be seen. The typical pathological alterations of B(a)P-induced forestomach tumor at the week 29 were that the number of papilloma and squamous cell carcinoma were both increased. The pathological changes of B(a)P-induced forestomach tumor are illustrated in Figure 2 and the dynamic processes of tumorigenesis in each period are shown in Table 1.
Figure 2.
Pathological observation of B(a)P-induced forestomach tumor (HE staining) in mice. A. The normal gastric mucosa × 200; B. The epithelium of gastric mucosa formed papillary projection × 100; C. Squamous tumor cells proliferated into connective tissues and they were polyhedral × 100; D. The mitotic figures (white arrow) in the squamous cell carcinoma × 400
Table 1.
Pathological alterations in each group at different periods of the experiment
| Groups |
Week 11 |
Week 16 |
Week 29 |
||||||
| H (%) | P (%) | S (%) | H (%) | P (%) | S (%) | H (%) | P (%) | S (%) | |
| 1. Succinic acid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. Succinic acid + B(a)P | 100 | 17.9 | 0 | 100 | 100 | 18.3 | 100 | 100 | 76.8 |
| 3. B(a)P | 100 | 9.6 | 0 | 100 | 100 | 19.9 | 100 | 100 | 70.4 |
| 4. 1.25 g/kgVES + B(a)P | 80.3 | 0 | 0 | 100 | 80.1 | 0 | 100 | 91.8 | 56.9 |
| 5. 2.5 g/kgVES + B(a)P | 79.7 | 0 | 0 | 100 | 60.4 | 0 | 100 | 90.3 | 49.8 |
| 6. 20 mg/kgVES(ip) + B(a)P | 69.7 | 0 | 0 | 100 | 49.7 | 0 | 100 | 80.7 | 18.4 |
H: Hyperplasia, P: Papillomas, S: Squamous cell carcinoma
Inhibitory effects of VES on B(a)P-induced forestomach tumorigenesis The treatment with 1mg/mouse-B(a)P twice a week for 4 weeks by ig in group 3 resulted in 100% incidence of forestomach tumors. There was an average of 5.4 tumors/mouse at week 29 of the experiment (Table 2, group 3). Similar results were obtained from group 2. The results in both groups showed that the succinic acid alone had no anticarcinogenic effect. Administration of 1.25 g/(kg b.w.) and 2.5 g/(kg b.w.) VES by ig 4 times/week for 4 weeks inhibited the number of B(a)P-induced forestomach tumors per mouse by 68.5% and 70.4%, respectively (Table 2, groups 4 and 5). When 20 mg/(kg b.w.) VES was injected intraperitoneally into the mice twice a week for 4 weeks, the number of B(a)P-induced forestomach tumors was inhibited by 79.6% (Table 2, group 6). These results suggested that VES could significantly decrease the number of B(a)P-induced tumors per mouse with a dose-dependent manner.
Table 2.
Inhibitory effects of VES on B(a)P induced forestomach tumorgenesis (x- ± sx-)
| Groups | No.of mice | Body weight (g) |
Total tumors |
|
| Tumors/mouse | % of mice with tumors | |||
| 1. Succinic acid | 12 | 39.5 ± 1.58 | 0 | 0 |
| 2. Succinic acid + B(a)P | 9 | 40.2 ± 1.12 | 5.3 ± 0.48 | 100 |
| 3. B(a)P | 10 | 40.6 ± 1.14 | 5.4 ± 0.32 | 100 |
| 4. 1.25 g/kgVES + B(a)P | 11 | 40.1 ± 1.22 | 1.7 ± 0.41a (68.5) | 81.8 (18.2) |
| 5. 2.5 g/kgVES + B(a)P | 13 | 39.9 ± 1.66 | 1.6 ± 0.34a | 76.9 (23.1) |
| 6. 20 mg/kgVES(ip) + B(a)P | 10 | 38.7 ± 1.06 | 1.1 ± 0.43a (79.6) | 50.0 (50.0) |
Compared with B(a)P group (group 3), P < 0.05 (using Student t test) Numbers in parentheses are % of inhibition compared to group 3.
