Abstract
Background:
Continuous interscalene blocks provide excellent analgesia after shoulder surgery. Although the safety of the ultrasound-guided in-plane approach has been touted, technical and patient factors can limit this approach. We developed a caudad-to-cephalad out-of-plane approach and hypothesized that it would decrease pain ratings due to better catheter alignment with the brachial plexus compared to the in-plane technique in a randomized, controlled study.
Objectives:
To compare an out-of-plane interscalene catheter technique to the in-plane technique in a randomized clinical trial.
Patients and Methods:
Eighty-four patients undergoing open shoulder surgery were randomized to either the in-plane or out-of-plane ultrasound-guided continuous interscalene technique. The primary outcome was VAS pain rating at 24 hours. Secondary outcomes included pain ratings in the recovery room and at 48 hours, morphine consumption, the incidence of catheter dislodgments, procedure time, and block difficulty. Procedural data and all pain ratings were collected by blinded observers.
Results:
There were no differences in the primary outcome of median VAS pain rating at 24 hours between the out-of-plane and in-plane groups (1.50; IQR, [0 - 4.38] vs. 1.25; IQR, [0 - 3.75]; P = 0.57). There were also no differences, respectively, between out-of-plane and in-plane median PACU pain ratings (1.0; IQR, [0 - 3.5] vs. 0.25; IQR, [0 - 2.5]; P = 0.08) and median 48-hour pain ratings (1.25; IQR, [1.25 - 2.63] vs. 0.50; IQR, [0 - 1.88]; P = 0.30). There were no differences in any other secondary endpoint.
Conclusions:
Our out-of-plane technique did not provide superior analgesia to the in-plane technique. It did not increase the number of complications. Our technique is an acceptable alternative in situations where the in-plane technique is difficult to perform.
Keywords: Peripheral Nerve, Brachial Plexus Block, Regional Anesthesia, Nerve Block, Shoulder Pain
1. Background
Continuous, ultrasound-guided blockade of the brachial plexus via an interscalene approach has already been shown to provide excellent analgesia after outpatient shoulder surgery (1, 2). Both in-plane and out-of-plane techniques have been described, with some studies supporting the in-plane technique (1, 3-5) and a single study that directly compared the in-plane to an out-of-plane approach concluding that the out-of-plane technique provided superior analgesia (6). The in-plane technique involves threading the catheter through the middle scalene muscle, which can be painful and challenging in some patients. It can also be difficult in morbidly obese patients. In an effort to address these challenges, we have developed an alternative out-of-plane technique that involves directing the needle and catheter in a caudad-to-cephalad direction, which should position the catheter into closer alignment with the superior portions of the brachial plexus and thus provide better analgesia for the shoulder. It also avoids insertion through the middle scalene muscle, a potential source of pain during the procedure, and does not require any posterolateral space next to the neck to perform the block, which can be a problem in patients with limited neck motion. Based on our clinical observations of this technique during the previous year and the potential for improved catheter alignment and proximity, as suggested by previous authors (7), we hypothesized that catheters placed with the out-of-plane technique would decrease visual analog scale (VAS) pain ratings by 2 cm on a 10-cm scale as measured at 24 hours compared to in-plane catheters.
2. Objectives
The objective of this study was to compare an ultrasound-guided out-of-plane technique for placement of interscalene catheters to the commonly performed in-plane technique.
3. Patients and Methods
The institutional review board of Thomas Jefferson University (Office of Human Research, Division of Human Subjects Protection, 1015 Chestnut St, Suite 1100, Philadelphia, PA 19107; Control #12D.328) approved the study on June 21, 2012 and all enrolled patients gave written informed consent. It was prospectively registered with clinicaltrials.gov (NCT01696188) on September 13, 2012 (available at https://clinicaltrials.gov/ct2/show/NCT01696188?term=interscalene+catheters&rank) (8) in accordance with our institutional review board regulations. The study population included all patients undergoing open shoulder surgery at our hospital from August 2012 through February 2014 who were scheduled to be admitted overnight. Inclusion criteria included: 18 years of age, English as native language, and American Society of Anesthesiologists Physical Status 1 - 3. Exclusion criteria included: patient refusal, contraindication to interscalene block (coagulopathy or infection at the block site or significant diaphragm dysfunction diagnosed by history or chest radiograph), and opioid tolerance (defined as taking greater than or equal to the equivalent of oxycodone 20 mg daily).
