INTRODUCTION
Our previous study has proved that Kupffer cells may have an inhibitory effect on the process of hepatocarcinogenesis[1], however, their inhibitory mechanism needs exploring deeply. We performed a comparative study on the expression of PCNA, Bax, p53 and apoptosis of liver cancer cells using immunohistochemical technology and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-dig oxigenin nick end labeling (TUNEL) in the diethylnitrosamine-induced hepatocellular carcinoma (HCC) in rats with or without pretreatment with gadolinium chloride or zymosan which might effectively block or enhance the activity of Kupffer cells in order to know the role of Kupffer cells in apoptosis in the experimental HCC and explore further the inhibitory mechanism of Kupffer cells on the process of hepatocarcinogenesis.
MATERIALS AND METHODS
Establishment of animal models and pathological examination
One hundred and forty male Sprague-Dawley rats were divided into six groups. ① DENA group, 40 rats received diethylnitrosamine (DENA) at a dose of 70 mg/kg in distilled water once/wk till wk15. ② GC + DENA group, gadolinium chloride was injected iv at a dose of 10 mg/kg once 2 wk to suppress Kupffer cells in 40 rats till wk15 and these rats simultaneously received DENA just as DENA group. ③ ZM + DENA group, zymosan was injected iv at a do se of 20 mg/kg once 2 wk to activate Kupffer cells till wk15 and DENA was received just as DENA group. ④ GC group, gadolinium chloride was injected iv at a dose of 10 mg/kg in 0.85% NaCl once 2 wk till wk 15. ⑤ ZM group, zymosan was injected iv at a dose of 20 mg/kg in 0.85% NaCl once 2 wk till wk15. ⑥ Control group, these rats were maintained on a standard laboratory diet and tap water. All rats were killed at the wk21 of hepatocarcinogenesis. The liver samples were taken, fixed in 40 mL/L paraformaldehyde and embedded in paraffin. Each specimen was cut into 5 μm serial slices, stained with hemotoxylin-eosin and subjected to histopathological examination.
Immunohistochemical staining
The SP method was used, the 1st antibody was mouse-anti-human PCNA monoclonal antibody (Calbiochem Co., dilution 1:50), rabbit-anti-human Bax (Santa Cruz, dilution 1:50) and mouse-anti-human p53 monoclonal antibody (Novocastra Laboratories, dilution 1:50). The SP kit was purchased from Boehringer Mannheim, Germany. DAB staining was used. The dark brown staining of nuclei was taken as PCNA and p53-positive reaction. The dark brown granules in cytoplasm were taken as Bax-positive reaction. We used PBS to replace the 1st antibody as negative control.
Terminal deoxynucleotydial transferase-mediated biotinylated-dutp nick end labeling method (TUNEL method)
In situ Cell Apoptosis Detection kit was purchased from Boehringer Mannheim, Germany. After deparaffinization and rehydration in ethanol, the sections were incubated with 0.2 mol/L HCl for 20 min at room temperature (RT). They were then deproteinized by incubation with 30 mg/L proteinase (in 50 mM Tris-HCl, pH8.0; 5 mM EDTA) for 30 min at RT. Terminal deoxynucleo tidyl transferase (TdT) and biotinylated dUTP in TdT buffer (30 mM Trizma base, pH7.2; 140 mM sodium cacodylate; 1 mM cobalt chloride) were added to cover the sections, which were then incubated for 60 min in a humid atmosphere at 37 °C. The sections then reacted with alkaline phasphatase (ALP) for 30 min at RT. Visulization was carried out with NBT/BCIP. Nuclei with clear blue staining were regarded as positive. TUNEL incubation solution without terminal deoxynucleo tidyl transferase was used as negative control.
