INTRODUCTION
The field of gastrointestinal hormones has expanded at a dizzying rate[1-4]. Gastrointestinal hormones as regulatory peptides that appear to be major components of bodily integration and have important regulatory actions on physiological function of the gastrointestinal tract. The successful isolation of some gastrointestinal hormones and the development of sensitive methods for their detection have led to the unexpected finding that they also exist in the brain. Recent studies[5-8] have indicated that some disorders of gastrointestinal tract are related to gastrointestinal hormones. We explored this relationship by measuring concentrations of gastrin, somatostatin, G cell and D cell.
MATERIALS AND METHODS
We used healthy adult male Sprague Dawley rats weighing 220 g ± 30 g. The rats were housed in individual cages in a temperature-controlled room (23 °C ± 2 °C) with a 12 h light-dark cycle. The rats were fed standard rat chow (provided by Experimental Animal Center, First Military Medical University) and water ad labium. Rats were acclimatized to the environment for 7 days prior to the experiments.
Reserpine was obtained from the Hongqi Pharmaceutical Factory (lot No. 960313). Si Junzi Tang, composed of ginseng, bighead, Fuling and Zhi Gancao, was prepared by routine method.
Fifty rats were randomly divided into five groups of ten rats each. Group A, the negative control group was injected with physiological saline, 0.5 mL·kg-1·d-1, ip and distilled water, 2 mL, twice daily, ig, for 14 days. Group B was injected with reserpine, 0.5 mL·kg-1·d-1, ip and distilled water, 2 mL, twice daily, ig, for 14 days. Group C was reserpine (0.5 mL·kg-1·d-1, ip) and Si Junzi Tang (2 mL, twice daily, ig), for 14 days. Group D was first injected with reserpine and distilled water for 14 days (similar to Group B). Group D was then injected with distilled water (2 mL twice daily, ig) for 6 days. Group E was first injected with reserpine and water for 14 days (similar to Group B). Group E was then injected with Si Junzi Tang (2 mL, twice daily, ig) for 6 days.
We collected samples of body fluids (intestinal juice[9], gastric juice[10], plasma) and tissues (gastric antrum, jejunum, hypothalamus[11]) after treatment was finished for each group. All samples were stored at -70 °C until analysis. All gastroduodenal sections collected were fixed with neutral-buffered 10% formalin and embedded in paraffin.
Gastrin levels in samples were measured with radioimmunoassay kits from the Chinese Atomic Energy Research Institute of Immunotechnology. Somatostatin levels were measured with radioimmunoassay kits from Dong-Ya Research Institute of Immunotechnology. G cells and D cells of gastroduodenal mucosa were analyzed with immunohistochemical technique (using polyclonal antibody to gastrin and somatostatin) and the Quantimet 500 image analysis system (Leica, USA). Staining was performed using a streptavidin/peroxidase kit (SPTM, ZYMED, USA). Immunostaining by replacing primary antibody with PBS was also conducted as a negative control. G cells and D cells of gastroduodenal mucosa were stained in continuous sections. Under magnification × 400, five randomly-selected cyclograms of each section were entered into the main computer of Quantimet 500 image analysis system from the photograph system. The main computer system analyzed graphs with corresponding software and obtained data of cell number, even square and even grey. The ratio of the cell number to square of G/D cells was calculated in continuous sections of G cells and D cells stained as follows: ratio of number of G/D cells = G cells number/D cells number; ratio of square on G/D cells = G cells square/D cells square.
Statistical analysis
Data were expressed as mean ± standard error of the mean. Experimental results were analyzed by analysis of variance and t tests for multiple comparisons. Statistical significance was determined at P < 0.05.
RESULTS
Levels of gastrin and somatostatin in body fluids and tissues
The levels of gastrin in all samples tested from Group B (treated with reserpine alone) were 20%-60% less than Group A (controls). Levels of gastrin in Group D (treated with reserpine, the water) were also less than in Group A, though higher than in Group B. Levels of somatostatin were 50%-200% higher the Group B than in Group A. Levels of somatostatin were also higher than in Group A, though not as high as in Group B (Table 1, Table 2, Table 3, Table 4).