Inhibitory effects of VES on the size of B(a)P-induced forestomach tumor Administration of 1.25 g/(kg b.w.) and 2.5 g/(kg b.w.) VES by ig significantly decreased the total tumor volume per mouse by 63.5% and 81.1%, respectively. Notably, the treatment with VES via ip at a much lower dose of 20 mg/(kg b.w.) and shorter time of twice a week for 4 weeks showed even higher rate of decreasing the total tumor volume per mouse (84.1%) (Table 3).
Table 3.
Inhibitory effects of VES on the size of B(a)P-induced forestomach tumors (x- ± sx-)
| Groups | No.of mice | Vol/tumor (mm3) | Total tumor Vol/mouse (mm3) |
| 1. Succinic acid | 12 | 0 | 0 |
| 2. Succinic acid + B(a)P | 9 | 31.5 ± 4.51 | 169.5 ± 33.42 |
| 3. B(a)P | 10 | 27.8 ± 8.52 | 150.2 ± 20.93 |
| 4. 1.25 g/kgVES + B(a)P | 11 | 31.7 ± 8.62 (0) | 54.8 ± 8.84b (63.5) |
| 5. 2.5 g/kgVES + B(a)P | 13 | 17.6 ± 5.54 (36.7) | 28.4 ± 8.32b |
| 6. 20 mg/kgVES (ip) + B(a)P | 10 | 21.7 ± 2.13 (21.9) | 23.9 ± 16.05b (84.1) |
Compared with the B(a)P group (group 3), P < 0.01 (using Student’s t test)ª¤Numbers in parentheses are % of inhibition of tumors compared to group 3.
DISCUSSION
The results of our study demonstrated that administration of VES to mice, by both ig. and ip., inhibited B(a)P-induced forestomach tumorigenesis during the initiation period. VES may be useful in the future as a chemopreventive agent of carcinogenesis. A study in vitro indicated that VES is able to inhibit the proliferation of tumor cells without adverse effects on non-tumor cells[27]. Kline et al[2] showed that VES did not inhibit the proliferation of normal murine bone marrow cells and the non-mitogen-stimulated normal avian B and T cells. They also reported that VES can retard the growth of MCF-7, a kind of human breast cancer cell, while it has no inhibitory effects on MCF-10A, a non-tumorigenic mammary cell. Since 1997, we have studied the antagonistic effects of VES on gastric cancer, and found the inhibitory effects of VES on the growth of human gastric cancer cell (SGC-7901), DNA synthesis arrest and induction of apoptosis[18,19]. These findings also supported the possibilities of VES as prospective chemopreventive and chemotherapeutic agent of tumors in the future. VES given intraperitoneally was more effective than given by ig. in lowering both the number and size of B(a)P-induced forestomach tumors even at a much lower dose. Therefore, it seems that VES administrated by ig. may be decomposed by some non-specific esterases which may normally exist in stomach, thus losing its original intact structure. VES contains a succinic acid moiety attached to the chroman head structure of RRR-α-tocopherol via an ester linkage. Esterification eliminates the hydroxy moiety that mediates RRR-α-tocopherol’s classical antioxidant properties that prevents VES from acting as an antioxidant, unless the esterified succinic acid moiety is removed by cellular esterases, thereby generating free RRR-α-tocopherol. After VES was proteolyzed into succinic acid and Vitamin E, succinic acid had no inhibitory effects on B(a)P-induced forestomach tumors, as shown in group 2. Previous studies have proved that the inhibitory effect of Vitamin E on tumors was performed through its antioxidant functions, and the inhibitory effects of natural vitamin E on human promyelocytic leukemia (HL-60) cell proliferation were much weaker than that of VES in vitro. All these data suggest that the inhibitory effects of VES may be attributed to its intact structure, as suggested by Fariss and co-workers[27].