Proliferating index (PI) and apoptosis index (AI)
PI and AI were determined by Leitz ASM 68K image analyzing system purchased from Germany. Five visual fields (orginal magnificantion, 10 × 40) from every positive slide would be chosen and the TUNEL and PCNA-positive parenchymal cells as well as the total number of parenchymal cells in the same field would be counted. PI or AI was expressed as the percentage of the number of PCNA or TUNEL-positive parenchymal cells in the total parenchymal cells of the same field.
Proliferating index (PI) = (number of PCNA-positive cells/number of total cells) × 100%
Apoptosis index (AI) = (number of TUNEL-positive cells/number of total cells) × 100%
Statistical analysis
Fisher′s exact test, Student′s test and Spearman rank correlation were employed.
RESULTS
Macroscopic observation and histopathological examination (at wk21 of hepatocarcinogenesis)
Meither the control group, nor the GC group and ZM group showed any changes macro and microscopically; on the contrary, the liver surface in DENA group and GC + DENA group were covered with a lot of white nodules. The diameter of the largest nodules was 0.5 cm in DENA group and 2.0 cm in GC + DENA group. These nodules were diagnosed as HCC histologically. Some white grey focal nodules scattered over the liver surface in ZM+DENA group, were also diagnosed as HCC with many apoptotic cells and apoptotic bodies.
Bax staining
Cytoplasm of cancer cells with clear brown staining was regarded as positive (Figure 1). The positive rates of Bax in ZM + DENA group, DENA group and GC + DENA group were 84.6% (11/13), 28.6% (2/7) and 27.3% (3/11) respectively. It was significantly higher in ZM + DENA group than that in DENA group (Fisher′s exact test, P < 0.05).
Figure 1.
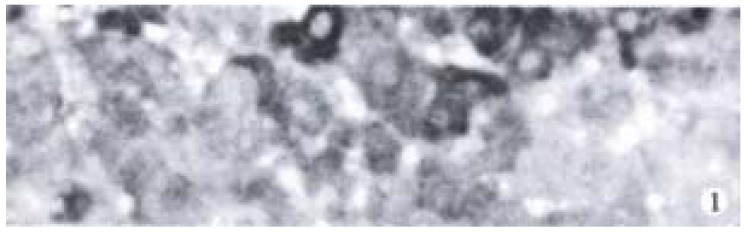
Expression of Bax in rat HCC in ZM + DENA group. SP × 400
p53 staining
A clear brown staining of the nuclei in cancer cells was regarded as positive (Figure 2). The positive rates of p53 in ZM + DENA group, DENA group and GC + DENA group were 76.9% (10/13), 14.3% (1/7) and 36.4% (4/11) respectively. It was markedly higher in ZM + DENA group than that in DENA group (Fisher′s exact test, P < 0.05).
Figure 2.
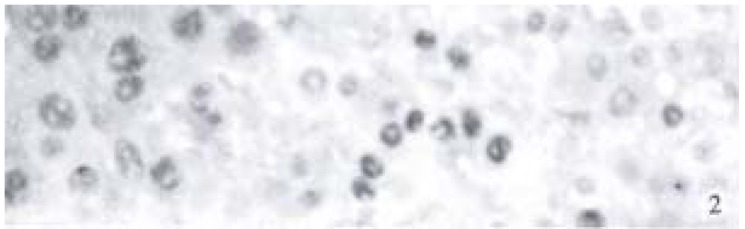
Expression of p53 in rat HCC in DENA group. SP × 400
PCNA staining
Nuclei with clear brown staining were regarded as positive (Figure 3).
Figure 3.
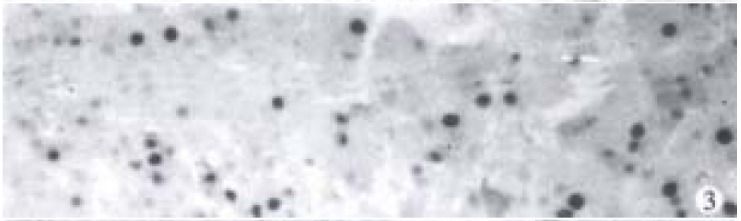
Expression of PCNA in rat HCC in DENA group. SP × 200
No expressions of Bax, p53 and PCNA in the control group, GC group and ZM group were found.