Table 1.
Gastrin in body fluids (n = 10, -x ± s, ng/L)
| Group | Plasma | Gastric juice | Intestinal juice |
| A | 168.40 ± 38.01 | 48.72 ± 12.55 | 147.36 ± 31.47 |
| B | 109.46 ± 40.88a | 30.78 ± 6.81a | 96.58 ± 14.36a |
| C | 150.38 ± 31.75 | 51.17 ± 7.56b | 171.19 ± 25.28b |
| D | 119.48 ± 30.21a | 33.03 ± 6.64ac | 122.57 ± 31.61bc |
| E | 179.84 ± 64.77bd | 54.29 ± 7.05bd | 79.01 ± 33.31abd |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Table 2.
Somatostatin in body fluids (n = 10, -x ± s, ng/L)
| Group | Plasma | Gastric juice | Intestinal juice |
| A | 17.93 ± 4.46 | 9.70 ± 2.14 | 13.43 ± 3.08 |
| B | 32.56 ± 7.91a | 29.21 ± 4.58a | 25.74 ± 4.16a |
| C | 18.24 ± 4.02b | 11.69 ± 2.53b | 15.30 ± 3.31b |
| D | 20.87 ± 4.68b | 22.56 ± 4.99abc | 24.16 ± 4.50ac |
| E | 16.98 ± 3.53b | 7.64 ± 2.26bcd | 13.85 ± 2.19bd |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Table 3.
Gastrin in tissues (n = 10, -x ± s, ng/g)
| Group | Gastric antrum | Jejunum | Hypothalamus |
| A | 503.77 ± 74.32 | 38.57 ± 8.14 | 107.85 ± 16.36 |
| B | 232.61 ± 53.88a | 22.47 ± 3.02a | 68.09 ± 13.40a |
| C | 493.75 ± 91.20b | 35.76 ± 6.41b | 102.79 ± 12.29b |
| D | 372.31 ± 54.35abc | 28.46 ± 5.48abc | 89.95 ± 11.11abc |
| E | 535.67 ± 57.58bd | 41.31 ± 7.27bd | 110.03 ± 12.94bd |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Table 4.
Somatostatin in tissues (n = 10, -x ± s, ng/g)
| Group | Gastric antrum | Jejunum | Hypothalamus |
| A | 175.19 ± 26.24 | 23.50 ± 6.36 | 43.96 ± 6.45 |
| B | 367.15 ± 42.30a | 47.31 ± 10.97a | 66.76 ± 6.55a |
| C | 207.23 ± 34.08b | 21.00 ± 5.66b | 45.56 ± 5.57b |
| D | 327.94 ± 46.68abc | 25.88 ± 7.57b | 59.45 ± 6.02abc |
| E | 184.94 ± 57.58bd | 17.61 ± 5.12b | 39.98 ± 5.40bd |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Levels of both gastrin and somatostatin in Groups C (treated concurrently with reserpine Si Junzi Tang) and E (treated first with reserpine then Si Junzi Tang) were similar to those in Group A, indicating that Si Junzi Tang may provide a protective effect against the functional disturbance caused by reserpine.
Expression of G cells and D cells in gastroduodenal mucosa
G cells of gastric antrum were mainly located in the lower 2/3 of the mucosa and rarely in the upper 1/3. G cells appeared round, elliptical, fusiform, triangular or irregular. They differed from the typical endocrine cells of gastroduodenum in that they produced long cytoplasmic and processed the end with small bulbous expansions on the putative effector cells. G cells gave off long cytoplasmic and processed terminate on D cells and also on enterochromaffin cells (Figure 1 and Figure 2). D cells of gastric antrum were mainly located in the lower 1/3 of the mucosa and rarely in the upper 2/3 (Figure 3). Appearance of D cells was similar to that of G cells. G cells and D cells of the duodenum were mainly distributed in the intestinal glands and their appearances were similar to those of the gastric antrum. The number and even square of G cells and D cells declined, whereas the even grey of G cells and the ratio of the number and square on G/D increased in functional disturbance of gastrointestinal tract (Table 5, Table 6, Table 7).
