Abstract
AIM: To demonstrate the relationship between H-ras oncogene and hepatocellular carcinoma (HCC) metastasis.
METHODS: Activated H-ras oncogene was transfected into SMMC 7721, a cell line derived from human HCC, by calcium phosphate transfection method. Some metastasis-related parameters were detected in vitro, including adhesion assay, migration assay, expression of collagenase IV (cIVase) and epidermal growth factor receptor (EGFR).
RESULTS: The abilities of H-ras-transfected cell clones in adhesion to laminin (LN) or fibronectin (FN), migration, cIVase secretion increased markedly, and the expression of EGFR elevated moderately. More importantly, these alterations were consistent positively with the expression of p21, the protein product of H-ras oncogene.
CONCLUSION: H-ras oncogene could induce the metastatic phenotype of HCC cell in vitro to raise its metastatic potential.
Keywords: liver neoplasms/pathology; carcinoma, hepatocellular/pathology; genes, ras; neoplasm metastasis
INTRODUCTION
Hepatocellular carcinoma (HCC) is common in China[1-6], and metastasis occurs early with poor prognosis[7-13]. Numerous studies on various human solid tumors have shown that H-ras oncogene is associated with tumor metastasis[14-21]. However, therelationship between H-ras and HCC metastasis remains an open question[22-25]. In the present study, we transferred activated H-ras genes into SMMC 7721, a cell line derived from human HCC, by the method of calcium phosphate transfection. The metastatic properties of ras-transfected clones were detected in vitro. This research was conducted to investigate the influence of H-ras oncogene on the metastatic characteristics of this liver cancer cell line from each link in the chain of tumor metastasis: adhesion-degration-migration, in order to reveal the relationship between H-ras oncogene and metastatic behavior of HCC cell.
METERIALS AND METHODS
Materials
Carrier plasmid pSV2-neo and recombinant plasmid pSV2-neo-ras (with activated H-ras DNA inserted at BamH I site) were gifts from Professor Luo, Director of Department of Biophysics, Fudan University. Human HCC cell line SMMC 7721 was provided by the Liver Cancer Institute, Zhongshan Hospital. Calcium phosphate transfection kit was purchased from Promega Company. DACO-p21 ras antibody, purchased from Sigma Company, could recognize specifically the 126-140 amino-acids of C-terminal. Antibodies of cIVase and EFGR were products of Oncogene Company.
Methods
Transfer of recombinant plasmid into SMMC 7721 The method of calcium transfection, was used according to the protocol in the kit. Transfected clones were selected by G418.
Southern blotting The presence of the transfected ras oncogene in the DNA of the clones was assessed by Southern blot. Briefly, total DNA was digested with BamH I, separated by electrophoresis in a 8 g·L-1 agarose gel, and transferred to nitrocellulose. The filter was then probed with H-ras-T24 DNA (6.6 kb BamH I fragment of plasmid pT24) which had been radiolabeled with[32P]dCTP. Following hybridization, the filter was washed and X-rayed.
Immunochemistry The avidin-biotin-peroxidase complex (ABC) method was employed to detect the expression of cIVase, EFGR and p21. The results were graded according to the percentage of positively stained cells: - less than 5%; + 5%-25%; ++ 25%-50%; +++ 50%-75%; and ++++ above 75%.
Cell adhesion assay The cell adhesion assay was performed as previously described by Busk et al. In brief, some wells of polystyrene 96-well flat-bottom microtiter plates were coated with increasing concentrations of laminin (LN) or fibronectin (FN), and additional wells with poly-lysine (positive control) or 10 g·L-1 bovin-serum albumin (BSA) (negative control) respectively. Cells were added after all the plates were blocked with 10 g·L-1 BSA. The plates were then incubated for 2 h at 37 °C in humidified CO2. Non-adherent cells were removed and the attached cells were fixed and stained. The relative number of cells in each well was evaluated by measuring the absorbance (A) at 595 nm with a Microplate Reader. The percentage of cells attached to the experimental wells was calculated according to the formula as follows: [A (experimental well) - A (mean of BSA-coated wells)]/[A (mean of poly-lysine well) - A (mean of BSA-coated wells)] × 100%
The data were expressed as -x ± sx of triplicate wells.