Apoptosis of cancer cells using TUNEL method
Apoptotic cells were observed in DENA group, GC + DENA group and ZM + DENA group by means of TUNEL method. The cells with clear nuclear labeling were defined as TUNEL-positive cells. Apoptotic cells were scattered in the cancer tissue (Figure 4).
Figure 4.
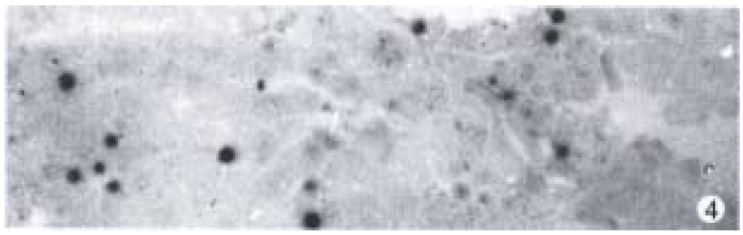
Apoptotic cells in rat HCC in ZM + DENA group. TUNEL × 200
Proliferating index (PI) and apoptosis index (AI) (Table 1)
Table 1.
PI and AI in ZM+DENA group, DENA group and GC + DENA group
| Groups | Number | PI (%, -x ± s) | AI (%, -x ± s) | PI/AI |
| ZM + DENA group | 13 | 15.22 ± 2.17b | 7.53 ± 1.61b | 2.20 |
| DENA group | 7 | 24.97 ± 2.53 | 4.36 ± 1.18 | 5.73 |
| GC + DENA group | 11 | 38.69 ± 3.17b | 2.52 ± 0.81b | 15.40 |
P < 0.01, vs DENA group, Fisher′s exact test.
Correlation between apoptosis index, Bax and p53 expression
There was a positive correlation between apoptosis index and Bax or p53 protein reactivity in ZM + DENA group, DENA group and GC + DENA group (Table 2).
Table 2.
Correlation between apoptosis index and Bax or p53 expression
| Groups | Bax-positive cases/total |
AI vs Bax |
p53-positive cases/total |
AI vs p53 |
||
| rs | P | rs | P | |||
| ZM + DENA group | 11/13 | 0.63 | < 0.05 | 10/13 | 0.73 | < 0.05 |
| DENA group | 2/7 | 0.79 | < 0.05 | 1/7 | 0.61 | |
| GC + DENA group | 3/11 | 0.74 | < 0.01 | 4/11 | 0.82 | < 0.05 |
Spearman rank correlation.
DISCUSSION
Gadolinium chloride is believed to be a specific suppressor of Kupffer cells[1,2]. Zymosan is an immunopotentiator and may be used to activate Kupffer cells[3]. We performed a comparative study on apoptosis in DENA-induced hepatocellular carcinoma in rats with or without pretreatment with gadolinium chloride or zymosan which might effectively block or enhance the activity of Kupffer cells in order to clarify whether the Kupffer cells play a role in apoptosis of the experimental hepatocarcinogenesis or not.