Figure 1.
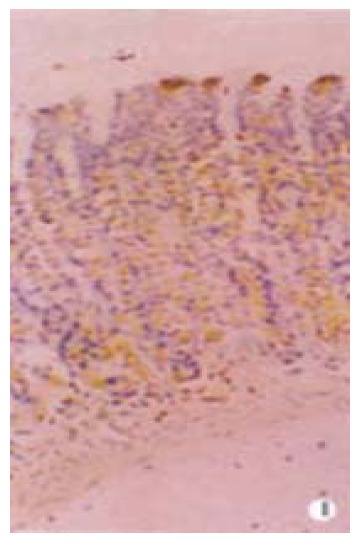
G cells of gastric antrum were mainly located in the lower 2/3 mucosa and rarely in the upper 1/3 mucosa. Magnification × 400
Figure 2.
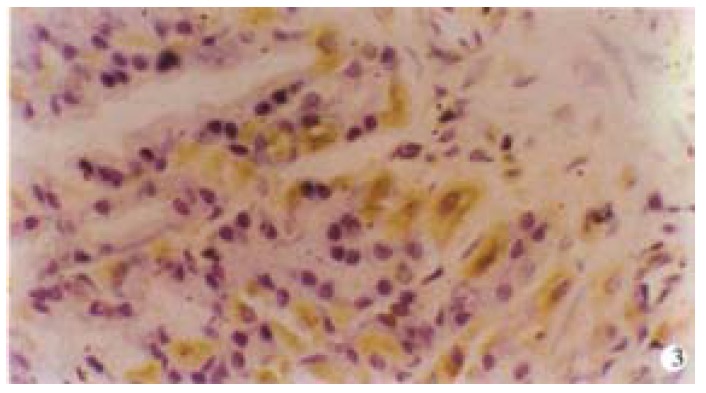
G cells appeared round, elliptical, fusiform, triangular or irregular. Magnification × 1000
Figure 3.
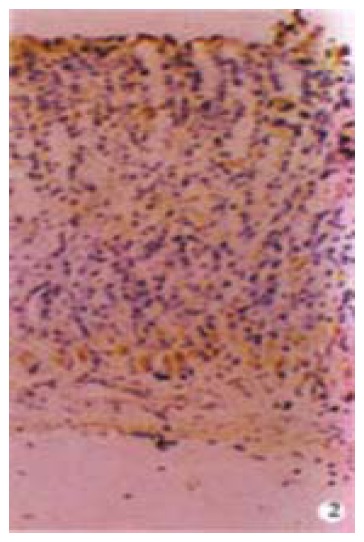
D cells of gastric antrum were mainly located in the lower 1/3 mucosa and rarely in the upper 2/3 mucosa. Magnification × 400
Table 5.
G cells and D cells of gastric antrum mucosa (n = 10, -x ± s)
| Group |
G cells |
D cells |
||||
| Number | Square (± 10-6 m2) | Even grey | Number | Square (× 10-6 m2) | Even grey | |
| A | 103.60 ± 12.33 | 98.05 ± 7.06 | 125.41 ± 2.29 | 15.67 ± 5.21 | 70.49 ± 8.96 | 124.04 ± 3.14 |
| B | 40.33 ± 5.53a | 93.47 ± 6.55 | 128.24 ± 3.03a | 5.70 ± 1.32a | 45.41 ± 5.27a | 115.38 ± 2.62a |
| C | 72.70 ± 10.14ab | 94.31 ± 5.77 | 125.90 ± 3.13b | 11.20 ± 2.55ab | 61.05 ± 7.11ab | 123.72 ± 3.10b |
| D | 50.63 ± 7.54abc | 87.54 ± 8.92abc | 123.15 ± 4.09abc | 8.30 ± 1.86abc | 43.54 ± 5.06ac | 123.27 ± 2.26b |
| E | 88.03 ± 10.37abcd | 97.16 ± 6.24d | 125.39 ± 2.22bd | 13.83 ± 3.11abcd | 72.71 ± 5.88bcd | 124.47 ± 2.46b |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Table 6.