A peripheral intravenous catheter was placed for all patients in the preoperative holding area after informed consent was obtained. Prior to the block midazolam was given at the discretion of the attending anesthesiologist. The blocks and catheters were placed by one of three fellowship-trained regional anesthesiologists or regional fellows under supervision by those same anesthesiologists. All had experience with both techniques. Patients were taken to the block rooms outside of the operating room (OR), where group allocation was revealed (in-plane or out-of-plane). Randomization was performed using sealed, unlabeled envelopes that were not opened until the patient reached the block room. The principal investigator (ES) generated the random sequence. One envelope was removed by a study team member for each patient from a bag containing all the remaining envelopes. After each patient was enrolled the bag was reshuffled. Although the anesthesiologist performing the block was aware of group allocation, all personnel performing post-block assessments, as well as all patients, were blinded. Personnel performing post-block assessments were not permitted in the block rooms during block performance and were not permitted to view any group allocation information that would reveal patient assignment.
3.1. Block Details Common to Both Approaches
After sterile preparation of the neck with chlorhexidine, the roots/trunks of the brachial plexus were identified by placing the ultrasound probe above and parallel to the clavicle, then sliding it the probe cephalad until the divisions of the brachial plexus united to form two or three hypoechoic structures (roots or trunks). An 18-g stimulating Tuohy needle (B Braun, Bethlehem, PA) and 20-g multi-orifice catheter (B Braun, Bethlehem, PA) were used for both the in-plane and out-of-plane approaches. The ultrasound probe was covered with a sterile sheath and the clinician performing the block wore a head cover, face mask, and sterile gloves. A nerve stimulator was attached and the current was adjusted to 1 mA. After placement of a skin wheal with 2% lidocaine, the Tuohy needle was inserted in the skin and advanced into the connective tissue between the two most superficial brachial plexus structures. If a biceps, shoulder, or triceps twitch was observed, the current was slowly decreased until the twitch disappeared. However, local anesthetic was injected based on ultrasound image, regardless of the presence or absence of a twitch with nerve stimulation. The catheter was then advanced blindly to 3 cm beyond the needle tip. After the catheter was inserted, the Tuohy needle was removed and catheter were secured using SurgiSeal skin sealant (Adhezion, Wyomissing, PA) and an EpiGuard catheter-securing device (Copenhagen Medlab, Copenhagen, Denmark). Our standard taping procedure makes identifying the block approach impossible.
Procedure time was from the skin wheal until removal of the Tuohy needle from the skin. A nurse who was not blinded recorded the time with a stopwatch.
3.2. In-Plane Approach Details
After the brachial plexus was identified, a skin wheal with lidocaine 2% was made laterally to the ultrasound probe (Figure 1A) and the Tuohy needle was inserted in-plane under continuous visualization and advanced to the target as described above. Thirty mL of ropivacaine 0.5% were injected through the needle with frequent aspiration and then the catheter was inserted blindly through the needle to a distance of 3 cm beyond the needle tip.
Figure 1. A) In-Plane Approach, B) Out-of-Plane Approach. The Skin Has Been Peeled Away to Show The Underlying Brachial Plexus and Possible Spatial Relationship Between The Catheter After it is Inserted and The Superior Portions of The Plexus.
3.3. Out-of-Plane Approach Details
After the brachial plexus identification and skin wheal placement and the Tuohy needle was inserted right under the center of the probe and advanced in a cephalad direction (Figure 1B) to the target. Needle advancement was confirmed with tissue movement and needle tip identification by ultrasound. If needle position was unclear, 1 - 2 mL of local anesthetic were injected to identify position and the needle was adjusted as necessary. Thirty mL of ropivacaine 0.5% was injected through the needle with frequent aspiration and then the catheter was then inserted blindly through the needle to a distance 3 cm beyond the needle tip.