Cell migration assay The wound assay described by Birch et al was used to determine the random migration capacity of various clones. Cells were plated into the wells of 24-well plates and incubated until the cultures were subconfluent. A wound track (approximately 4 mm in size) was scored in each well. Replicate wells were terminated at 8, 16 and 24 h after wounding by fixing and staining the cell cultures with 10 g·L-1 crystal violet in methanol. The stained cells were then examined under an inverted microscope.
RESULTS
Identification of transfected cell clones
The four clones transfected with recombinant plasmid pSV2-neo ras (named RC1, RC2, RC3 and RC4) and the two clones transfected with carrier plasmid pSV2-neo (named NC1 and NC2), along with SMMC 7721, were tested for both Southern blot analysis and p21 expression. The presence of the transfected ras oncogene in RC1-RC4 was confirmed by Southern blotting, while it was absent in NC1, NC2 and SMMC 7721. The immunochemistry staining showed that the percentage of positive stained cells of RC1-RC4 was 71%, 76%, 55% and 49%, respectively. However, it was less than 5% in SMMC 7721, NC1 and NC2. The staining grade of these cell clones is presented in Table 1. The results showed that H-ras DNA had been transferred into SMMC 7721 successfully and it could express its protein product normally.
Table 1.
Expression of p21, cIVase and EGFR in different cell clones
|
Staining grade |
|||||||
| SMMC 7721 | NC1 | NC2 | RC1 | RC2 | RC3 | RC4 | |
| p21 | - | - | - | +++ | ++++ | +++ | ++ |
| cIVase | + | + | + | +++ | ++++ | +++ | +++ |
| EGFR | + | + | + | +++ | +++ | ++ | ++ |
Detection of metastasis-related parameters
Adhesive ability When ras transfected clones were assessed for their ability to bind LN or FN, it was found that there was a substantial difference in the adhesive capabilities of these variants (Figure 1). The attachment percentage of RC1-RC4 to LN increased by different degree as compared with SMMC 7721, the maximal was up to 69.7%, 74.4%, 38.5% and 55% respectively. Similar results were observed in adhesion assay to FN. The adhesive capabilities of NC1 and NC2 had no significant difference from that of SMMC 7721, suggesting that the carrier plasmid itself had no effect on cell metastatic properties.
Figure 1.

Attachment of different cell clones to LN or FN (-x ± sx). A: To increasing concentrations (0, 0.8, 1.6, 2.4 and 3 mg·L-1) of LN B: To increasing concentrations (0, 5, 10, 20 and 30 mg·L-1) of FN
Migration assay The migration of the different clonal lines was analyzed by using the "wound" system in vitro. Wounds of approximately 4 mm were made in subconfluent monolayers of the different clones and cells were allowed to migrate into the cell-free area over a 24 h period. Representative experiments using three clones are illustrated in Figure 2. The cell-free areas were filled up with cells within 24 h in the tests of RC1-RC4, but they still remained empty even after 24 h in the tests of SMMC 7721, NC1 and NC2.
Figure 2.
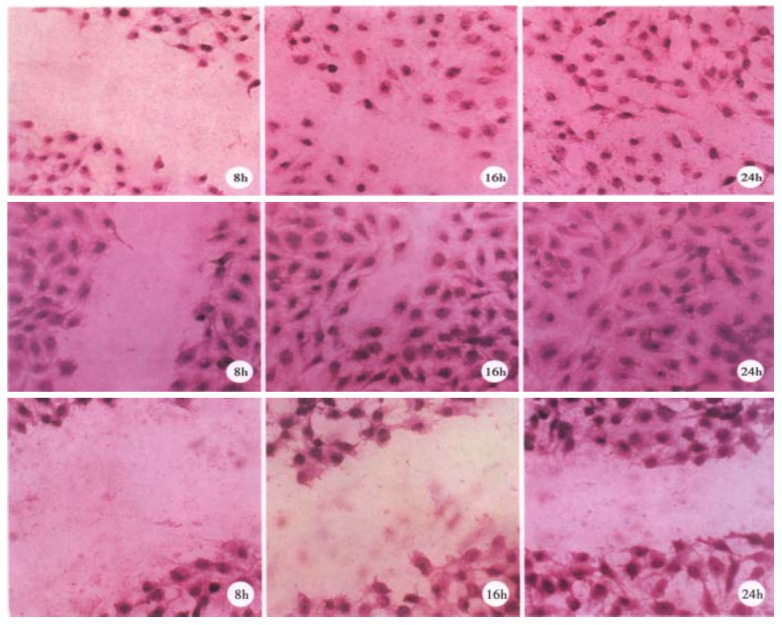
Migration ability of representative clones. Subconfluent monolayers of the clones were "wounded" at time 0. The cells were allowed to migration into the cell-free area for 24 h then fixed and stained with crystal violet. A: RC1; B: RC2; C: SMMC 7721
Expression of cIVaes and EGFR The expressions of cIVaes and EGFR were significantly different before and after ras transfection (Table 1) and these alterations were consistent positively with the expression of p21.