G cells and D cells of jejunum mucosa (n = 10, -x ± s)
| Group |
G cells |
D cells |
||||
| Number | Square (× 10-6 m2) | Even grey | Number | Square (× 10-6 m2) | Even grey | |
| A | 63.57 ± 10.16 | 81.99 ± 9.75 | 125.44 ± 3.14 | 7.80 ± 2.58 | 60.56 ± 7.26 | 125.82 ± 1.70 |
| B | 29.43 ± 7.11a | 78.49 ± 6.94 | 130.24 ± 2.01a | 3.13 ± 1.14a | 41.80 ± 5.14a | 111.29 ± 3.26a |
| C | 54.83 ± 10.03ab | 73.63 ± 6.55ab | 124.58 ± 2.31b | 6.77 ± 1.98b | 53.92 ± 6.86ab | 125.94 ± 2.58b |
| D | 35.37 ± 9.16abc | 62.18 ± 6.07abc | 122.34 ± 2.56abc | 5.10 ± 1.92abc | 44.52 ± 6.02ac | 123.80 ± 2.96b |
| E | 65.10 ± 9.63bcd | 77.44 ± 7.69ad | 125.80 ± 2.45bd | 7.53 ± 2.26bd | 61.77 ± 10.27bcd | 125.47 ± 2.76abcd |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
Table 7.
Ratio of the number and square on G/D cells (n = 10, -x ± s)
| Group |
Gastric antrum |
Jejunum |
||
| Number (cells) | Square | Number (cells) | Square | |
| A | 6.48 ± 0.77 | 1.42 ± 0.24 | 8.43 ± 1.57 | 1.39 ± 0.20 |
| B | 7.20 ± 0.54a | 2.05 ± 0.37a | 9.82 ± 2.71a | 1.87 ± 0.30a |
| C | 6.47 ± 0.69 | 1.55 ± 0.26b | 8.25 ± 1.46b | 1.38 ± 0.21b |
| D | 5.95 ± 1.13b | 2.01 ± 0.30ac | 7.33 ± 1.16b | 1.18 ± 0.25bc |
| E | 6.81 ± 0.61d | 1.29 ± 0.13abcd | 8.53 ± 1.87b | 1.24 ± 0.25b |
P < 0.05 vs A,
P < 0.05 vs B,
P < 0.05 vs C,
P < 0.05 vs D.
DISCUSSION
Gastrin and somatostatin are both important gut hormones[12-21]. Edkins first discovered gastrin in 1905. However, the existence of gastrin was questioned until the middle of the 19th century. Gastrin is mostly distributed in the mucosa of gastric antrum, the mucosa of the jejunum, and the central nervous system. Gastrin has a wide range of biological actions[22-25]. The most potent actions of gastrin are stimulation of gastric acid secretion[26-29] and antral smooth muscle activity.
Somatostatin was originally isolated from extracts of sheep hypothalamus as a growth hormone inhibiting factor. In addition to its marked effect on GH secretion, the peptide[30-33] possesses a surprising range of biological effects paralleled by an equally wide but characteristic anatomical distribution. Somatostatin also appears to inhibit the secretion of many gastrointestinal hormones and may be an important regulator for gastrointestinal functions[34-37].