3.4. Maintenance of Anesthesia and Continuous Interscalene Block
General anesthesia with an endotracheal tube was administered for the intraoperative period per surgeon request, unless the patient requested a regional anesthetic. General anesthesia was induced with propofol 1 - 2 mg/kg, rocuronium, and fentanyl 1 - 2 mcg/kg. Fentanyl was given as indicated by the intraoperative anesthesia team for signs of sympathetic response to surgery. Maintenance of anesthesia was with sevoflurane or desflurane. If only regional anesthesia was chosen, a propofol infusion was given for patient comfort.
The continuous interscalene block infusion was started in the PACU and consisted of 0.2% ropivacaine at 10 cc/hour. Boluses were 5 cc of 0.2% ropivacaine and were given as needed by the acute pain team for numerical rating scale pain scores greater than 5 out of 10.
3.5. Post-Block Assessments
Beginning at five minutes after removal of the Tuohy needle from the skin, a blinded study assistant assessed sensory and motor function every five minutes until block onset, which was defined as a sensory score of 0 or 1 in both C5 and C6 dermatomes. Sensation in the C5-8 dermatomes was assessed on a scale of 0 - 2, with 0 = no sensation, 1 = decreased sensation, and 2 = normal sensation. Deltoid and biceps strength were both assessed on a scale of 0 - 3, with 0 = no motor function, 1 = significant weakness (unable to move against resistance), 2 = mild weakness (able to move against resistance but decreased), and 3 = normal strength. Sensory and motor examinations were repeated in the PACU, at 24 hours, and at 48 hours.
VAS pain ratings at rest were assessed in the post-anesthesia care unit (PACU), at 24 hours, and 48 hours after block placement. For the VAS assessments, patients were asked to make a mark on a 10-cm line corresponding to their pain level, with “0” being “no pain at all” and “10” being “the worst pain possible.” Interscalene catheters were inspected and categorized as “in place” or “dislodged.” A “dislodged” catheter was any catheter for which the catheter tip was visible outside of the skin. Patients were questioned about the presence of nausea, vomiting, dizziness, and tinnitus, all of which were recorded as “yes” or “no” responses. If patients were discharged before 48 hours, post-hospital assessments were not made. Patients were given either a fentanyl or morphine PCA without restriction in the PACU and until postoperative day 1, after which either oral oxycodone or oral hydromorphone was given as needed. All opioids received (oral and IV) were recorded and converted to IV morphine equivalents using an online opioid conversion scale.
3.6. Statistical Analysis
For determination of sample size, a power analysis was performed based on the primary objective, which was to evaluate VAS pain scores between the out-of-plane and in-plane continuous interscalene block techniques. The investigators agreed that a minimum of two points in pain reduction at 24 hours, on a 10-cm VAS pain scale, would be considered a clinically relevant difference. Twenty-four hours was chosen as the primary endpoint because a primary block with 30 cc of ropivacaine 0.5% wears off in virtually all patients by then and analgesia at that time solely reflects the catheter. The sample size was determined using a power analysis for a one-way fixed effects analysis of variance with two levels assuming a two-point difference between the two means, with a hypothesized standard deviation of three. With 37 subjects per treatment group (N = 74), this study has 80% power to detect a difference in VAS scores among the two groups. The criterion for significance (alpha) has been set 0.05 and the analysis of variance is non-directional. Approval for an additional 10 patients was requested to account for patient dropouts. Statistical analyses were performed using Systat version 13 (Systat Software, Inc., San Jose, CA) and SPSS Software, Ver. 20.0 (IBM Corporation, Armonk, NY). P Values reported were from independent samples t-test for normally distributed continuous data or the Pearson chi square or Fisher exact tests, as appropriate, for categorical data. Continuous data are reported as means with 95% confidence interval (CI); VAS ratings and morphine consumption were analyzed using the Kruskal-Wallis test for independent samples. VAS ratings and morphine consumption are reported as medians with interquartile ranges.
4. Results
Out of a total of 130 patients who were assessed for eligibility from August 2012 through June 2014, a total of 84 patients were randomized, of which 41 were in-plane and 43 were out-of-plane. Two patients, one from each group, were excluded from analysis after randomization (see Figure 2): patient #1 was later found to be taking greater than 20 mg daily of oxycodone and patient #2 had extremely limited neck motion and the in-plane approach was technically difficult, so an out-of-plane technique was performed successfully. There were no differences in demographics, American Society of Anesthesiologists (ASA) Physical Status, and surgical characteristics between the groups (Table 1). All but one patient, an 89-year-old ASA Physical Status 3 female who requested a regional anesthetic, were given general anesthesia.