DISCUSSION
The process of tumor invasion and metastasis can be divided into three steps at molecular level: adhesion, degration and migration. This consecutively complex process involves many kinds of cytokines, enzymes and cell surface receptors[26-29]. Ras gene has been implicated in these processes through the signal transduction pathway[30-42]. In malignant tumors, cell-matrix interactions are very important for tumor invasion and metastasis. LN and FN, major components of the basement membrane, are involved in several biologic activities. We investigated the adhesive abilities of H-ras transfected SMMC 7721 cells to LN and FN. The results showed that the adhesive abilities of different cell clones raised in different degree. The reason that the adhesive ability of RC3 to LN had no significant increase as against SMMC 7721, may be contributed to the heterogeneity of transfected clones. Some data have shown that the property of transfected clones is not expressed stably and that heterogeneity may develope during the growth of the clones. Experimental studies with several different tumors have suggested that the instability causing the heterogeneity of metastatic properties is due to a variety of genetic and epigenetic processes.
cIVase is also associated with tumor metastasis[43]. It is considered that activated or overexpressed H-ras gene can induce the secretion and synthesis of cIVase directly. Ura et al revealed that the transcription level of cIVase gene was obviously higher in BEAS-2B cells transformed by H-ras gene than in their parent cells. The cIVase secretion ability of these cells increased, and metastatic behavior in vitro was positively related to cIVase secretion in vivo. Our results showed that the cIVase expression level increased markedly following ras-transfection, the percentage of positively stained cells increased 2 to 5 times after transfection. It was indicated that oncogenic p21 ras may upregulate translational efficiency by activating the eukaryotic translation initiation factor 4E (eIF-4E), thereby enhancing the protein expression of ras-induciable genes.
EGFR is known to be interrelated with and interact on ras gene in cell signal transduction pathway[44-47]. It has some effects on tumor cell attachment, secretion of proteolytic enzymes, cytoskeleton structure and cell migration[48-52]. We found that the expression of EGFR in ras-transfected clones increased moderately, and the expressions of EGFR and cIVase had definite relevance to p21 expression.
In summary, we have demonstrated that H-ras oncogene can induce the metastatic phenotype of human HCC cell in vitro, to raise its metastatic potential. The detections of some metastasis-related parameters, such as cell adhesion ability, migration ability, expressions of cIVase and EGFR may have predictive value in the metastatic potential of HCC clinically.
Footnotes
Qing Wang, earned master degree from Shanghai Medical University in 1996, now a senior lecturer of microbiology, specialized in the role of oncogenes on tumor metastasis, having 8 papers published.