Upon functional disturbance of the gastrointestinal tract, we inferred[22-25] that the inhibition of the release of gastrin might lead to a decrease of basal and maximal gastric acid secretion and inhibition of the secretion of gastric proteinase and inner factor. We further anticipated a decrease in blood flow to the upper digestive tract mucosa, which would lead to a reduction in nutrition and proliferation of gastric mucosal cells. Somatostatin[30-33] inhibited not only secretion of basal gastric acid and gastric proteinase, but also the effect of gastrin on gastric acid secretion. Some studies have demonstrated that motilin[34] had a potent actions on the smooth muscle of the stomach, the duodenum, and the colon, which could enhance gastric emptying rate, colonic motility and nutrition absorption. We observed that somatostatin inhibited the gastric emptying rate, colonic motility and nutritional absorption by inhibiting motlin secretion following administration of reserpine. We inferred that decrease of gastrin release, increase of somatostatin release, and mutual regulatory disturbance can lead to a functional disturbance of gastrointestinal tract. Treatment with Si Junzi Tang, appeared to regulate the levels of gastrin and somatostatin in body fluids and tissues. Following administration of Si Junzi Tang, both gastrin and somatostatin remained or returned to normal level in the experimental functional disturbance of gastrointestinal tract model. This result indicates that Si Junzi Tang may be used to treat disorders of gastrointestinal hormones secretion.
Somatostatin[38] is also produced by endocrine-like (D) cells of the gut and pancreas and by peripheral nerve cells. Its actions are not restricted to the hypothalamo-hypophyseal system, somatostatin may also inhibit the release of several gastrointestinal and pancreatic hormones and affect on gastric HCl and pancreatic enzyme secretion. The bulk of evidence[38-40] suggests that somatostatin is delivered directly onto the membranes of G cells and parietal cells. In agreement with the cytochemical observations of D cells processing termination on G cells. Studies on the isolated perfused stomach have shown that infusion of somatostatin antiserum results in a brisk increase of gastrin release to up to 70 percent of maximally stimulated levels. These data suggest that G cells may be under tonic inhibitory control by somatostatin.
The reserpine-induced functional disturbance caused the number of G cells, D cells, and the even square of D cells to decline, whereas the even grey of G cells evaluated and ratio of the number and square on G/D increased[39-40]. These data suggest that high levels of somatostatin detected in body fluids and tissues were partly due to the release of somatostatin by D cells. High levels of somatostatin resulted in a decrease of gastrin release. This might explain the changes observed in G cells and D cells.
The study confirmed that changes of gastrin and somatostatin are associated with gastrointestinal disorder, which is one of the important causes for pathogenesis of the gastrointestinal tract.
Footnotes
Yong-Li Yao, graduated from First Military Medical University with a master degree in 1998. She now works in the Institute of Gastrointestinal Diseases, Nanfang Hospital as a doctoral candidate majoring gastrointestinal diseases and gastrointestinal hormones, she has published 8 papers.
Supported by the Military Science Foundation of China, No. 96M060
Edited by Jason Carr
References
- 1.Nauck MA. Is glucagon-like peptide 1 an incretin hormone. Diabetologia. 1999;42:373–379. doi: 10.1007/s001250051165. [DOI] [PubMed] [Google Scholar]
- 2.Pan XZ, Cai LM. Current status of Gastrointestinal hormones. Shijie Huaren Xiaohua Zazhi. 1999;7:464–466. [Google Scholar]