The mechanisms by which VES has the inhibitory effects on chemically induced forestomach tumorigenesis in mice are still unknown. VES may influence the metabolic activation and detoxification of carcinogens as well as the postinitiation phase of carcinogenesis[28-30]. B(a)P is a suspected human carcinogen and is known to cause tumors in the forestomach of mice. Singh and Hu et al[31-33] reported that GST plays a major role in the detoxification of the ultimate carcinogen of B(a)P (+) anti-BPDE. In our lab, VES has been found to increase the activity of glutathione S transferase (GST) which is phase II metabolic enzyme and inhibits the activity of ethoxyresorufin O-deethylase (EROD) of liver S-9 fraction in mice, a phaseIxenobiotic-metabolizing enzyme. This was the same as the results of Wu[34,35] and Seng[36]. During the developing process of cancer, the immune functions will be decreased[37-39]. Of course, this is a very complex process and it may be involved in many factors[40-42], such as IL-2, IL-4[43,44], TNF[45,46], TGF-β[47-52] and so on. In 1993, Kline et al[52] found that RRR-α-tocopheryl succinate could induce IL-2 production by avian splenic T lymphocytes and murine EL-4 thymic lymphoma cells. And they suggested that VES-induced IL-2 production may involve a mechanism other than antioxidant effects of Vitamin E. In addition, administration of VES to mice increased the serum levels of IL-2 and IL-4 (unpublished data). The information indicates that the mechanisms of VES in vivo may be related to the activation pathway of B(a)P metabolism in liver and regulation of the immune system.
Our findings that VES can inhibit chemically induced carcinogenesis in the forestomach of mice suggest a need for further studies on pharmacology and toxicology to determine whether VES may be a useful chemopreventive agent against human gastric carcinogenesis.
ACKNOWLEDGEMENTS
We would like to thank Dr. Wei Ping Yu for his technical assistance, and Dr. Dong Lai Wu for reading the pathological slides and taking the pathological photos. We are also grateful to Dr. Love for his critical review of this manuscript.
Footnotes
Supported by National Natural Science Foundation of China, No.39870662
Edited by Ma JY
References
- 1.Bendich A, Machlin LJ. Safety of oral intake of vitamin E. Am J Clin Nutr. 1988;48:612–619. doi: 10.1093/ajcn/48.3.612. [DOI] [PubMed] [Google Scholar]
- 2.Kline K, Yu WP, Zhao BH, Israel K, Charpentier A, Simmons-Menchaca M, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Prasad KN, editor. Santamaria L and Willams RM, eds. Nutrients in cancer prevention and treatment. Totowa NY: Humana; 1995. pp. 39–56. [Google Scholar]
- 3.Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111(Pt 4):435–441. doi: 10.1242/jcs.111.4.435. [DOI] [PubMed] [Google Scholar]
- 4.Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145–1151. doi: 10.1016/s0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- 5.Israel K, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits the proliferation of human prostatic tumor cells with defective cell cycle/differentiation pathways. Nutr Cancer. 1995;24:161–169. doi: 10.1080/01635589509514404. [DOI] [PubMed] [Google Scholar]
- 6.Kline K, Yu WP, Sanders BG. Vitamin E: Mechanisms of action as tumor cell growth inhibitors. In: Prasad KN, Cole WC, eds. Cancer and nutrition. IOS Press; 1998. pp. 37–53. [DOI] [PubMed] [Google Scholar]
- 7.Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171–185. doi: 10.1080/01635589509514405. [DOI] [PubMed] [Google Scholar]
- 8.Turley JM, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate modulation of human promyelocytic leukemia (HL-60) cell proliferation and differentiation. Nutr Cancer. 1992;18:201–213. doi: 10.1080/01635589209514221. [DOI] [PubMed] [Google Scholar]
- 9.Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668–2675. [PubMed] [Google Scholar]
- 10.Yu WP, Simmons-Menchaca M, You HH, Brown P, Birrer MJ, Sanders BG, Kline K. RRR-α -Tocopheryl Succinate induction of prolonged activation of c-jun amino-terminal kinase and c-jun during induction of apoptosis in human MDA-MB-435 breast can-cer cells. Mol Carcinogenesis. 1998;22:247–257. [PubMed] [Google Scholar]