Edited by Ma JY
References
- 1.Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234–238. doi: 10.3748/wjg.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. World J Gastroenterol. 1998;4(Suppl 2):4–7. [Google Scholar]
- 3.Wu MC. Clinical research advances in primary liver cancer. World J Gastroenterol. 1998;4:471–474. doi: 10.3748/wjg.v4.i6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo D, Liu QF, Gove C, Naomov N, Su JJ, Williams R. Analysis of N-ras gene mutation and p53 gene expression in human hepatocellular carcinomas. World J Gastroenterol. 1998;4:97–99. doi: 10.3748/wjg.v4.i2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai WX, Zheng H, Ye QL. Combined measurement of serum tumor markers in patients with hepatocellular carcinoma. World J Gastroenterol. 1998;4:181–182. doi: 10.3748/wjg.v4.i2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol. 1999;5:111–115. doi: 10.3748/wjg.v5.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhang BH, Qian GX, Chen H, Wu MC. Blood AFPmRNA in patients with distant metastasis of human hepatocellular carcinoma (HCC) Huaren Xiaohua Zazhi. 1998;6(Suppl 7):143–144. [Google Scholar]
- 9.Hong ZY, Yu JL, Zhang YS, Gao Y. Relationship between the expression of matrix metalloproteinase-9, CD34 and the invasion-metastasis of hepatocellular cancer (HCC) Shijie Huaren Xiaohua Zazhi. 2001;9:170–174. [Google Scholar]
- 10.Zhang BH, Liu Y, Qian GX, Chen H, Wu MC. The prognostic significance of detection of AFPmRNA and AFP after HCC resected. Huaren Xiaohua Zazhi. 1998;6(Suppl 7):125–126. [Google Scholar]
- 11.Zhao SL, Pan XF, Lü XP. Primary hepatic carcinoma with extrahepatic metastasis and secondary hepatic carcinoma. World J Gastroenterol. 1998;4(Suppl 2):90–91. [Google Scholar]
- 12.Wang JH, Lin G, Yan ZP, Wang XL, Cheng JM, Li MQ. Stage II surgical resection of hepatocellular carcinoma after TAE: a report of 38 cases. World J Gastroenterol. 1998;4:133–136. doi: 10.3748/wjg.v4.i2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 14.Scharovsky OG, Rozados VR, Gervasoni SI, Matar P. Inhibition of ras oncogene: a novel approach to antineoplastic therapy. J Biomed Sci. 2000;7:292–298. doi: 10.1007/BF02253247. [DOI] [PubMed] [Google Scholar]
- 15.Boivin-Angèle S, Lefrançois L, Froment O, Spiethoff A, Bogdanffy MS, Wegener K, Wesch H, Barbin A, Bancel B, Trépo C, et al. Ras gene mutations in vinyl chloride-induced liver tumours are carcinogen-specific but vary with cell type and species. Int J Cancer. 2000;85:223–227. doi: 10.1002/(sici)1097-0215(20000115)85:2<223::aid-ijc12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Tsunematsu S, Saito H, Sato R, Morizane T, Ishii H. Role of H-ras gene in chronic liver damage in mice. By using transgenic mice carrying a human C-H-ras proto-oncogene without mutations. Biochem Mol Biol Int. 1997;42:371–379. doi: 10.1080/15216549700202771. [DOI] [PubMed] [Google Scholar]
- 17.Cochet O, Kenigsberg M, Delumeau I, Virone-Oddos A, Multon MC, Fridman WH, Schweighoffer F, Teillaud JL, Tocqué B. Intracellular expression of an antibody fragment-neutralizing p21 ras promotes tumor regression. Cancer Res. 1998;58:1170–1176. [PubMed] [Google Scholar]
- 18.Fujita M, Norris DA, Yagi H, Walsh P, Morelli JG, Weston WL, Terada N, Bennion SD, Robinson W, Lemon M, et al. Overexpression of mutant ras in human melanoma increases invasiveness, proliferation and anchorage-independent growth in vitro and induces tumour formation and cachexia in vivo. Melanoma Res. 1999;9:279–291. doi: 10.1097/00008390-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kim MS, Son MW, Kim WB, In Park Y, Moon A. Apicidin, an inhibitor of histone deacetylase, prevents H-ras-induced invasive phenotype. Cancer Lett. 2000;157:23–30. doi: 10.1016/s0304-3835(00)00465-1. [DOI] [PubMed] [Google Scholar]