Figure 2. Consort Flow Diagram.
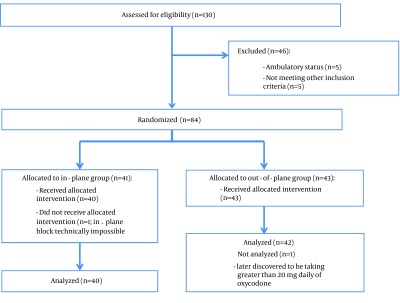
Table 1. Patient Demographics and Surgical Characteristics.
| Variables | In-Plane (n = 40) | Out-of-Plane (n = 42) | P |
|---|---|---|---|
| Gender, F:M | 25:15 | 17:25 | 0.051 |
| Age, y a | 67.1 (63.8-70.4) | 63.8 (60.2-67.4) | 0.188 |
| BMI, kg/m 2 a | 29.1 (27.5-30.7) | 31.12 (28.9-33.3) | 0.151 |
| ASA Physical Status, I/II/III | 1/21/18 | 0/24/18 | 0.560 |
| Home analgesic use b,c | 22 (55) | 22 (52.4) | 0.828 |
| Surgery Type c | 0.942 | ||
| Open rotator cuff repair | 1 (2.5) | 1 (2.4) | |
| Total shoulder arthroplasty | 24 (60) | 28 (66.7) | |
| Reverse total shoulder arthroplasty | 10 (25) | 9 (21.4) | |
| Revision total shoulder arthroplasty | 1 (2.5) | 2 (4.7) | |
| Shoulder hemi-arthroplasty | 1 (2.5) | 1 (2.4) | |
| ORIF humerus fracture | 3 (7.5) | 1 (2.4) | |
| Anesthesia duration, min a | 131.5 (130.1 - 132.9) | 124.7 (123.6 - 125.8) | 0.243 |
| Surgery time, min a | 90.33 (89.2 - 91.5) | 84.6 (83.7 - 85.5) | 0.223 |
aValues are presented as Mean (95% Confidence Interval).
bIncluded opioids, cyclooxygenase-2 inhibitors, and nonsteroidal anti-inflammatory drugs.
cValues are presented as No. (%).
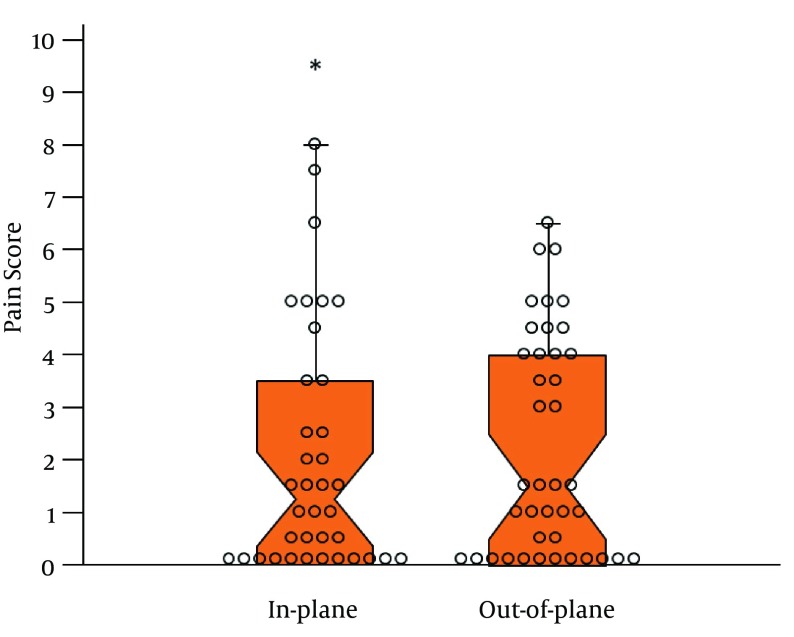
For the primary outcome of VAS pain ratings at 24 hours, the out-of-plane technique was not superior to the in-plane technique, respectively (median VAS 1.50; IQR, [0 - 4.28] vs. 1.25; IQR, [0 - 3.75], P = 0.570; Table 2 ; Figure 3). In the PACU, there was no difference in median VAS pain ratings (1.0; IQR, [0 - 3.50] vs. 0.25; IQR, [0 - 2.50]; P = 0.079; Table 2), and at 48 hours there was no difference in median VAS pain ratings (1.25; IQR, [1.25 - 2.63] vs. 0.50; IQR, [0 - 1.88], P = 0.301; Table 2). There was no difference in median morphine consumption at 24 hours between the out-of-plane and in-plane groups, respectively (22.50 mg; IQR, [12.00 - 45.00] vs. 15.70 mg; IQR [5.00 - 44.20]; P = 0.189; Table 3). For other secondary outcomes, there were no differences in block difficulty, skin punctures, traumatic blocks, local anesthetic spread, inadvertent vascular punctures, final nerve stimulation current, and