- 3.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 4.Straus E. Gastrointestinal hormones. Mt Sinai J Med. 2000;67:54–57. [PubMed] [Google Scholar]
- 5.MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999–1006. doi: 10.1093/ajcn/69.5.999. [DOI] [PubMed] [Google Scholar]
- 6.Zaĭtsev VT, Boĭko VV, Savvi SA, Taraban IA, Belozerov IA, Butkevich AIu. [The meaning of duodenostasis in the intestinal phase gastric secretion disorders in bleeding duodenal ulcer] Klin Khir. 1999;(3):5–6. [PubMed] [Google Scholar]
- 7.Coll P, Guttormsen AB, Berstad A. [Gastrointestinal disease with elevated plasma homocysteine level] Tidsskr Nor Laegeforen. 1999;119:3577–3579. [PubMed] [Google Scholar]
- 8.Yao YL, Zhang WD, Song YG. Relationship between Spleen deficiency and gastrointestinal hormones. Xin Xiaohuabingxue Zazhi. 1997;5:728–729. [Google Scholar]
- 9.Elson CO, Ealding W, Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- 10.Ye FX. Experimental zoology in medicine. First edition. Beijing: The People's Medical Publishing House; 1995. p. 227. [Google Scholar]
- 11.Lü XF, Lu RX, Ye XQ. Changes of β-endorphin in hypothalamus of yang insufficiency model and function of restoring yang drug. Zhongyi Zazhi. 1994;35:619–620. [Google Scholar]
- 12.Sawada M, Dickinson CJ. The G cell. Annu Rev Physiol. 1997;59:273–298. doi: 10.1146/annurev.physiol.59.1.273. [DOI] [PubMed] [Google Scholar]
- 13.Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979;205:1393–1395. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- 14.Dockray GJ. Topical review. Gastrin and gastric epithelial physiology. J Physiol. 1999;518(Pt 2):315–324. doi: 10.1111/j.1469-7793.1999.0315p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Weerth A, Bläker M, von Schrenck T. [Receptors for cholecystokinin and gastrin] Z Gastroenterol. 1999;37:389–401. [PubMed] [Google Scholar]
- 16.Epelbaum J, Dournaud P. [Somatostatin: a ubiquitous peptide] C R Seances Soc Biol Fil. 1998;192:597–606. [PubMed] [Google Scholar]
- 17.Reubi JC, Schaer JC, Markwalder R, Waser B, Horisberger U, Laissue J. Distribution of somatostatin receptors in normal and neoplastic human tissues: recent advances and potential relevance. Yale J Biol Med. 1997;70:471–479. [PMC free article] [PubMed] [Google Scholar]
- 18.Tuo BG, Yan YH, Ge ZL, Ou GW, Zhao K. Ascorbic acid secretion in the human stomach and the effect of gastrin. World J Gastroenterol. 2000;6:704–708. doi: 10.3748/wjg.v6.i5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie B, He SW, Wang XD. Effect of gastrin on protein kinase C and its subtype in human colon cancer cell line SW480. World J Gastroenterol. 2000;6:304–306. doi: 10.3748/wjg.v6.i2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lü B, Ye ZY, Zhu XL, Zhang Q, Du WD, Xiang BK. Study on gastrointestinal hormones in tissues and blood of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1429–1430. [Google Scholar]
- 21.Watson SA, Durrant LG, Morris DL. Growth-promoting action of gastrin on human colonic and gastric tumour cells cultured in vitro. Br J Surg. 1988;75:342–345. doi: 10.1002/bjs.1800750416. [DOI] [PubMed] [Google Scholar]
- 22.Ryberg B, Tielemans Y, Axelson J, Carlsson E, Håkanson R, Mattson H, Sundler F, Willems G. Gastrin stimulates the self-replication rate of enterochromaffinlike cells in the rat stomach. Effects of omeprazole, ranitidine, and gastrin-17 in intact and antrectomized rats. Gastroenterology. 1990;99:935–942. doi: 10.1016/0016-5085(90)90610-d. [DOI] [PubMed] [Google Scholar]
- 23.Eissele R, Patberg H, Koop H, Krack W, Lorenz W, McKnight AT, Arnold R. Effect of gastrin receptor blockade on endocrine cells in rats during achlorhydria. Gastroenterology. 1992;103:1596–1601. doi: 10.1016/0016-5085(92)91183-5. [DOI] [PubMed] [Google Scholar]