Proliferating cell nuclear antigen (PCNA) is an auxiliary protein of DNA polymerize-delta. It accumulates little in the resting stage cell, but prominently in the nuclei of proliferating cells during G1-late phase and S-phase and decreases in G2-phase and M-phase. It is associated with cell proliferation an d regarded as a biological marker for cell proliferation[4]. Bax is a Mr21000 protein with extensive amino acid homology with Bcl-2. This protein has been shown to form heterodimers with apoptosis inhibiting protein, Bcl2, so it can induce cell apoptosis[5]. When DNA is injured by toxicant, wild-type p53 drives cell to G1 and arrests or inhibits cell proliferation until DNA is repaired. When nuclear DNA is badly damaged or cannot be rep aired, wild-type p53 induces transcription of apoptotic genes and drives the cell to be in apoptosis[6]. Gottlieb et al[7] thought that overexpression of p53 was related to cell apoptosis. Zhao et al[8] observed that there was a positive correlation between apoptosis index and p53 protein, and supported the role of p53 in regulating apoptosis in human HCC. The positive correlation between apoptosis index and p53 protein immunoreactivity observed in our study also supports these findings. A new method to detect apoptosis in situ, terminal deoxynucleotidyl transferase-mediated (TdT) dUTP-digoxigenin nick end labeling (TUNEL) was recently developed by Gavrieli et al[9], and was applied to detect cell apoptosis of human HCC and other tumors[10].
Our results showed that the positive rates of Bax and p53 protein were markedly higher in ZM + DENA group than in DENA group with significant differences. Proliferating index and apoptosis index respectively increased or decreased in ZM +DENA group, DENA group and GC + DENA group successively. These results demonstrated that the blockage of Kupffer cells with gadolinium chloride might suppress cell apoptosis and the activation of Kupffer cells with zymosan might promote cell apoptosis in the experimental hepatocarcinogenesis.
One of the characteristic features of cancer is the continuous growth and the ratio of cell proliferation and cell apoptosis determine the fate of tumor growth[11-13]. In normal tissues, apoptosis is an efficient way of eliminating transformed cells. However, inability of cells to undergo apoptosis may advance their development, both by allowing the accumulation of dividing cells and by impairing the elimination of genetic mutants that may harbor malignant potential[11,12]. Our results proved that Kupffer cells could promote apoptosis in the experimental hepatocarcinogenesis and revealed further that they played an inhibitory role in hepatocarcinogenesis through inducing apoptosis of tumor cells.
Footnotes
Dr. Hai-Zhen Zhu, graduated from Tongji Medical University in 1997, engaged in the researches of antitumor of Kupffer cell under the instruction of Professor You Bing Ruan, having four papers published.
Edited by You DY
proofread by Sun SM
References
- 1.Zhu H, Ruan Y, Wu Z. [The influence of Kupffer cells on experimental hepatocarcinogenesis] Zhonghua Binglixue Zazhi. 1998;27:102–104. [PubMed] [Google Scholar]
- 2.Kukan M, Vajdová K, Horecký J, Nagyová A, Mehendale HM, Trnovec T. Effects of blockade of Kupffer cells by gadolinium chloride on hepatobiliary function in cold ischemia-reperfusion injury of rat liver. Hepatology. 1997;26:1250–1257. doi: 10.1002/hep.510260524. [DOI] [PubMed] [Google Scholar]
- 3.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 4.Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci USA. 1985;82:3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung WC, Chuang LY. Induction of apoptosis by sphingosine-1-phosphate in human hepatoma cells is associated with enhanced expression of bax gene product. Biochem Biophys Res Commun. 1996;229:11–15. doi: 10.1006/bbrc.1996.1750. [DOI] [PubMed] [Google Scholar]
- 6.Mitry RR, Sarraf CE, Wu CG, Pignatelli M, Habib NA. Wild-type p53 induces apoptosis in Hep3B through up-regulation of bax expression. Lab Invest. 1997;77:369–378. [PubMed] [Google Scholar]
- 7.Gottlieb TM, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Zimmermann A. Apoptosis in human hepatocellular carcinoma and in liver cell dysplasia is correlated with p53 protein immunoreactivity. J Clin Pathol. 1997;50:394–400. doi: 10.1136/jcp.50.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hino N, Higashi T, Nouso K, Nakatsukasa H, Tsuji T. Apoptosis and proliferation of human hepatocellular carcinoma. Liver. 1996;16:123–129. doi: 10.1111/j.1600-0676.1996.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 11.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 12.Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA. 1991;88:3412–3415. doi: 10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]