- 20.Weijzen S, Velders MP, Kast WM. Modulation of the immune response and tumor growth by activated Ras. Leukemia. 1999;13:502–513. doi: 10.1038/sj.leu.2401367. [DOI] [PubMed] [Google Scholar]
- 21.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou H, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 22.Guo HB, Zhang QS, Chen HL. Effects of H-ras and v-sis overexpression on N-acetylglucosaminyltransferase V and metastasis-related phenotypes in human hepatocarcinoma cells. J Cancer Res Clin Oncol. 2000;126:263–270. doi: 10.1007/s004320050341. [DOI] [PubMed] [Google Scholar]
- 23.Chao HK, Tsai TF, Lin CS, Su TS. Evidence that mutational activation of the ras genes may not be involved in aflatoxin B(1)-induced human hepatocarcinogenesis, based on sequence analysis of the ras and p53 genes. Mol Carcinog. 1999;26:69–73. doi: 10.1002/(sici)1098-2744(199910)26:2<69::aid-mc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XD. Prevention and treatment of recurrences and metastases of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:260–261. [Google Scholar]
- 25.Zheng X, Lin Z, Liu Y. [Relationships between intrahepatic metastasis of hepatocellular carcinoma and in situ microenvironment, and the abundance of nm23-H1 H-ras mRNA] Zhonghua Zhongliu Zazhi. 1998;20:15–17. [PubMed] [Google Scholar]
- 26.Wittekind C. [Current concepts of metastasis classification] Zentralbl Chir. 2000;125 Suppl 1:1–4. [PubMed] [Google Scholar]
- 27.Ogata R. Type IV collagen and laminin enhance the motility, adhesion, and proliferation of hepatoma cells. Kurume Med J. 1998;45:11–20. doi: 10.2739/kurumemedj.45.11. [DOI] [PubMed] [Google Scholar]
- 28.Genda T, Sakamoto M, Ichida T, Asakura H, Hirohashi S. Loss of cell-cell contact is induced by integrin-mediated cell-substratum adhesion in highly-motile and highly-metastatic hepatocellular carcinoma cells. Lab Invest. 2000;80:387–394. doi: 10.1038/labinvest.3780043. [DOI] [PubMed] [Google Scholar]
- 29.Magee T, Marshall C. New insights into the interaction of Ras with the plasma membrane. Cell. 1999;98:9–12. doi: 10.1016/S0092-8674(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 30.Olson MF, Marais R. Ras protein signalling. Semin Immunol. 2000;12:63–73. doi: 10.1006/smim.2000.0208. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Alcoceba R, del Peso L, Lacal JC. The Ras family of GTPases in cancer cell invasion. Cell Mol Life Sci. 2000;57:65–76. doi: 10.1007/s000180050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glennon TM, Villà J, Warshel A. How does GAP catalyze the GTPase reaction of Ras A computer simulation study. Biochemistry. 2000;39:9641–9651. doi: 10.1021/bi000640e. [DOI] [PubMed] [Google Scholar]
- 33.Arase Y, Hiwasa T, Hasegawa R, Nomura J, Ito H, Suzuki N. Prevention of v-Ha-ras-dependent apoptosis by PDGF coordinates in phosphorylation of ERK and Akt. Biochem Biophys Res Commun. 2000;267:33–39. doi: 10.1006/bbrc.1999.1857. [DOI] [PubMed] [Google Scholar]
- 34.Zuber J, Tchernitsa OI, Hinzmann B, Schmitz AC, Grips M, Hellriegel M, Sers C, Rosenthal A, Schäfer R. A genome-wide survey of RAS transformation targets. Nat Genet. 2000;24:144–152. doi: 10.1038/72799. [DOI] [PubMed] [Google Scholar]
- 35.Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–969. [PubMed] [Google Scholar]
- 36.Joneson T, Fulton JA, Volle DJ, Chaika OV, Bar-Sagi D, Lewis RE. Kinase suppressor of Ras inhibits the activation of extracellular ligand-regulated (ERK) mitogen-activated protein (MAP) kinase by growth factors, activated Ras, and Ras effectors. J Biol Chem. 1998;273:7743–7748. doi: 10.1074/jbc.273.13.7743. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzo PS, Kung JW, Bottorff DA, Garfield SH, Stone JC, Blumberg PM. Phorbol esters modulate the Ras exchange factor RasGRP3. Cancer Res. 2001;61:943–949. [PubMed] [Google Scholar]