muscle twitch location (Table 4). There was no difference in mean procedure time for the out-of-plane and in-plane groups, respectively (257.8 seconds; 95% CI, [238.1 - 277.4] vs. 296.1 seconds; 95% CI, [255.2 - 336.9]; P = 0.093). There were no differences in the proportion of patients in each group with sensory or motor block at any time during the study (Table 3). There were also no differences in the number of catheter dislodgments and catheter boluses needed in the PACU and at 24 and 48 hours (Table 2).
Table 2. Pain Ratings and Postoperative Analgesic Interventionsa.
| Variables | In-Plane (n = 40) | Out-of-Plane (n = 42) | P b |
|---|---|---|---|
| VAS score at rest, cm c,d | |||
| PACU | 0.3 (0 - 2.5) | 1.0 (0 - 3.5) | 0.079 |
| 24 h | 1.3 (0 - 3.8) | 1.5 (0 - 4.5) | 0.570 |
| 48 h | 0.5 (0 - 1.9) | 1.3 (1.25 - 2.6) | 0.301 |
| Patients with VAS score at rest > 4 e | |||
| 24 h | 9 (23.7) | 15 (37.5) | 0.225 |
| 48 h | 5 (15.6) | 3 (10.0) | 0.709 |
| Morphine consumption, mg d | |||
| PACU | 0.0 (0 - 1.5) | 0.0 (0 - 5.0) | 0.130 |
| 24 h (cumulative) | 15.7 (5.0 - 44.2) | 22.5 (12.0 - 45.0) | 0.189 |
| 48 h (cumulative) | 25.0 (12.5 - 65.0) | 35.3 (24.8 - 70.7) | 0.208 |
| Incidence of catheter boluses before 24 h f | |||
| 0 | 19/40 (47.5) | 18/42 (42.9) | |
| > 1 | 21/40 (52.5) | 24/42 (57.1) | 0.825 |
| Incidence of catheter boluses 24 - 48 h f | |||
| 0 | 34/37 (91.9) | 35/38 (92.1) | |
| > 1 | 3/37 (8.1) | 3/38 (7.9) | 1.00 |
| Catheter dislodgments ≤ 24 h f | 4/40 (10) | 1/42 (2.4) | 0.149 |
| Catheter dislodgments ≤ 48 h f | 4/40 (10) | 5/42 (11.9) | 0.626 |
aAbbreviations: IV = intravenous.
bP Value based on Fisher’s exact test for categorical variables or Student’s t-test for continuous variables (normal distribution). For VAS and opioid consumption, P Value was based on independent samples Kruskal-Wallis test.
cVAS range is 0 (no pain) to 10 cm (worst pain imaginable).
dValues are presented as Median (IQR).
eValues are presented as No. (%).
fValues are presented as Fraction (%).
Figure 3. Notched Dox Plot Illustrating the Median VAS Pain Scores on a 10-cm Scale at 24 Hours for the In-Plane and Out-of-Plane Groups.