- 11.Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137–149. doi: 10.1080/01635589609514436. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92–101. doi: 10.1080/01635589709514508. [DOI] [PubMed] [Google Scholar]
- 13.Boscoboinik D, Szewczyk A, Azzi A. Alpha-tocopherol (vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch Biochem Biophys. 1991;286:264–269. doi: 10.1016/0003-9861(91)90039-l. [DOI] [PubMed] [Google Scholar]
- 14.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917–1928. [PubMed] [Google Scholar]
- 15.Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881–890. [PubMed] [Google Scholar]
- 16.Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953–961. [PubMed] [Google Scholar]
- 17.Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163–170. doi: 10.1006/jsre.2000.5948. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Ren Y, Guo J. The effects of Vitamin E Succinate on the cyclic regulation protein of human gastric cancer cells. Weisheng Dulixue Zazhi. 1998;12:203–207. [Google Scholar]
- 19.Liu BH, Wu K. Study on the growth inhibition of Vitamin E Succinate in human gastric cancer cell. Aibian Jibian Tubian. 2000;12:79–81. [Google Scholar]
- 20.Wu K, Guo J, Shan YJ, Liu BH. Effects of Vitamin E succinate on inducing apoptosis of human gastric carcinoma cells. Weisheng Dulixue Zazhi. 1999;13:84–87. [Google Scholar]
- 21.Schwartz J, Shklar G. The selective cytotoxic effect of carotenoids and alpha-tocopherol on human cancer cell lines in vitro. J Oral Maxillofac Surg. 1992;50:367–373; discussion 373-374. doi: 10.1016/0278-2391(92)90400-t. [DOI] [PubMed] [Google Scholar]
- 22.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 23.Ji XL, Cheng YQ, Wang SQ. Gastroendoscopic biopsy diagnosis of mucosa-associated lymphoid tissue lyphoma. China Natl J New Gastroenterol. 1995;1:30–32. [Google Scholar]
- 24.Yin GY, He XF, Yin YF. Clinical and experimental study on gas-tric mucosal pathology, DNA, cAMP, and trace elements of pixu patients. China Natl J New Gastroenterol. 1996;2:44–50. [Google Scholar]
- 25.Yu JY, D' Adda T. Quantitative ultrastructure analysis of neuroen-docrine cells of gastric mucosa in normal and pathological conditions. China Natl J New Gastroenterol. 1996;2:155–157. [Google Scholar]
- 26.Zhang XC, Zhao FZ, Shan PY, Dai X, Tian DL. Ultrastructural effect of xiaopiling chongji on gastric precancerous lesions in rats. Xin Xiaohuabingxue Zazhi. 1996;4:669–671. [Google Scholar]
- 27.Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346–3351. [PubMed] [Google Scholar]
- 28.Gudi VA, Singh SV. Effect of diallyl sulfide, a naturally occurring anti-carcinogen, on glutathione-dependent detoxification enzymes of female CD-1 mouse tissues. Biochem Pharmacol. 1991;42:1261–1265. doi: 10.1016/0006-2952(91)90263-5. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Matsui M, Shirouzu Y, Fujita H, Yamana H, Shirouzu K. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann Surg. 1998;227:485–491. doi: 10.1097/00000658-199804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava SK, Hu X, Xia H, Awasthi S, Amin S, Singh SV. Metabolic fate of glutathione conjugate of benzo[a]pyrene-(7R,8S)-diol (9S,10R)-epoxide in human liver. Arch Biochem Biophys. 1999;371:340–344. doi: 10.1006/abbi.1999.1475. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Singh SV. Glutathione S-transferases of female A/J mouse lung and their induction by anticarcinogenic organosulfides from garlic. Arch Biochem Biophys. 1997;340:279–286. doi: 10.1006/abbi.1997.9915. [DOI] [PubMed] [Google Scholar]
- 32.Xia H, Pan SS, Hu X, Srivastava SK, Pal A, Singh SV. Cloning, expression, and biochemical characterization of a functionally novel alpha class glutathione S-transferase with exceptional activity in the glutathione conjugation of (+)-anti-7,8-dihydroxy-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Arch Biochem Biophys. 1998;353:337–348. doi: 10.1006/abbi.1998.0668. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava SK, Singhal SS, Hu X, Awasthi YC, Zimniak P, Singh SV. Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys. 1999;366:89–94. doi: 10.1006/abbi.1999.1217. [DOI] [PubMed] [Google Scholar]