- 38.Park BJ, Park JI, Byun DS, Park JH, Chi SG. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 2000;60:3031–3038. [PubMed] [Google Scholar]
- 39.Shibayama H, Anzai N, Braun SE, Fukuda S, Mantel C, Broxmeyer HE. H-ras is involved in the inside-out signaling pathway of interleukin-3-induced integrin activation. Blood. 1999;93:1540–1548. [PubMed] [Google Scholar]
- 40.Delgado MD, Vaqué JP, Arozarena I, López-Ilasaca MA, Martínez C, Crespo P, León J. H-, K- and N-ras inhibit myeloid leukemia cell proliferation by a p21WAF1-dependent mechanism. Oncogene. 2000;19:783–790. doi: 10.1038/sj.onc.1203384. [DOI] [PubMed] [Google Scholar]
- 41.Futatsugi N, Hata M, Hoshino T, Tsuda M. Ab initio study of the role of lysine 16 for the molecular switching mechanism of Ras protein p21. Biophys J. 1999;77:3287–3292. doi: 10.1016/S0006-3495(99)77159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giehl K, Skripczynski B, Mansard A, Menke A, Gierschik P. Growth factor-dependent activation of the ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene. 2000;19:2930–2942. doi: 10.1038/sj.onc.1203612. [DOI] [PubMed] [Google Scholar]
- 43.Bu W, Tang ZY, Ye SL, Liu KD, Huang XW, Gao DM. Relationship between type IV collagenase and the invasion and metastasis of hepatocellular carcinoma. Zhonghua Xiaohua Zazhi. 1999;19:13–15. [Google Scholar]
- 44.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Lacaci I, Kannan S, De Santis M, Bianco C, Kim N, Wallace-Jones B, Ebert AD, Wechselberger C, Salomon DS. RAS transformation causes sustained activation of epidermal growth factor receptor and elevation of mitogen-activated protein kinase in human mammary epithelial cells. Int J Cancer. 2000;88:44–52. doi: 10.1002/1097-0215(20001001)88:1<44::aid-ijc7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh ET, Shepherd FA, Tsao MS. Co-expression of epidermal growth factor receptor and transforming growth factor-alpha is independent of ras mutations in lung adenocarcinoma. Lung Cancer. 2000;29:151–157. doi: 10.1016/s0169-5002(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 47.Charvat S, Chignol MC, Souchier C, Le Griel C, Schmitt D, Serres M. Cell migration and MMP-9 secretion are increased by epidermal growth factor in HaCaT-ras transfected cells. Exp Dermatol. 1998;7:184–190. doi: 10.1111/j.1600-0625.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 48.Hisaka T, Yano H, Haramaki M, Utsunomiya I, Kojiro M. Expressions of epidermal growth factor family and its receptor in hepatocellular carcinoma cell lines: relationship to cell proliferation. Int J Oncol. 1999;14:453–460. doi: 10.3892/ijo.14.3.453. [DOI] [PubMed] [Google Scholar]
- 49.Kira S, Nakanishi T, Suemori S, Kitamoto M, Watanabe Y, Kajiyama G. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17:177–182. doi: 10.1111/j.1600-0676.1997.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamazaki K, Yunoki Y, Tagashira H, Mimura T, Mori M, Orita K. Epidermal growth factor receptor in human hepatocellular carcinoma. Cancer Detect Prev. 1997;21:355–360. [PubMed] [Google Scholar]
- 51.Tang Z, Qin L, Wang X, Zhou G, Liao Y, Weng Y, Jiang X, Lin Z, Liu K, Ye S. Alterations of oncogenes, tumor suppressor genes and growth factors in hepatocellular carcinoma: with relation to tumor size and invasiveness. Chin Med J (Engl) 1998;111:313–318. [PubMed] [Google Scholar]
- 52.Shen Y, Vogel I, Kalthoff H. [Comparative study of metastasis-associated characteristics of tumor cells with different metastatic capacities] Zhonghua Zhongliu Zazhi. 2000;22:201–204. [PubMed] [Google Scholar]