Table 3. Sensory and Motor Assessmentsa.
| Post-Block Sensory and Motor Assessmentb,c | In-Plane (n = 40) | Out-of-Plane (n = 42) | Pd |
|---|---|---|---|
| 5 min | |||
| C5 | 24 (63.2) | 27 (69.2) | 0.635 |
| C6 | 21 (55.3) | 22 (56.4) | 1.00 |
| C7 | 17 (44.7) | 16 (41.0) | 0.820 |
| C8 | 13 (34.2) | 10 (25.6) | 0.462 |
| Arm/shoulder abduction | 22 (84.6) | 27 (90.0) | 0.693 |
| Bicep flexion | 20 (74.1) | 26 (81.3) | 0.544 |
| 10 min | |||
| C5 | 32 (84.2) | 35 (89.7) | 0.517 |
| C6 | 32 (84.2) | 36 (90.0) | 0.512 |
| C7 | 29 (76.3) | 22 (55.5) | 0.059 |
| C8 | 21 (55.3) | 17 (42.5) | 0.365 |
| Arm/shoulder abduction | 25 (92.6) | 33 (100) | 0.198 |
| Bicep flexion | 24 (85.7) | 34 (97.1) | 0.162 |
| PACU | |||
| C5 | 37 (97.4) | 40 (97.6) | 1.00 |
| C6 | 37 (97.4) | 41 (100) | 0.481 |
| C7 | 35 (92.1) | 39 (95.1) | 0.667 |
| C8 | 28 (73.7) | 31 (75.6) | 1.00 |
| 24 hours | |||
| C5 | 31 (86.1) | 35 (87.5) | 1.00 |
| C6 | 26 (72.2) | 27 (67.5) | 0.803 |
| C7 | 20 (55.6) | 17 (42.5) | 0.358 |
| C8 | 13 (36.1) | 13 (32.5) | 0.811 |
| 48 Hours | |||
| C5 | 22 (73.3) | 22 (75.9) | 1.00 |
| C6 | 18 (60.0) | 10 (34.5) | 0.069 |
| C7 | 8 (26.7) | 3 (10.3) | 0.181 |
| C8 | 8 (26.7) | 4 (13.8) | 0.333 |
aValues are presented as No. (%).
bSensory block was recorded as “yes” with pinprick score ≤ 1 or “no” with pinprick test score of 2.
cMotor block was recorded as “yes” or “no” based on a motor test score of ≤ 1 or ≥ 2. Unable to assess postoperative motor block due to presence of sling on arm.
dP Values are based on two-tailed Fisher’s exact test.
Table 4. Procedure Detailsa.
| Variables | In-plane (n=40) | Out-of-plane (n=42) | Pb |
|---|---|---|---|
| Number of punctures c | 1.15 ± 0.36 | 1.14 ± 0.42 | 0.930 |
| Ease of Visualization, easy:moderate:difficult | 33:6:1 | 29:11:2 | 0.365 |
| Deltoid contraction present d | 27 (67.5) | 27 (64.3) | 0.224 |
| Biceps contraction present d | 14 (35) | 14 (33.3) | 0.226 |
| Ending NS current, mA c | 0.48 (0.16) | 0.44 (0.16) | 0.424 |
| LA spread, good:moderate:poor | 32:8:0 | 38:4:0 | 0.180 |
| Procedural time, s e | 296 (255 - 337) | 257 (238 – 277) | 0.093 |
| Inadvertent vascular punctures d | 4 (10) | 4 (9.5) | 0.942 |
aAbbreviations: LA = Local anesthetic; NS = Nerve stimulation
bP Value based on independent samples t-test for continuous variables (normal distribution) and Pearson’s chi square analysis for categorical variables.
cValues are presented as mean ± SD.
dValues are presented as No. (%).
eValues are presented as mean (95% CI).
Individual data points are shown by open circles. The median pain scores are represented by the narrowest part of the box and the notches depict the upper and lower 95% confidence intervals of the median. The overlap of the 95% confidence intervals reflects that no statistically significant differences were observed between the two groups. The interquartile ranges are shown by the whiskers and one outlier score (asterisk).
5. Discussion
The principal finding of this study was that our out-of-plane approach was not superior to the in-plane approach. As a result, our out-of-plane technique should not replace the in-plane technique as the accepted standard. We formulated our hypothesis based on the possibility of improved catheter alignment with the brachial plexus that may occur with the out-of-plane technique and powered the study to detect a clinically meaningful 2-cm difference in VAS pain ratings. Although we failed to show this difference, we believe the median VAS pain scores of less than 2 in both groups clearly demonstrate that excellent analgesia was obtained in both groups and as a result our out-of-plane technique could be considered in situations where the standard in-plane technique is technically difficult. In our practice, these situations include patients with morbid obesity, limited neck mobility, and aberrant anatomy. However, like any peripheral nerve block technique, it requires significant practice and experience to master.