- 24.Kusyk CJ, McNiel NO, Johnson LR. Stimulation of growth of a colon cancer cell line by gastrin. Am J Physiol. 1986;251:G597–G601. doi: 10.1152/ajpgi.1986.251.5.G597. [DOI] [PubMed] [Google Scholar]
- 25.Wu P, Rui J, Xia XH, Yuan P, Zhou G, Wu CY. Gastrin stimulated growth of colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:501–503. [Google Scholar]
- 26.Kovacs TO, Walsh JH, Maxwell V, Wong HC, Azuma T, Katt E. Gastrin is a major mediator of the gastric phase of acid secretion in dogs: proof by monoclonal antibody neutralization. Gastroenterology. 1989;97:1406–1413. doi: 10.1016/0016-5085(89)90383-1. [DOI] [PubMed] [Google Scholar]
- 27.Chuang CN, Tanner M, Chen MC, Davidson S, Soll AH. Gastrin induction of histamine release from primary cultures of canine oxyntic mucosal cells. Am J Physiol. 1992;263:G460–G465. doi: 10.1152/ajpgi.1992.263.4.G460. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman SH. Ontogeny of gastric acid secretion in the rat: evidence for multiple response systems. Science. 1982;217:75–77. doi: 10.1126/science.6211765. [DOI] [PubMed] [Google Scholar]
- 29.Waldum HL, Sandvik AK, Brenna E, Petersen H. Gastrin-histamine sequence in the regulation of gastric acid secretion. Gut. 1991;32:698–701. doi: 10.1136/gut.32.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Chiba T, Yamada T. Mechanisms for direct inhibition of canine gastric parietal cells by somatostatin. J Biol Chem. 1987;262:14190–14196. [PubMed] [Google Scholar]
- 31.Koerker DJ, Ruch W, Chideckel E, Palmer J, Goodner CJ, Ensinck J, Gale CC. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science. 1974;184:482–484. doi: 10.1126/science.184.4135.482. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto E, Haruma K, Hata J, Tani H, Sumii K, Kajiyama G. Effects of octreotide, a somatostatin analogue, on gastric function evaluated by real-time ultrasonography. Aliment Pharmacol Ther. 1997;11:177–184. doi: 10.1046/j.1365-2036.1997.128298000.x. [DOI] [PubMed] [Google Scholar]
- 33.Koop H, Bothe E, Eissele R, Dionysius J, Arnold R. Somatostatin-gastrin interactions in the rat stomach. Res Exp Med (Berl) 1988;188:115–121. doi: 10.1007/BF01852267. [DOI] [PubMed] [Google Scholar]
- 34.Yao YL, Song YG, Zhang WD. Study on the relationship between motilin and enterorrhea of spleen deficiency. Shijie Huaren Xiaohua Zazhi. 1999;7:432–433. [Google Scholar]
- 35.Yao YL, Song YG, Zhang WD, Zhang DQ. Relationship between gastrin, somatostatin and functional disturbance of gastrointestinal tract. Shijie Huaren Xiaohua Zazhi. 1999;7:1016–1017. [Google Scholar]
- 36.Yao YL, Song YG, Zhang WD. Experimental study on the relationship between gastrin and somatostatin in body fluid and spleen asthenia syndrome. Zhongguo Zhongxiyi Jiehe Piwei Zazhi. 2000;8:333–335. [Google Scholar]
- 37.Zhang WD, Yao YL, Song YG. Variation in content of gastrin and somatostatin in experimental model of spleen asthenia syndrome and its significance. Zhongguo Zhongxiyi Jiehe Piwei Zazhi. 1998;6:223–225. [Google Scholar]
- 38.Glória H, Portela-Gomes M, Grimelius L, Ahlman H, Ferra MA. Effects of adrenalectomy on serotonin-, somatostatin-, and gastrin-immunoreactive cells in rat gastrointestinal tract. Dig Dis Sci. 1997;42:1216–1220. doi: 10.1023/a:1018802123553. [DOI] [PubMed] [Google Scholar]
- 39.Yao YL, Song YG, Zhang WD. Variations of G cells and D cells in experimental spleen asthenia and its significance. Zhongguo Zhongxiyi Jiehe Piwei Zazhi. 1999;7:8–11. [Google Scholar]
- 40.Yao YL, Song YG, Zhang WD. Changes and significance of G cells and D cells of experimental functional disturbance of gastrointestinal tract model. Zhongguo Zuzhi Huaxue Yu Xibao Huaxue Zazhi. 2000;9:293–296. [Google Scholar]