- 34.Wu K, Leslie CL, Stacey NH. Effects of mutagenic and non-mutagenic aniline derivatives on rat liver drug-metabolizing enzymes. Xenobiotica. 1989;19:1275–1283. doi: 10.3109/00498258909043179. [DOI] [PubMed] [Google Scholar]
- 35.Wu K, Bonin AM, Leslie CL, Baker RS, Stacey NH. Genotoxicity and effects on rat liver drug-metabolizing enzymes by possible substitutes for 4,4'-methylene bis(2-chloroaniline) Carcinogenesis. 1989;10:2119–2122. doi: 10.1093/carcin/10.11.2119. [DOI] [PubMed] [Google Scholar]
- 36.Seng JE, Gandy J, Turturro A, Lipman R, Bronson RT, Parkinson A, Johnson W, Hart RW, Leakey JE. Effects of caloric restriction on expression of testicular cytochrome P450 enzymes associated with the metabolic activation of carcinogens. Arch Biochem Biophys. 1996;335:42–52. doi: 10.1006/abbi.1996.0480. [DOI] [PubMed] [Google Scholar]
- 37.Araghi-Niknam M, Zhang Z, Jiang S, Call O, Eskelson CD, Watson RR. Cytokine dysregulation and increased oxidation is prevented by dehydroepiandrosterone in mice infected with murine leukemia retrovirus. Proc Soc Exp Biol Med. 1997;216:386–391. doi: 10.3181/00379727-216-44186. [DOI] [PubMed] [Google Scholar]
- 38.Gammon MD, John EM, Britton JA. Recreational and occupational physical activities and risk of breast cancer. J Natl Cancer Inst. 1998;90:100–117. doi: 10.1093/jnci/90.2.100. [DOI] [PubMed] [Google Scholar]
- 39.Mullins DW, Burger CJ, Elgert KD. Paclitaxel enhances macrophage IL-12 production in tumor-bearing hosts through nitric oxide. J Immunol. 1999;162:6811–6818. [PubMed] [Google Scholar]
- 40.Liu Y, Lu MZ, Pan BR. Cells proliferation and regulation in diges-tive tumors. Xin Xiaohuabingxue Zazhi. 1997;5:532–533. [Google Scholar]
- 41.Yuan Y, Dong M, Lu P, Wang XJ, Jin CL, He AG. Restriction fragment length polymorphism of pepsinogen C gene in patients with stomach carcinoma and in its high risk population. China Natl J New Gastroenterol. 1996;2:223–225. [Google Scholar]
- 42.Yuan Y, Lin HZ, Zhang YC, Wang XJ, Wu YQ, Gao H, Wang L, Liu YH, Lu F, Lou SQ. Study on the pathogenetic effect of salted pork from a high risk area of stomach cancer in China. China Natl J New Gastroenterol. 1997;3:93–94. doi: 10.3748/wjg.v3.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belli F, Mascheroni L, Gallino G, Lenisa L, Arienti F, Melani C, Colombo MP, Parmiani G, Cascinelli N. Active immunization of metastatic melanoma patients with IL-2 or IL-4 gene transfected, allogeneic melanoma cells. Adv Exp Med Biol. 1998;451:543–545. doi: 10.1007/978-1-4615-5357-1_84. [DOI] [PubMed] [Google Scholar]
- 44.Saijo Y, Hong X, Tanaka M, Tazawa R, Liu SQ, Saijo K, Ohno T, Koike K, Ohkuda K, Satoh K, et al. Autologous high-killing cytotoxic T lymphocytes against human lung cancer are induced using interleukin (IL)-1beta, IL-2, IL-4, and IL-6: possible involvement of dendritic cells. Clin Cancer Res. 1999;5:1203–1209. [PubMed] [Google Scholar]
- 45.Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82:395–402. doi: 10.1002/(sici)1097-0142(19980115)82:2<403::aid-cncr21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- 47.Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 48.Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 49.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–197. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang ZH, Chen YL, Wang CD, Chen YG. Expressions of TGF-β 1 and TGF-β receptor I in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 1999;7:507–509. [Google Scholar]
- 51.Deng DH, Luo JY, Zhu HH, Li DL, Wang KM. The expression of TGF-β 1 in squamous oesophagus cancer and heteroproliferation. Xin Xiaohuabingxue Zazhi. 1997;5:465. [Google Scholar]
- 52.Kline K, Sanders BG. RRR-alpha-tocopheryl succinate inhibition of lectin-induced T cell proliferation. Nutr Cancer. 1993;19:241–252. doi: 10.1080/01635589309514255. [DOI] [PubMed] [Google Scholar]