There were also no differences in the incidence of catheter dislodgments between the two techniques. Our dislodgment numbers (20% of in-plane patients and 14% of out-of-plane patients) are similar to those of Capdevila et al., who reported technical block failure rates of 13% and 17% in the two regional anesthesia groups (9). The lack of a difference between the groups in catheter dislodgments suggests that it may depend less on the approach and more on the fixation technique. Particularly in superficial locations, such as the interscalene groove, some peripheral nerve catheters will inevitably become dislodged.
Our results are in contrast to those of Fredrickson et al., who compared out-of-plane to in-plane, ultrasound-guided interscalene continuous blocks and found that patients in the out-of-plane group were more frequently pain-free in the recovery room and consumed less tramadol in the first 24 hours postoperatively (6). Those authors also reported a statistically significant decrease in the number of catheters with “difficult threading” in the out-of-plane group, as well as decreased median procedure time (9 minutes vs. 6.5 minutes), while we found no difference in mean procedure time. It should be noted that our out-of-plane technique differed from that of Fredrickson et al. because we inserted the needle and threaded the catheter in a caudad-to-cephalad direction, whereas they threaded their catheters in a cephalad-to-caudad direction (Figure 1B) (6). In addition, a single anesthesiologist who was more experienced with the out-of-plane technique performed all the blocks in their study, potentially affecting procedure time. One patient who was excluded from our final analysis after randomization was switched from the in-plane group to the out-of-plane group due to limited neck motion and technical difficulty with the in-plane technique. It is that type of patient where an alternate technique could be considered. Of note, all four patients in our study whose procedure time exceeded 8 minutes were in the in-plane group, suggesting that in certain patients significant difficulty was encountered with that technique. These outliers had procedure times that would affect clinical practice.
The out-of-plane approach described here may concern some clinicians due to a perceived similarity between our technique and Winnie’s classic approach (10). As Sardesai and colleagues demonstrated (11), Winnie’s approach can increase the likelihood of needle entrance into the intravertebral foramen. However, as seen in Figure 1B, our approach directs the needle and catheter cephalad and the discrepancy angle would be much larger than that of Winnie’s technique, making intraforamenal entry very unlikely. We did not experience any complications specifically related to this approach, such as epidural or intrathecal catheter insertion or vertebral artery puncture or placement, which we believe was largely due to strict adherence to monitoring of catheter insertion depth at 3 cm. One may argue that ultrasound visualization of catheter insertion would have helped ensure that catheters did not migrate into unwanted areas, such as the epidural space. However, as Ilfeld and colleagues pointed out, perineural catheters rarely stay within the two-dimensional plane of the ultrasound beam as they are advanced, and therefore this would not prevent such migration (7).
We believe a strength of our study is the inclusion of several different practitioners with variable experience levels with both regional anesthesia and the out-of-plane technique specifically. This represents clinical practice where both seasoned and novice anesthesiologists perform blocks. Despite this variability in skill level, excellent analgesia was obtained in both groups.
Our study is not without limitations. Because it was not possible to blind the clinician performing the block, it is possible that a preference for one technique over the other could have influenced block attempts or procedure time. Variability in the skill of the operator could also have affected block results, and we did not randomize the clinician performing the block, but patient randomization should have minimized this. The use of sealed envelopes in a bag could introduce the possibility of selection bias during randomization. Second, final catheter position was not observed with ultrasound and the primary block was injected through the needle before catheter insertion, so it is possible that some catheters may not have been in the ideal expected location. However, this limitation would apply equally to both out-of-plane and in-plane groups and, as mentioned earlier, ultrasound visualization of a three-dimensional catheter is not reliable. Finally, our study was not powered to detect rare complications of interscalene blocks, such as vertebral artery or epidural placement of catheters. Although both have been described in case reports (12, 13), they are unusual complications and our results should be interpreted in this context.
In conclusion, our out-of-plane approach for interscalene catheter placement was not superior to the in-plane approach more commonly used but it can be performed in the same amount of time without increasing the risk of complications and considered as a reasonable alternative in appropriate patients in whom the in-plane technique is not.
Acknowledgments
The authors would like to acknowledge Angelo Andonakakis for the artwork and Matthew Ramsey and Charles Getz for allowing participation of their patients.
Footnotes
Authors’ Contribution:Eric Schwenk designed the study, analyzed the data, and wrote the manuscript. Kishor Gandhi designed the study, analyzed the data, and wrote the manuscript. Jaime Baratta designed the study and helped conduct the study. Marc Torjman analyzed the data. Jaeyoon Chung helped collect the data and conduct the study. Richard Epstein analyzed the data and helped write the manuscript. Benjamin Vaghari helped conduct the study. David Beausang helped conduct the study. Elird Bojaxhi helped conduct the study. Bernadette Grady helped conduct the study.
Funding/Support:This study was funded by department funds only.
References
- 1.Mariano ER, Afra R, Loland VJ, Sandhu NS, Bellars RH, Bishop ML, et al. Continuous interscalene brachial plexus block via an ultrasound-guided posterior approach: a randomized, triple-masked, placebo-controlled study. Anesth Analg. 2009;108(5):1688–94. doi: 10.1213/ane.0b013e318199dc86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fredrickson MJ, Ball CM, Dalgleish AJ. Analgesic effectiveness of a continuous versus single-injection interscalene block for minor arthroscopic shoulder surgery. Reg Anesth Pain Med. 2010;35(1):28–33. doi: 10.1097/AAP.0b013e3181c771bd. [DOI] [PubMed] [Google Scholar]
- 3.Antonakakis JG, Sites BD, Shiffrin J. Ultrasound-guided posterior approach for the placement of a continuous interscalene catheter. Reg Anesth Pain Med. 2009;34(1):64–8. doi: 10.1016/AAP.0b013e3181933a53. [DOI] [PubMed] [Google Scholar]
- 4.Mariano ER, Loland VJ, Ilfeld BM. Interscalene perineural catheter placement using an ultrasound-guided posterior approach. Reg Anesth Pain Med. 2009;34(1):60–3. doi: 10.1097/AAP.0b013e3181933af7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandefo I, Bernard JM, Elstraete V, Lebrun T, Polin B, Alla F, et al. Patient-controlled interscalene analgesia after shoulder surgery: catheter insertion by the posterior approach. Anesth Analg. 2005;100(5):1496–8. doi: 10.1213/01.ANE.0000149901.42804.92. [DOI] [PubMed] [Google Scholar]
- 6.Fredrickson MJ, Ball CM, Dalgleish AJ. Posterior versus anterolateral approach interscalene catheter placement: a prospective randomized trial. Reg Anesth Pain Med. 2011;36(2):125–33. doi: 10.1097/aap.0b013e31820d5ee6. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123–6. doi: 10.1097/AAP.0b013e3181d245a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings KC, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107(3):446–53. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila X, Dadure C, Bringuier S, Bernard N, Biboulet P, Gaertner E, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566–73. doi: 10.1097/00000542-200609000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455–66. [PubMed] [Google Scholar]
- 11.Sardesai AM, Patel R, Denny NM, Menon DK, Dixon AK, Herrick MJ, et al. Interscalene brachial plexus block: can the risk of entering the spinal canal be reduced? A study of needle angles in volunteers undergoing magnetic resonance imaging. Anesthesiology. 2006;105(1):9–13. doi: 10.1097/00000542-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gaus P, Heb B, Tanyay Z, Muller-Breitenlohner H. [Epidural malpositioning of an interscalene plexus catheter]. Anaesthesist. 2011;60(9):850–3. doi: 10.1007/s00101-011-1900-5. [DOI] [PubMed] [Google Scholar]
- 13.Faust A, Fournier R, Hagon O, Hoffmeyer P, Gamulin Z. Partial sensory and motor deficit of ipsilateral lower limb after continuous interscalene brachial plexus block. Anesth Analg. 2006;102(1):288–90. doi: 10.1213/01.ane.0000183638.76874.73. [DOI] [PubMed] [Google Scholar]